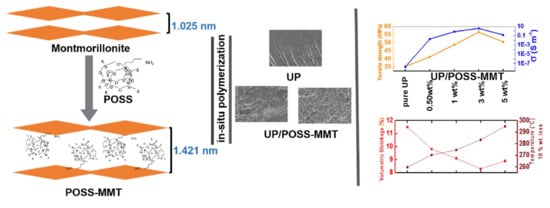
Effect of POSS-Modified Montmorillonite on Thermal, Mechanical, and Electrical Properties of Unsaturated Polyester Nanocomposites
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis of POSS-Modified MMT (POSS-MMT)
2.3. Synthesis of UP Resin
2.4. Synthesis of UP/MMT-POSS Nanocomposites via In-Situ Polymerization
2.5. Characterization
3. Results and Discussion
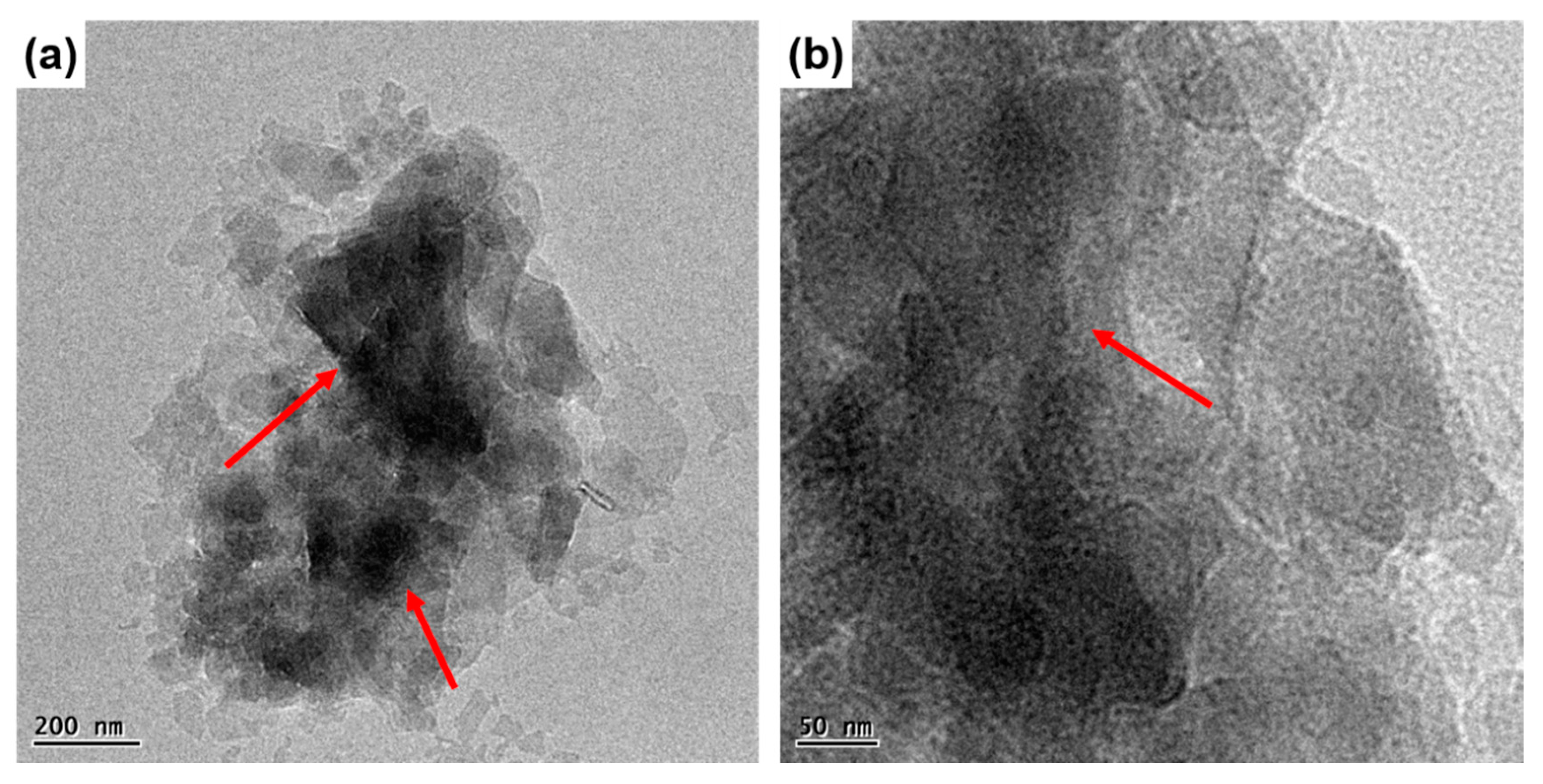
3.1. Structural and Morphological Analysis of POSS-Modified MMT
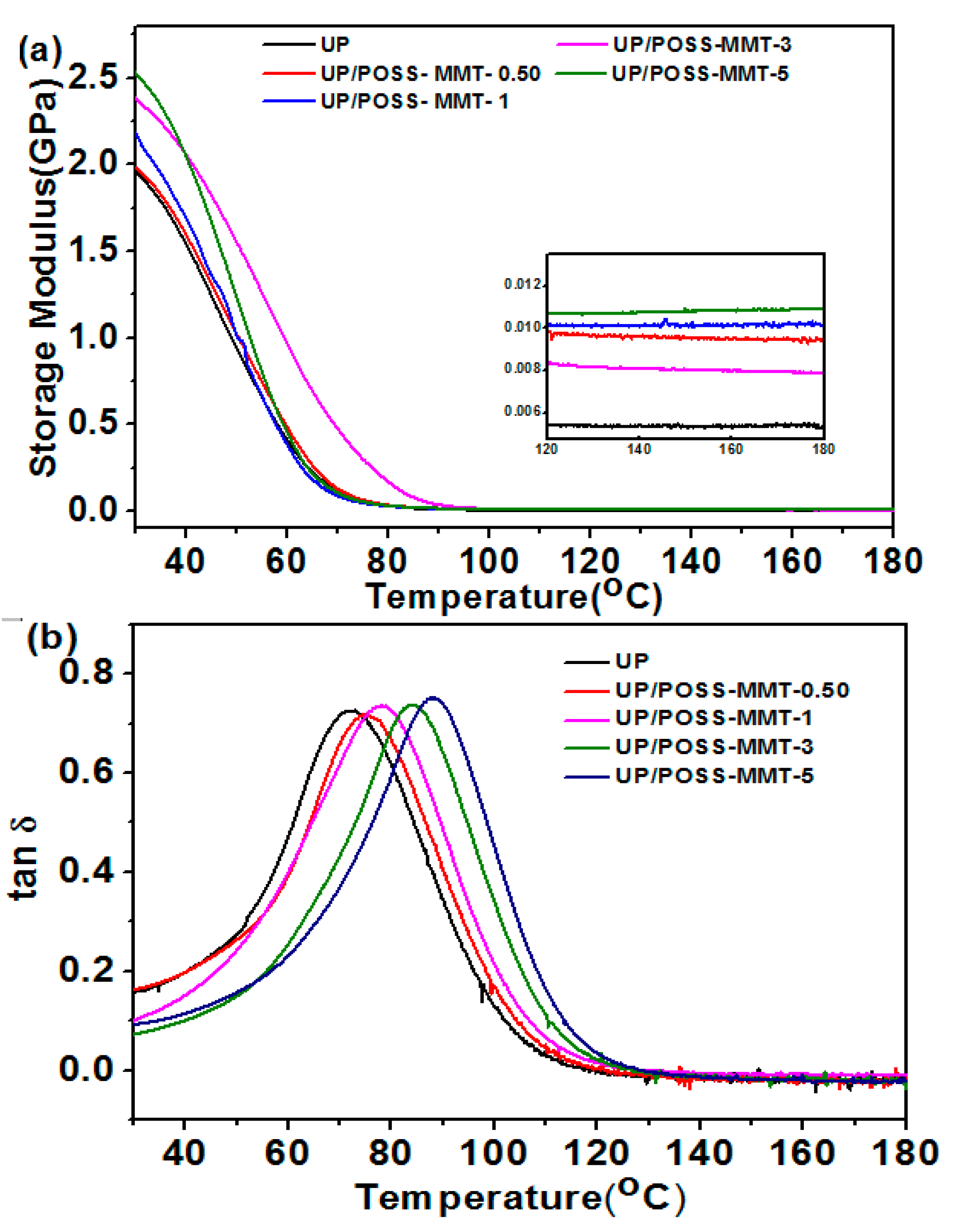
3.2. Thermal Properties of UP/POSS-MMT Nanocomposites
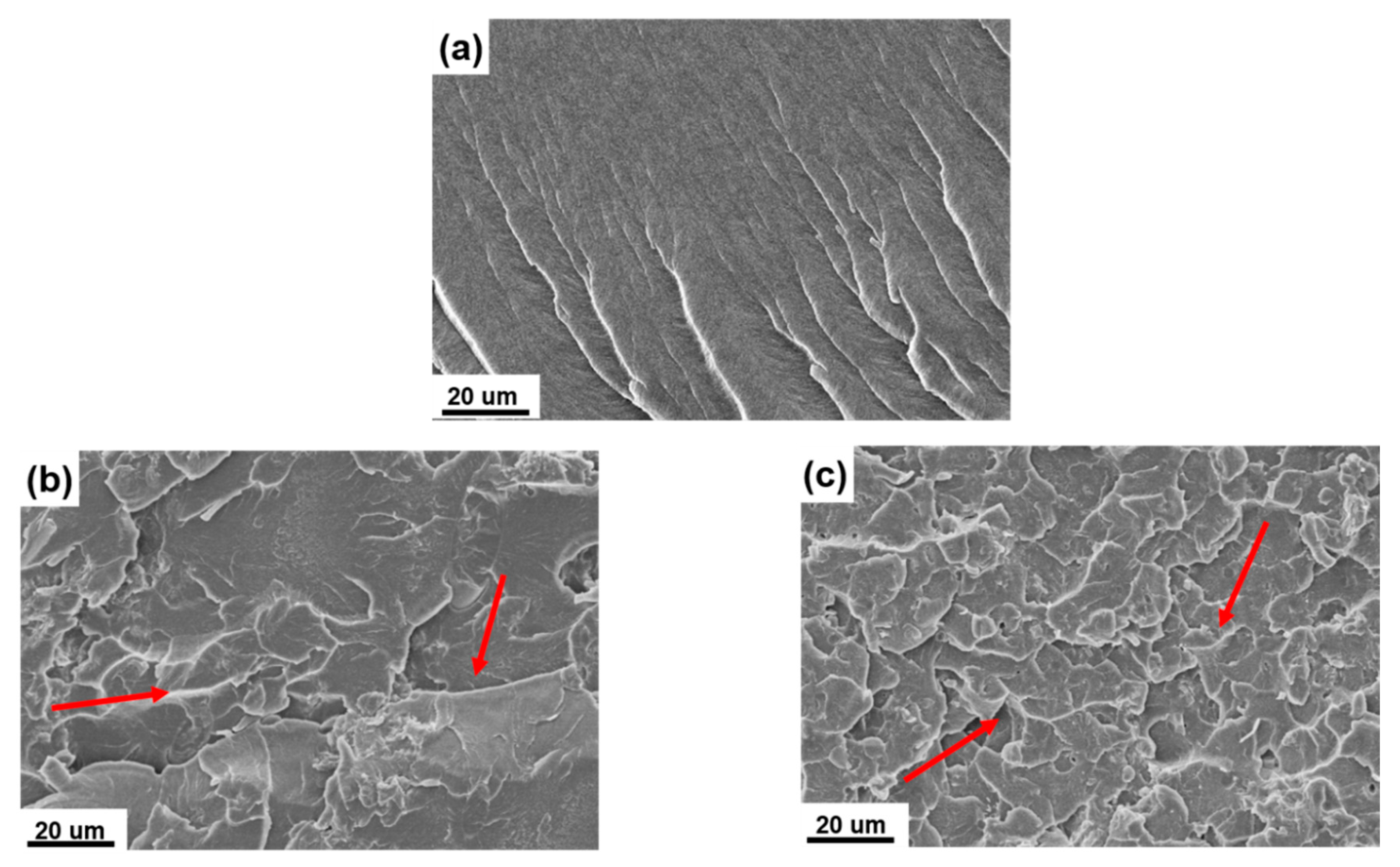
3.3. Morphological Properties of UP/POSS-MMT Nanocomposites
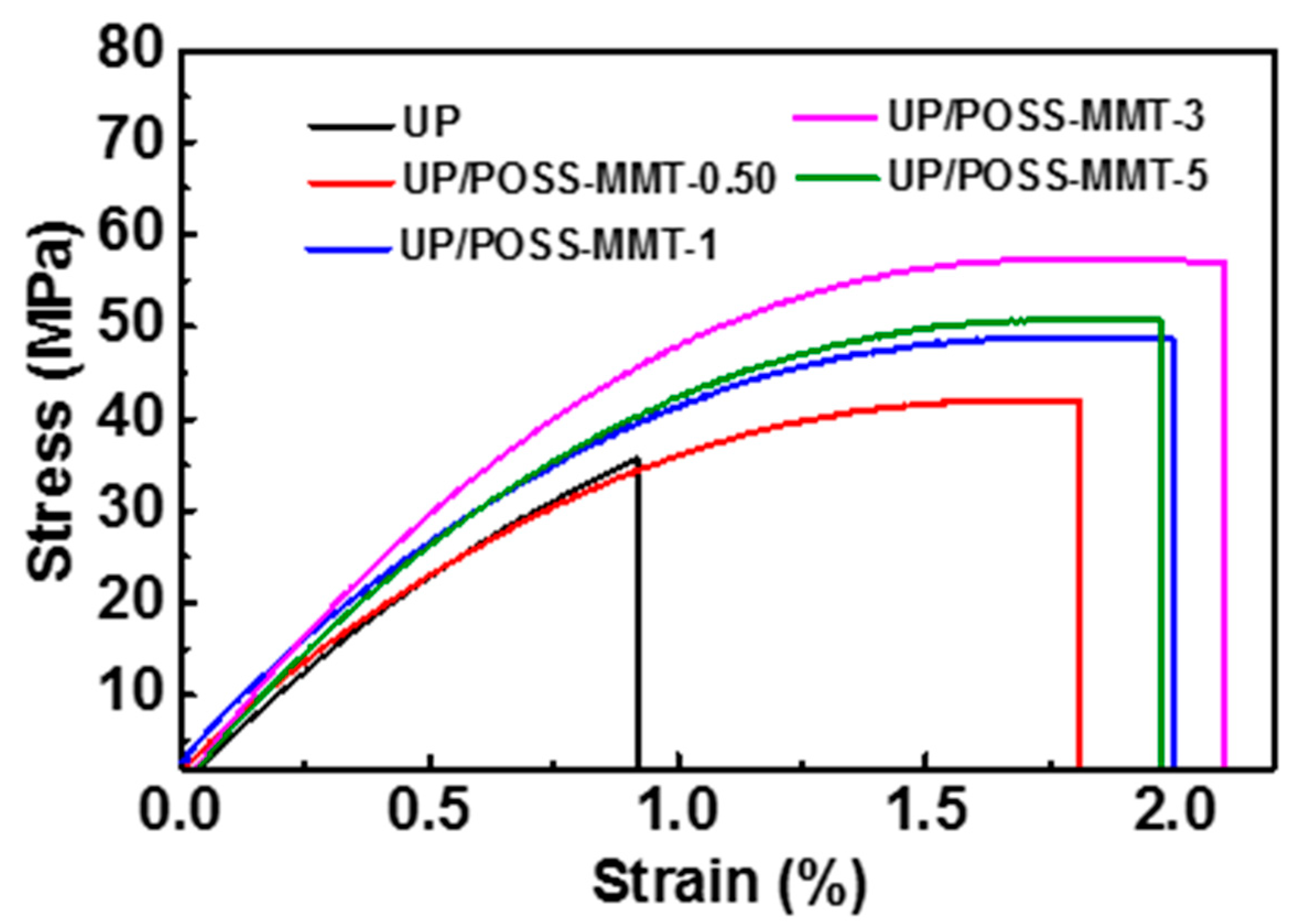
3.4. Mechanical Properties of UP/POSS-MMT Nanocomposites
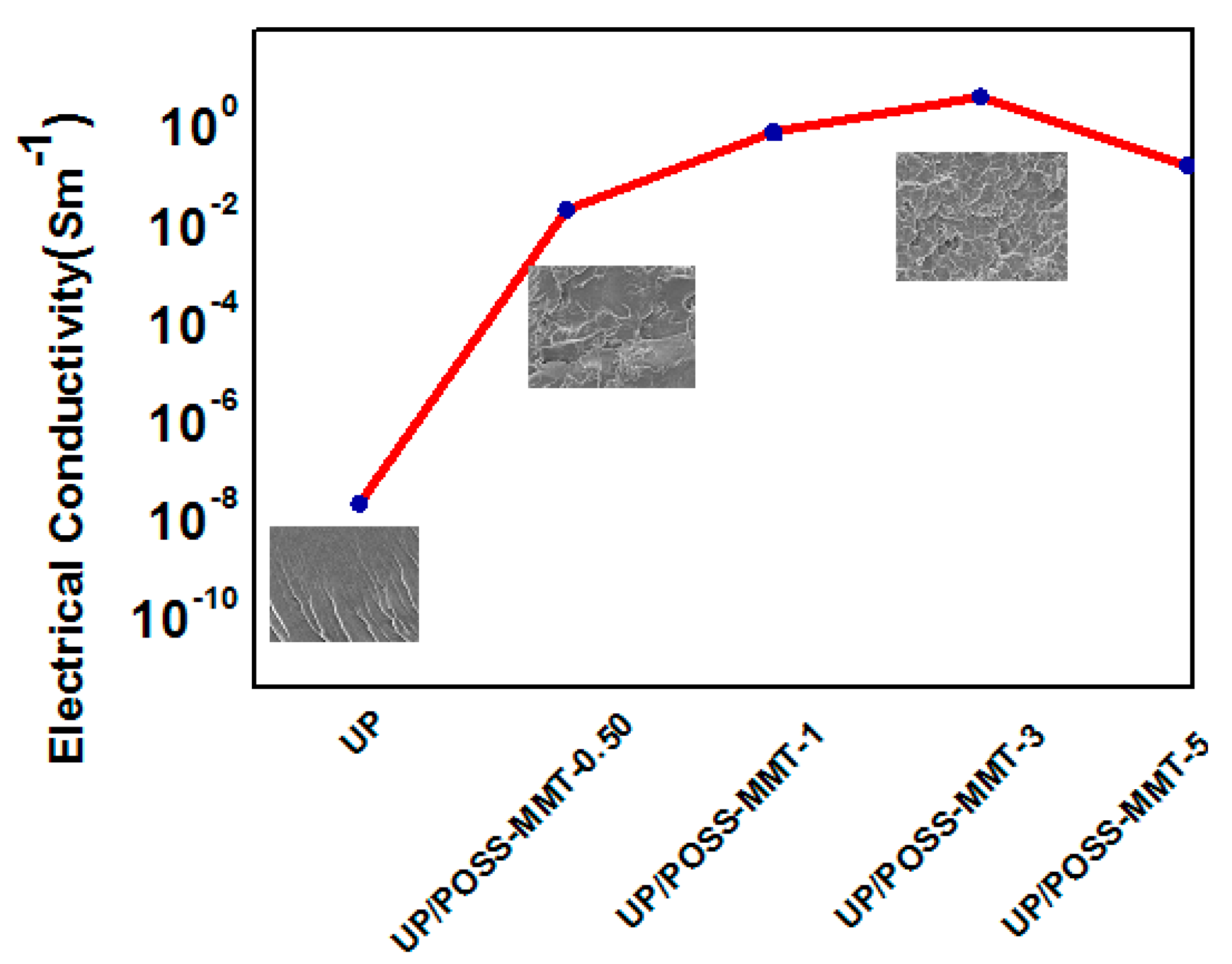
3.5. Electrical Properties of UP/POSS-MMT Nanocomposites
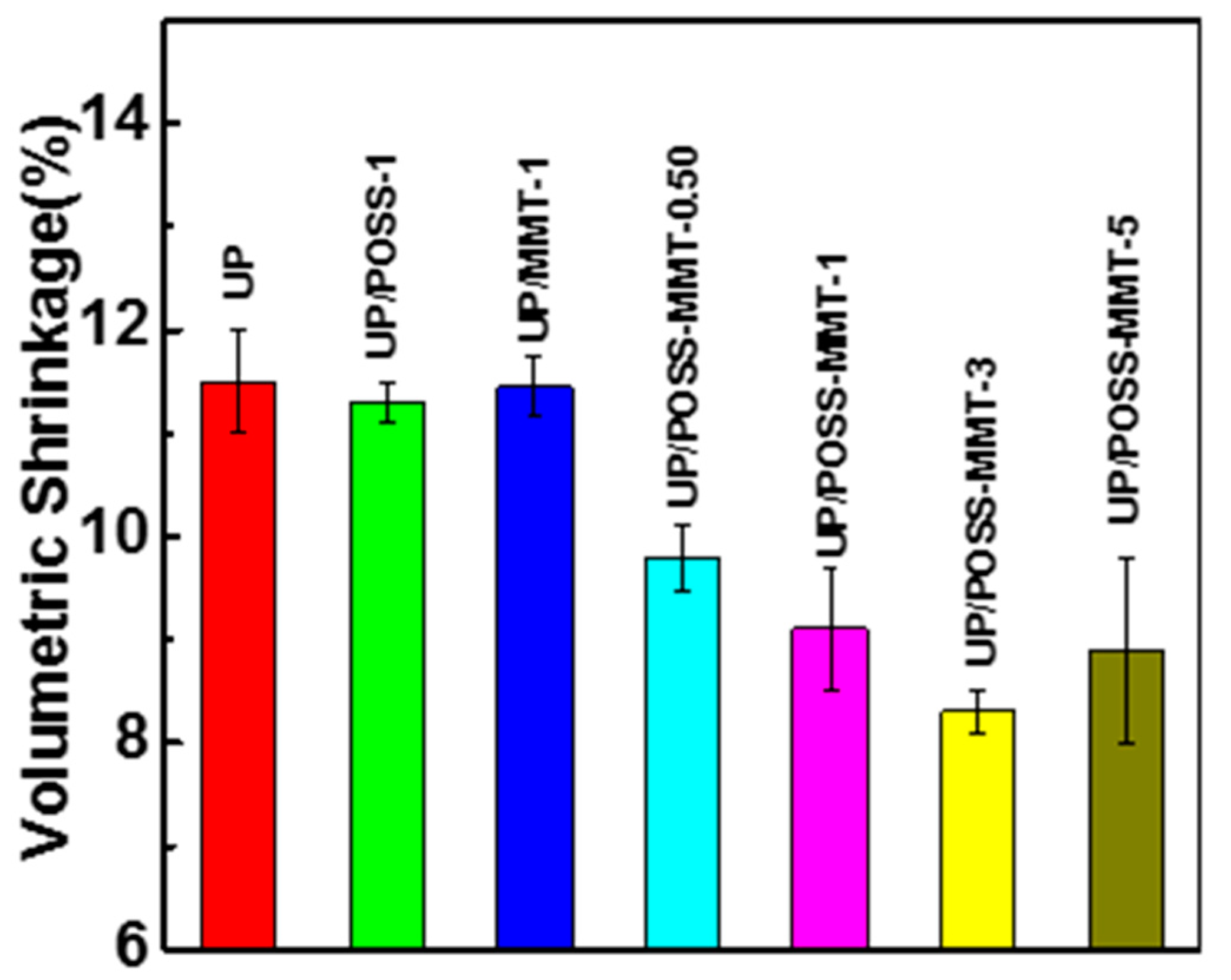
3.6. Shrinkage wt.% Loss of UP/POSS-MMT Nanocomposites
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fernandez, M.J.; Fernandez, M.D. Effect of Organic Modifier and Clay Content on Non-Isothermal Cold Crystallization and Melting Behavior of Polylactide/Organovermiculite Nanocomposites. Polymers 2020, 12, 364. [Google Scholar] [CrossRef] [PubMed]
- Nikolaidis, A.K.; Achilias, D.S.; Karayannidis, G.P. Synthesis and Characterization of PMMA/Organomodified Montmorillonite Nanocomposites Prepared by in Situ Bulk Polymerization. Ind. Eng. Chem. Res. 2011, 50, 571–579. [Google Scholar] [CrossRef]
- Msaadi, R.; Yilmaz, G.; Allushi, A.; Hamadi, S.; Ammar, S.; Chehimi, M.M.; Yagci, Y. Highly Selective Copper Ion Imprinted Clay/Polymer Nanocomposites Prepared by Visible Light Initiated Radical Photopolymerization. Polymers 2019, 11, 286. [Google Scholar] [CrossRef] [PubMed]
- Bee, S.L.; Abdullah, M.A.A.; Bee, S.T.; Sin, L.T.; Rahmat, A.R. Polymer Nanocomposites Based on Silylated-Montmorillonite: A Review. Prog. Polym. Sci. 2018, 85, 57–82. [Google Scholar] [CrossRef]
