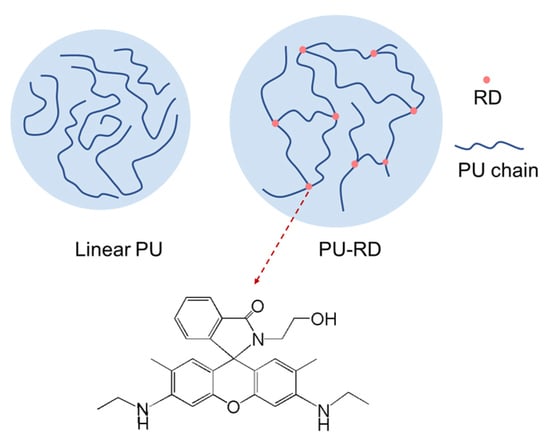
A Fluorescent Polyurethane with Covalently Cross-Linked Rhodamine Derivatives
Abstract
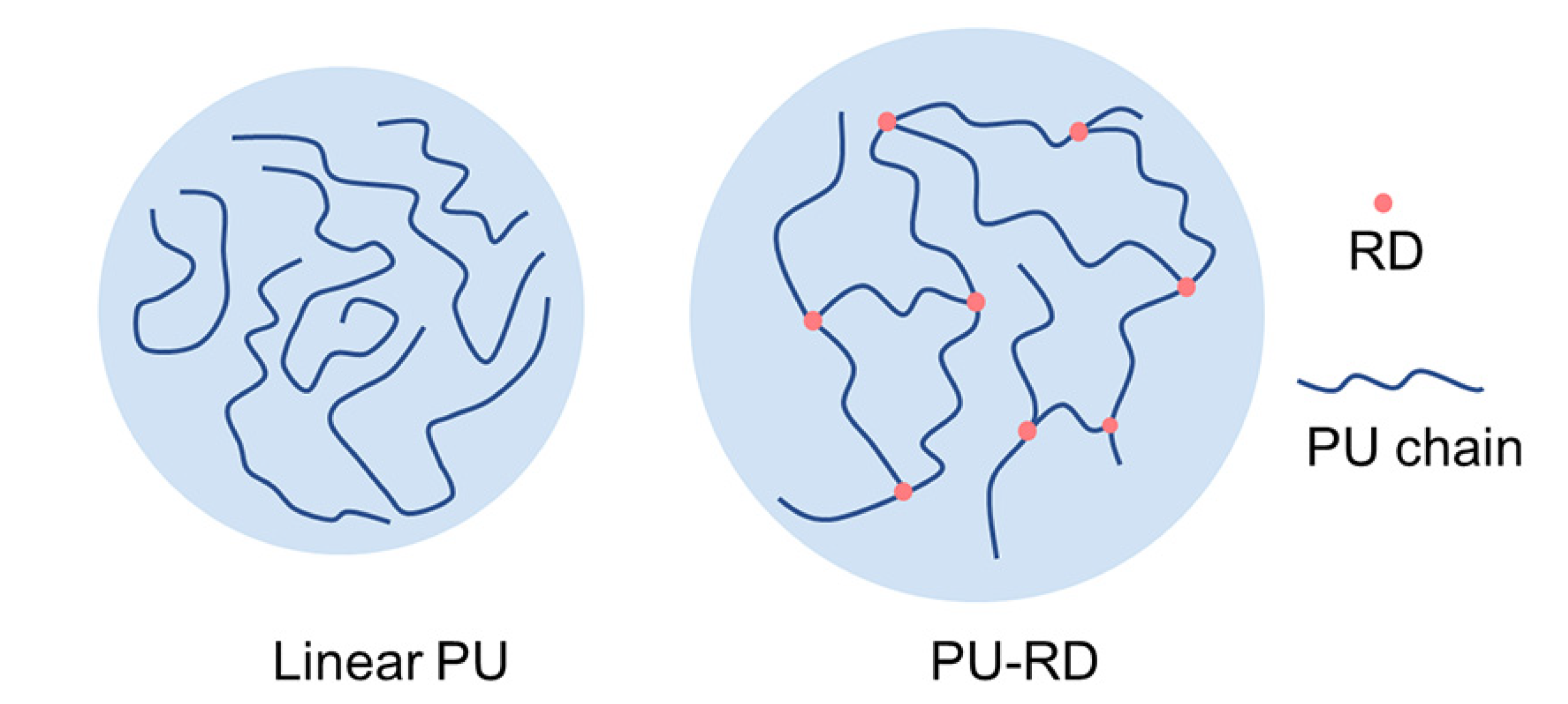
1. Introduction
2. Experiment
2.1. Materials
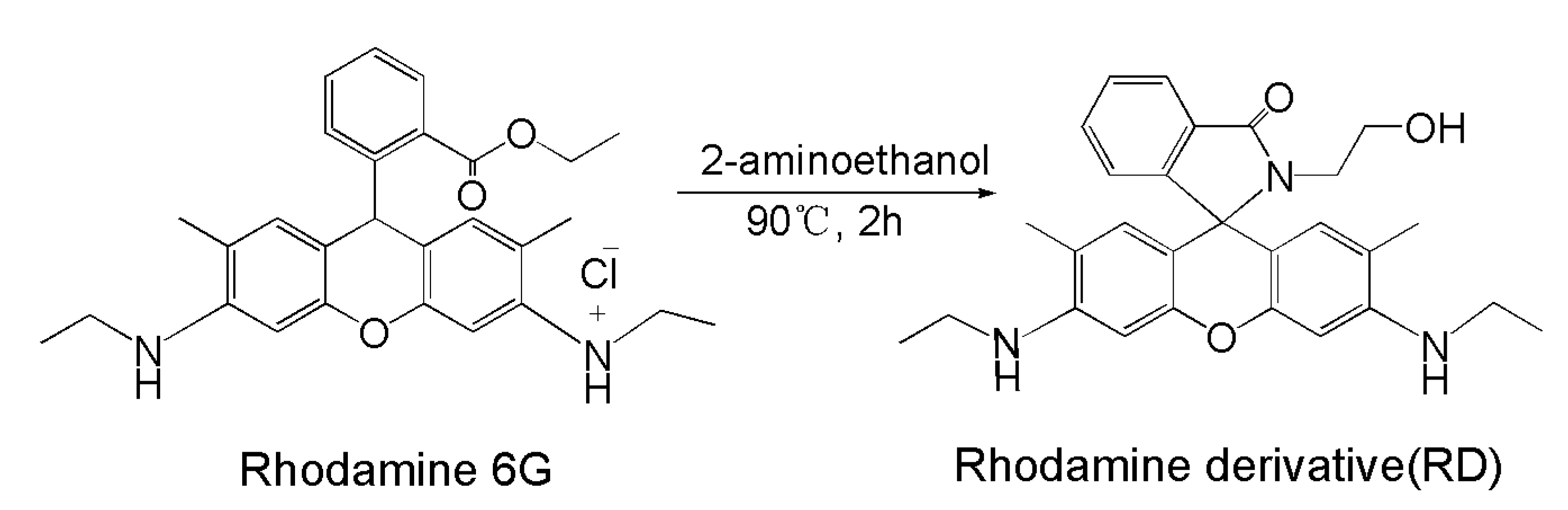
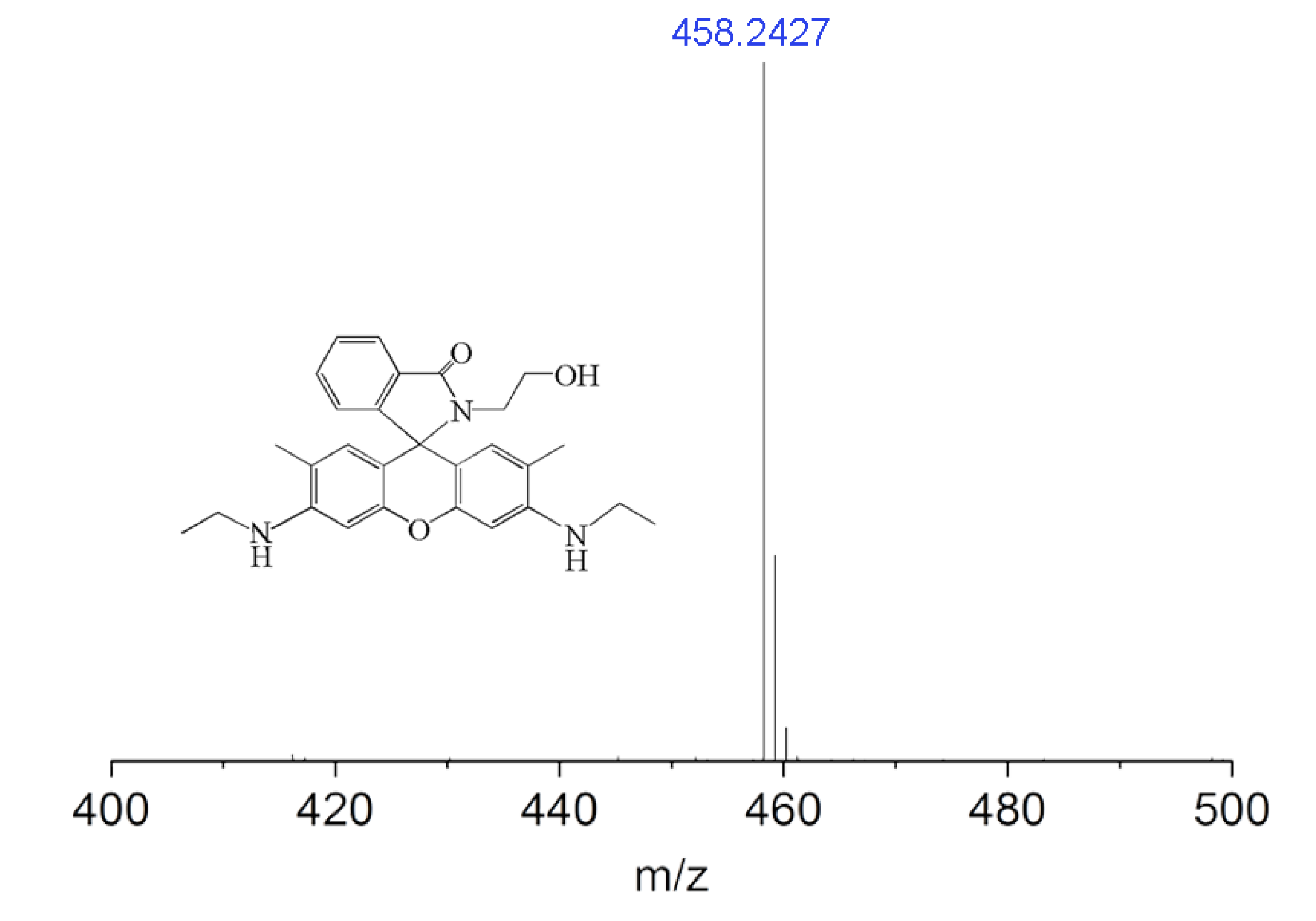