- Chiaradia, V.; Hanay, S.B.; Kimmins, S.D.; Oliveira, D.D.; Araujo, P.H.H.; Sayer, C.; Heise, A. Crosslinking of Electrospun Fibres from Unsaturated Polyesters by Bis-Triazolinediones (TAD). Polymers 2019, 11, 1808. [Google Scholar] [CrossRef]
- Chu, F.; Zhang, D.; Hou, Y.; Qiu, S.; Wang, J.; Hu, W.; Song, L. Construction of Hierarchical Natural Fabric Surface Structure Based on Two-Dimensional Boron Nitride Nanosheets and Its Application for Preparing Biobased Toughened Unsaturated Polyester Resin Composites. Acs Appl. Mater. Interfaces 2018, 10, 40168–40179. [Google Scholar] [CrossRef]
- Hu, D.; Jia, Z.; Li, J.; Zhong, B.; Fu, W.; Luo, Y.; Jia, D. Characterization of Waste Printed Circuit Boards Nonmetals and Its Reutilization as Reinforcing Filler in Unsaturated Polyester Resin. J. Polym. Environ. 2018, 26, 1311–1319. [Google Scholar] [CrossRef]
- Ollier, R.; Rodriguez, E.; Alvarez, V. Unsaturated Polyester/Bentonite Nanocomposites: Influence of Clay Modification on Final Performance. Compos. Part A Appl. Sci. Manuf. 2013, 48, 137–143. [Google Scholar] [CrossRef]
- Laatar, F.; Jayan, M.; Padmanabhan, P.; Ben Romdhane, M.R.; Srasra, E. Unsaturated Polyester-Nanoclay Nanocomposite Coatings for Effective Corrosion Protection of Steel. J. Coat. Technol. Res. 2018, 15, 293–301. [Google Scholar] [CrossRef]
- Lin, J.; Zhong, B.; Jia, Z.; Hu, D.; Ding, Y.; Luo, Y.; Jia, D. In-Situ Fabrication of Halloysite Nanotubes/Silica Nano Hybrid and Its Application in Unsaturated Polyester Resin. Appl. Surf. Sci. 2017, 407, 130–136. [Google Scholar] [CrossRef]
- Kumar Gudapati, S.P.; Chidambaranathan, S.; Ratna Prasad, A.V. Influence of Nanoclay on Thermal Properties of Wild Cane Grass Fiber Reinforced Polyester Composites. Mater. Today Proc. 2020, 23, 632–636. [Google Scholar] [CrossRef]
- Chaeichian, S.; Wood-Adams, P.M.; Hoa, S.V. In Situ Polymerization of Polyester-Based Hybrid Systems for the Preparation of Clay Nanocomposites. Polymer (Guildf.) 2013, 54, 1512–1523. [Google Scholar] [CrossRef]
- Huang, R.; Zhang, X.; Li, H.; Zhou, D.; Wu, Q. Bio-Composites Consisting of Cellulose Nanofibers and Na+ Montmorillonite Clay: Morphology and Performance Property. Polymers 2020, 12, 1448. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Liu, Y.; Lv, K.; Han, S.; Gao, S.; Yu, G. Prominent Crystallization Promotion Effect of Montmorillonite on PTT/PC Blends with PTT as the Continuous Phase. Polymers 2020, 12, 541. [Google Scholar] [CrossRef] [PubMed]
- Shah, K.J.; Shukla, A.D.; Shah, D.O.; Imae, T. Effect of Organic Modifiers on Dispersion of Organoclay in Polymer Nanocomposites to Improve Mechanical Properties. Polymer (Guildf.) 2016, 97, 525–532. [Google Scholar] [CrossRef]
- Chieruzzi, M.; Miliozzi, A.; Kenny, J.M. Effects of the Nanoparticles on the Thermal Expansion and Mechanical Properties of Unsaturated Polyester/Clay Nanocomposites. Compos. Part A Appl. Sci. Manuf. 2013, 45, 44–48. [Google Scholar] [CrossRef]
- Bur, H. Property Enhancement in Unsaturated Polyester Nanocomposites by Using a Reactive Intercalant for Clay Modification. J. Appl. Polym. Sci. 2013, 129, 3247–3254. [Google Scholar] [CrossRef]
- Dinakaran, K.; Deveraju, S.; Alagar, M. Unsaturated Polyester Resin Clay Hybrid Nanocomposites. In Thermoset Nanocomposites; Mittal, V., Ed.; Wiley: Hoboken, NJ, USA, 2013; pp. 129–146. [Google Scholar] [CrossRef]
- Danaei, S.; Poorabdollah, M.; Rajabi, L. Investigation of Diffusion and Cure Kinetic in Nanoclay-Reinforced Unsaturated Polyester Resin. Thermochim. Acta 2017, 651, 34–42. [Google Scholar] [CrossRef]
- Strakowska, A.; Czlonka, S.; Strzelec, K. POSS compounds as a modifiers for rigid polyurethane foams (composites). Polymers 2019, 11, 1092. [Google Scholar] [CrossRef]
- Kaneko, Y.; Coughlin, E.B.; Gunji, T.; Itoh, M.; Matsukawa, K.; Naka, K. Silsesquioxanes: Recent Advancement and Novel Applications. Int. J. Polym. Sci. 2012, 2012, 453821. [Google Scholar] [CrossRef]
- Hojiyev, R.; Ulcay, Y.; Hojamberdiev, M.; Çelik, M.S.; Carty, W.M. Hydrophobicity and Polymer Compatibility of POSS-Modified Wyoming Na-Montmorillonite for Developing Polymer-Clay Nanocomposites. J. Colloid Interface Sci. 2017, 497, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Shivakumar, R.; Bolker, A.; Tsang, S.H.; Atar, N.; Verker, R.; Gouzman, I.; Hala, M.; Moshe, N.; Jones, A.; Grossman, E.; et al. POSS enhanced 3D graphene-Polyimide film for atomic oxygen endurance in Low Earth Orbit space environment. Polymer (Guildf.) 2020, 191, 122270. [Google Scholar] [CrossRef]
- Kuo, S.W.; Fu, H.K.; Yeh, D.R.; Chang, F.C. Properties Enhancement of PS Nanocomposites through the POSS Surfactants. J. Nanomater. 2008, 2008, 739613. [Google Scholar] [CrossRef]
- Abate, L.; Bottino, F.A.; Cicala, G.; Chiacchio, M.A.; Ognibene, G.; Blanco, I. Polystyrene Nanocomposites Reinforced with Novel Dumbbell-Shaped Phenyl-POSSs: Synthesis and Thermal Characterization. Polymers 2019, 11, 1475. [Google Scholar] [CrossRef]
- Jones, I.K.; Zhou, Y.X.; Jeelani, S.; Mabry, J.M. Effect of Polyhedral-Oligomeric-Sil-Sesquioxanes on Thermal and Mechanical Behavior of SC-15 Epoxy. Express Polym. Lett. 2008, 2, 494–501. [Google Scholar] [CrossRef]
- Fernandez, M.D.; Guzman, D.J.; Ramos, J.R.; Fernandez, M.J. Effect of Alkyl Chain Length in POSS Nanocage on Non-Isothermal Crystallization Behavior of PCL/Amino-POSS Nanocomposites. Polymers 2019, 11, 1719. [Google Scholar] [CrossRef] [PubMed]
- Mishra, K.; Gidley, D.; Singh, R.P. Influence of Self-Assembled Compliant Domains on the Polymer Network and Mechanical Properties of POSS-Epoxy Nanocomposites under Cryogenic Conditions. Eur. Polym. J. 2019, 116, 283–290. [Google Scholar] [CrossRef]
- Cobos, M.; Ramos, J.R.; Guzman, D.J.; Fernandez, M.D.; Fernandez, M.J. PCL/POSS Nanocomposites: Effect of POSS Derivative and Preparation Method on Morphology and Properties. Polymers 2019, 11, 33. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Liu, L.; Long, J.; Xing, L.; Huang, Y.; Wu, Z.; Yan, X.; Guo, Z. Reinforced Unsaturated Polyester Composites by Chemically Grafting Amino-POSS onto Carbon Fibers with Active Double Spiral Structural Spiralphosphodicholor. Compos. Sci. Technol. 2014, 100, 158–165. [Google Scholar] [CrossRef]
- Divakaran, N.; Kale, M.B.; Senthil, T.; Mubarak, S.; Dhamodharan, D.; Wu, L.; Wang, J. Novel Unsaturated Polyester Nanocomposites via Hybrid 3D POSS-Modified Graphene Oxide Reinforcement:Electro-Technical Application Perspective. Nanomaterials 2020, 10, 260. [Google Scholar] [CrossRef]
- Bi, D.; Li, Q.; Chen, G.X. Synthesis of Polyhedral Oligomeric Silsesquioxane-Modified Organic Montmorillonites and Their Nanocomposites with Poly(l-Lactide). Appl. Clay Sci. 2014, 87, 34–39. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, W.; Zheng, S. Montmorillonite Intercalated by Ammonium of Octaaminopropyl Polyhedral Oligomeric Silsesquioxane and Its Nanocomposites with Epoxy Resin. Polymer (Guildf.) 2005, 46, 157–165. [Google Scholar] [CrossRef]
- Teo, J.K.H.; Toh, C.L.; Lu, X. Catalytic and Reinforcing Effects of Polyhedral Oligomeric Silsesquioxane (POSS)-Imidazolium Modified Clay in an Anhydride-Cured Epoxy. Polymer (Guildf.) 2011, 52, 1975–1982. [Google Scholar] [CrossRef]
- Zhao, F.; Bao, X.; McLauchlin, A.R.; Gu, J.; Wan, C.; Kandasubramanian, B. Effect of POSS on Morphology and Mechanical Properties of Polyamide 12/Montmorillonite Nanocomposites. Appl. Clay Sci. 2010, 47, 249–256. [Google Scholar] [CrossRef]
- Yeo, J.; Lee, J.; Hwang, S. Effects of Lignin on the Volume Shrinkage and Mechanical Properties of a Styrene/Unsaturated Polyester/Lignin Ternary Composite System. Compos. Part B Eng. 2017, 130, 167–173. [Google Scholar] [CrossRef]
- Barkoula, N.M.; Alcock, B.; Cabrera, N.O.; Peijs, T. Fatigue Properties of Highly Oriented Polypropylene Tapes and All-polypropylene Composites. Polym. Polym. Compos. 2008, 16, 101–113. [Google Scholar] [CrossRef]
- Al-Khanbashi, A.; El-Gamal, M.; Moet, A. Reduced Shrinkage Polyester-Montmorillonite Nanocomposite. J. Appl. Polym. Sci. 2005, 98, 767–773. [Google Scholar] [CrossRef]
- Xu, L.; Lee, L.J. Effect of Nanoclay on Shrinkage Control of Low Profile Unsaturated Polyester (UP) Resin Cured at Room Temperature. Polymer (Guildf). 2004, 45, 7325–7334. [Google Scholar] [CrossRef]
- Schwab, J.J.; Lichtenhan, J.D. Polyhedral Oligomeric Silsesquioxane (POSS)-Based Polymers. Appl. Organomet. Chem. 1998, 12, 707–713. [Google Scholar] [CrossRef]
- Wu, S.; Wang, L.; Wu, L.; Weng, Z.; Zhou, Y. Unsaturated Polyester/Montmorillonite Nanocomposites with Improved Mechanical and Thermal Properties Fabricated by in Situ Polymerization. J. Appl. Polym. Sci. 2017, 134, 1–9. [Google Scholar] [CrossRef]
- Diani, J.; Liu, Y.; Gall, K. Finite Strain 3D Thermoviscoelastic Constitutive Model for Shape Memory Polymers. Polym. Eng. Sci. 2006, 46, 486–492. [Google Scholar] [CrossRef]
- Zhan, Z.; Liang, G.; Ren, P.; Wang, J. Curing Behavior of Epoxy/POSS/DDS Hybrid Systems. Poly. Compos. 2008, 29, 77–83. [Google Scholar] [CrossRef]
- Perrin, F.X.; Bruzaud, S.; Grohens, Y. Structure and thermal behaviour of polyhedral oligomeric modified montmorillonite. Appl. Clay. Sci. 2010, 49, 113–119. [Google Scholar] [CrossRef]
- Szabo, A.; Gournis, D.; Karakassides, M.A.; Petridis, D. Clay-Aminopropylsiloxane compositions. Chem. Mater. 1998, 10, 639–645. [Google Scholar] [CrossRef]
- Yei, D.R.; Kuo, S.W.; Su, Y.C.; Chang, F.C. Enhanced Thermal Properties of PS Nanocomposites Formed from Inorganic POSS-Treated Montmorillonite. Polymer (Guildf.) 2004, 45, 2633–2640. [Google Scholar] [CrossRef]
- Lee, J.H.; Jeong, Y.G. Preparation and Crystallization Behavior of Polylactide Nanocomposites Reinforced with POSS-Modified Montmorillonite. Fibers Polym. 2011, 12, 180–189. [Google Scholar] [CrossRef]
- Venkatesha, N.J.; Prakasha, a.S.J.; Bhat, Y.S. The Active Site Accessibility Aspect in Montmorillonite for Ketone Yield in Ester Rearrangement. Catal. Sci. Technol. 2015, 5, 1629–1637. [Google Scholar] [CrossRef]
- Ervithayasuporn, V.; Chanmungkalakul, S.; Churinthorn, N.; Jaroentomeechai, T.; Hanprasit, S.; Sodkhomkhum, R.; Kaewpijit, P.; Kiatkamjornwong, S. Modifying Interlayer Space of Montmorillonite with Octakis(3-(1-Methylimidazolium)Propyl) Octasilsesquioxane Chloride. Appl. Clay Sci. 2019, 171, 6–13. [Google Scholar] [CrossRef]
- Divakaran, N.; Zhang, X.; Kale, M.B.; Senthil, T.; Mubarak, S.; Dhamodharan, D.; Wu, L.; Wang, J. Fabrication of Surface Modified Graphene Oxide/Unsaturated Polyester Nanocomposites via in-Situ Polymerization: Comprehensive Property Enhancement. Appl. Surf. Sci. 2020, 502, 144164. [Google Scholar] [CrossRef]
- Ejder-Korucu, M.; Gürses, A.; Karaca, S. Poly(Ethylene Oxide)/Clay Nanocomposites: Thermal and Mechanical Properties. Appl. Surf. Sci. 2016, 378, 1–7. [Google Scholar] [CrossRef]
- Shettar, M.; Achutha Kini, U.; Sharma, S.S.; Hiremath, P. Study on Mechanical Characteristics of Nanoclay Reinforced Polymer Composites. Mater. Today Proc. 2017, 4, 11158–11162. [Google Scholar] [CrossRef]
- Wang, F.; Wu, Y.; Huang, Y. High strength, thermostable and fast drying hybrid transparent membranes with POSS nanoparticles aligned on aramid nanofibers. Compos. Part A 2018, 110, 154–161. [Google Scholar] [CrossRef]
- Zare, Y.; Rhee, K.Y. A Two-Step Technique for Tensile Strength of Montmorillonite/Polymer Nanocomposites Assuming Filler Morphology and Interphase Properties. Appl. Clay Sci. 2017, 150, 42–46. [Google Scholar] [CrossRef]
- Ajorloo, M.; Fasihi, M.; Ohshima, M.; Taki, K. How Are the Thermal Properties of Polypropylene/Graphene Nanoplatelet Composites Affected by Polymer Chain Configuration and Size of Nanofiller. Mater. Des. 2019, 181, 108068. [Google Scholar] [CrossRef]
- Huang, J.C.; He, C.B.; Xiao, Y.; Mya, K.Y.; Dai, J.; Siow, Y.P. Polyimide/POSS Nanocomposites: Interfacial Interaction, Thermal Properties and Mechanical Properties. Polymer (Guildf.) 2003, 44, 4491–4499. [Google Scholar] [CrossRef]
- Nezakati, T.; Tan, A.; Seifalian, A.M. Enhancing the Electrical Conductivity of a Hybrid POSS-PCL/Graphene Nanocomposite Polymer. J. Colloid Interface Sci. 2014, 435, 145–155. [Google Scholar] [CrossRef]
- Boček, J.; Matějka, L.; Mentlík, V.; Trnka, P.; Šlouf, M. Electrical and Thermomechanical Properties of Epoxy-POSS Nanocomposites. Eur. Polym. J. 2011, 47, 861–872. [Google Scholar] [CrossRef]
- Birtane, H.; Esmer, K.; Madakbas, S.; Kahraman, M.V. Structural and Dielectric Properties of POSS Reinforced Polyimide Nanocomposites. J. Macromol. Sci. Part. A Pure Appl. Chem. 2019, 56, 245–252. [Google Scholar] [CrossRef]
- Beheshty, M.H.; Vafayan, M.; Poorabdollah, M. Shrinkage Control and Kinetics Behaviour of Clay-Unsaturated Polyester Nanocomposites. Iran. Polym. J. Engl. Ed. 2006, 15, 841–849. [Google Scholar]



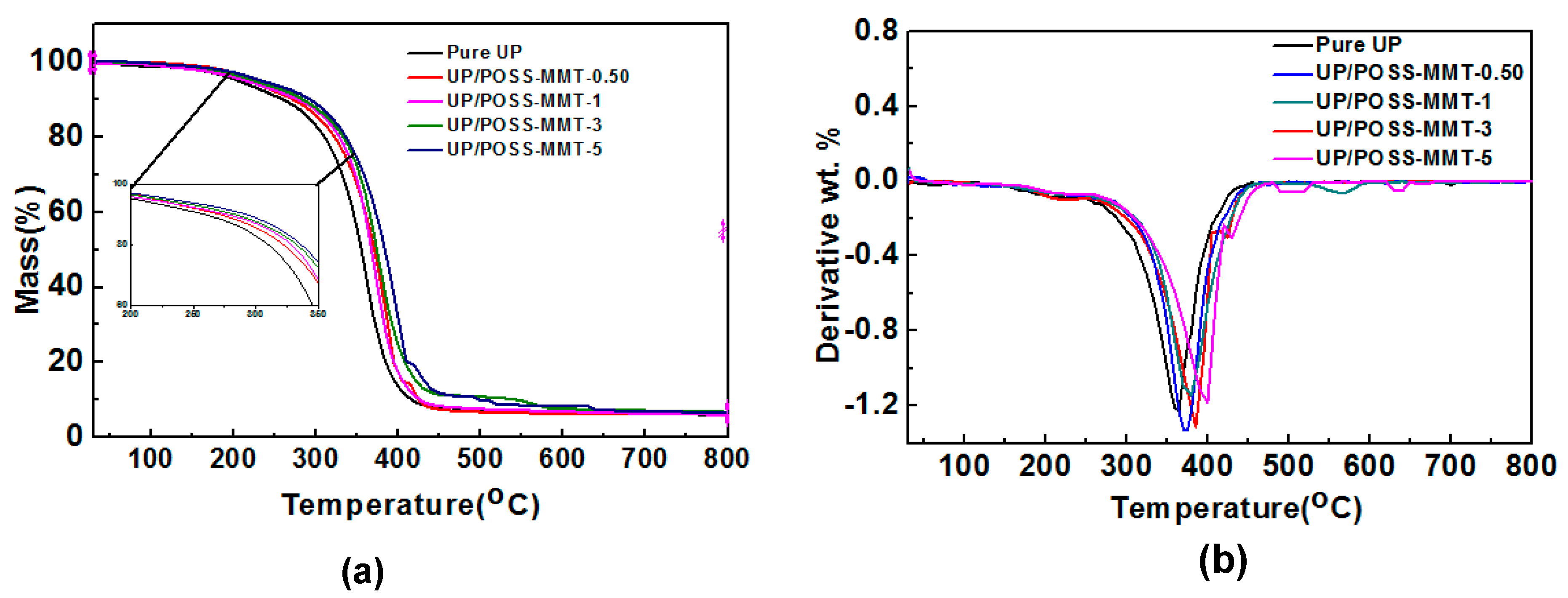
| Sample Name | T10 * (°C) | T50 * (°C) | Residual (%) |
|---|---|---|---|
| UP | 258.4 | 356.2 | 6.34 |
| UP/POSS-MMT-0.50 | 266.6 | 370.1 | 6.25 |
| UP/POSS-MMT-1 | 279.7 | 371.3 | 6.40 |
| UP/POSS-MMT-3 | 284.5 | 376.4 | 7.35 |
| UP/POSS-MMT-5 | 295.4 | 381.3 | 6.98 |
| Sample Name | Tensile Strength (MPa) | Elongation at Break (%) | Storage Modulus at 30 °C (MPa) | Tg * (°C) |
|---|---|---|---|---|
| UP | 35.2 ± 0.3 | 0.92 ± 0.015 | 1956 | 72.1 |
| UP/POSS-MMT-0.05 | 41.9 ± 1.4 | 1.81 ± 0.001 | 1980 | 74.8 |
| UP/POSS-MMT-1 | 48.6 ± 2.5 | 1.99 ± 0.023 | 2152 | 78.7 |
| UP/POSS-MMT-3 | 56.8 ± 1.9 | 1.97 ± 0.030 | 2393 | 83.8 |
| UP/POSS-MMT-5 | 50.2 ± 2.4 | 2.21 ± 0.100 | 2538 | 88.0 |
| Sample Name | Electrical Conductivity (S m−1) |
|---|---|
| UP | 2.12 × 10−8 ± 0.0003 |
| UP/POSS-MMT-0.50 | 1.96 × 10−2 ± 0.009 |
| UP/POSS-MMT-1 | 0.7500 ± 0.030 |
| UP/POSS-MMT-3 | 3.846 ± 0.135 |
| UP/POSS-MMT-5 | 0.153 ± 0.025 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Divakaran, N.; B. Kale, M.; Dhamodharan, D.; Mubarak, S.; Wu, L.; Wang, J. Effect of POSS-Modified Montmorillonite on Thermal, Mechanical, and Electrical Properties of Unsaturated Polyester Nanocomposites. Polymers 2020, 12, 2031. https://doi.org/10.3390/polym12092031
Divakaran N, B. Kale M, Dhamodharan D, Mubarak S, Wu L, Wang J. Effect of POSS-Modified Montmorillonite on Thermal, Mechanical, and Electrical Properties of Unsaturated Polyester Nanocomposites. Polymers. 2020; 12(9):2031. https://doi.org/10.3390/polym12092031
Chicago/Turabian StyleDivakaran, Nidhin, Manoj B. Kale, Duraisami Dhamodharan, Suhail Mubarak, Lixin Wu, and Jianlei Wang. 2020. "Effect of POSS-Modified Montmorillonite on Thermal, Mechanical, and Electrical Properties of Unsaturated Polyester Nanocomposites" Polymers 12, no. 9: 2031. https://doi.org/10.3390/polym12092031
APA StyleDivakaran, N., B. Kale, M., Dhamodharan, D., Mubarak, S., Wu, L., & Wang, J. (2020). Effect of POSS-Modified Montmorillonite on Thermal, Mechanical, and Electrical Properties of Unsaturated Polyester Nanocomposites. Polymers, 12(9), 2031. https://doi.org/10.3390/polym12092031