2.2. Synthesis of Rhodamine Derivative (RD)
2.3. Synthesis of Fluorescent Waterborne Polyurethane (PU-RD)
2.4. Measurements
3. Results and Discussion
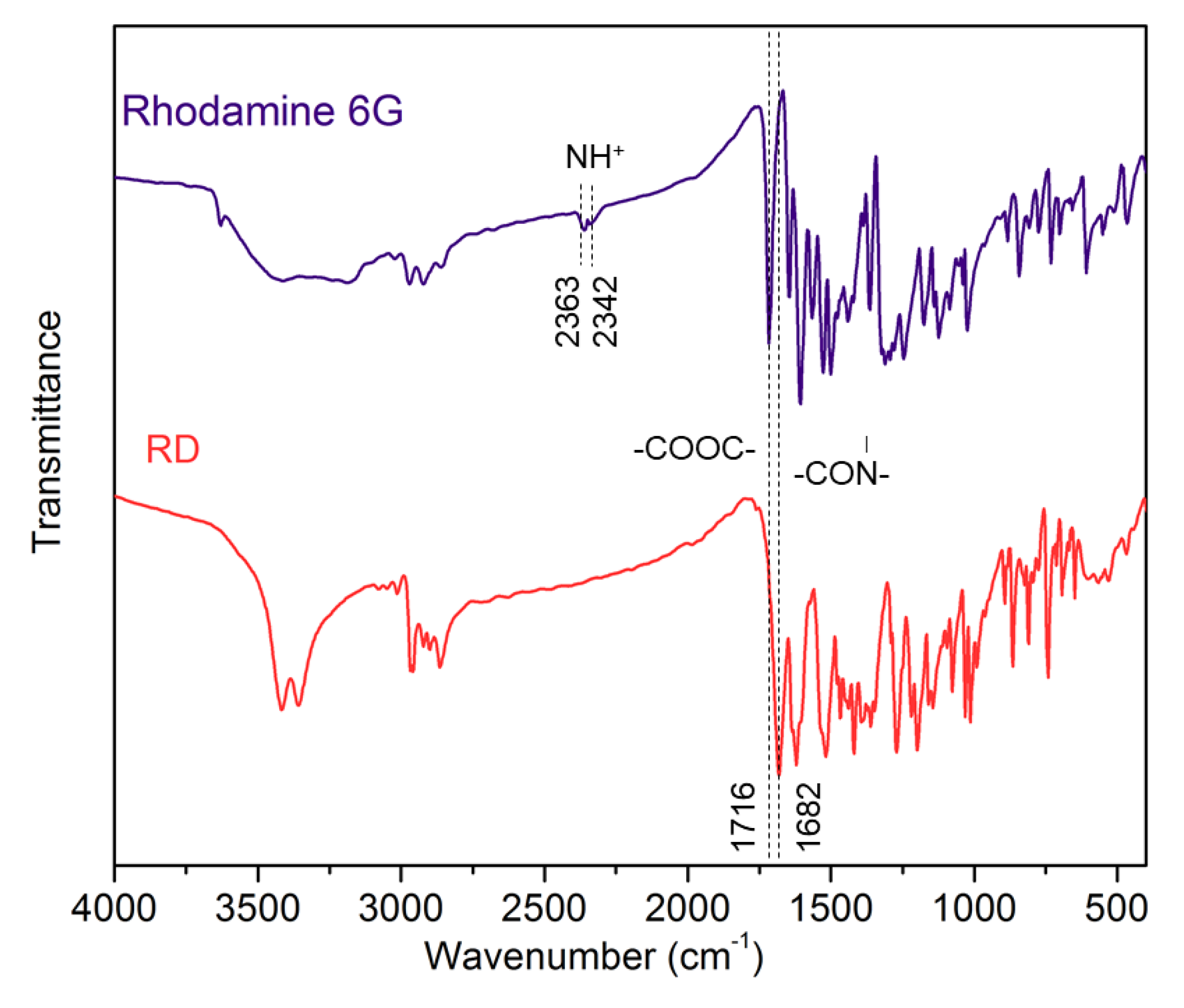
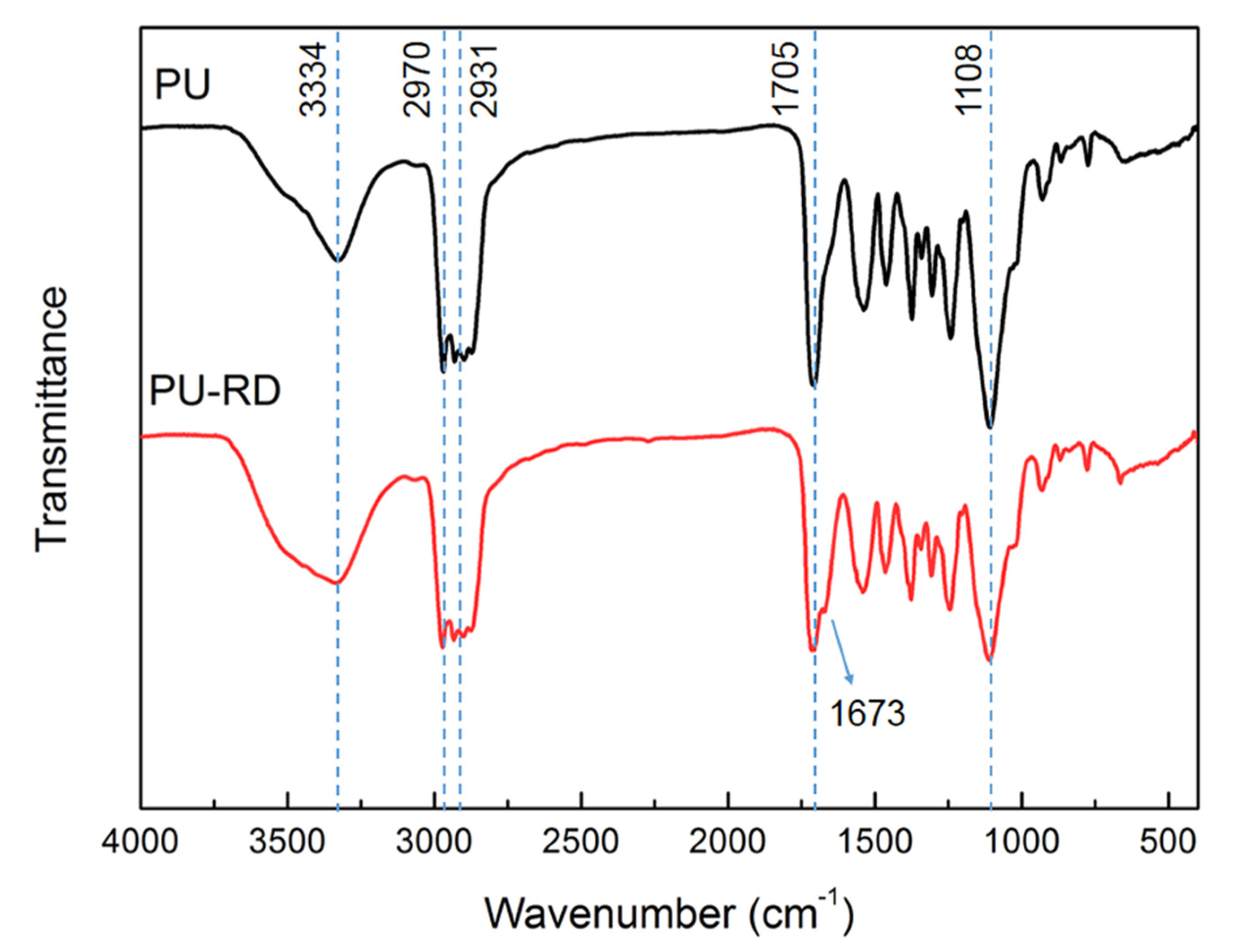
3.1. Structure Characterization
3.2. Fluorescent Properties
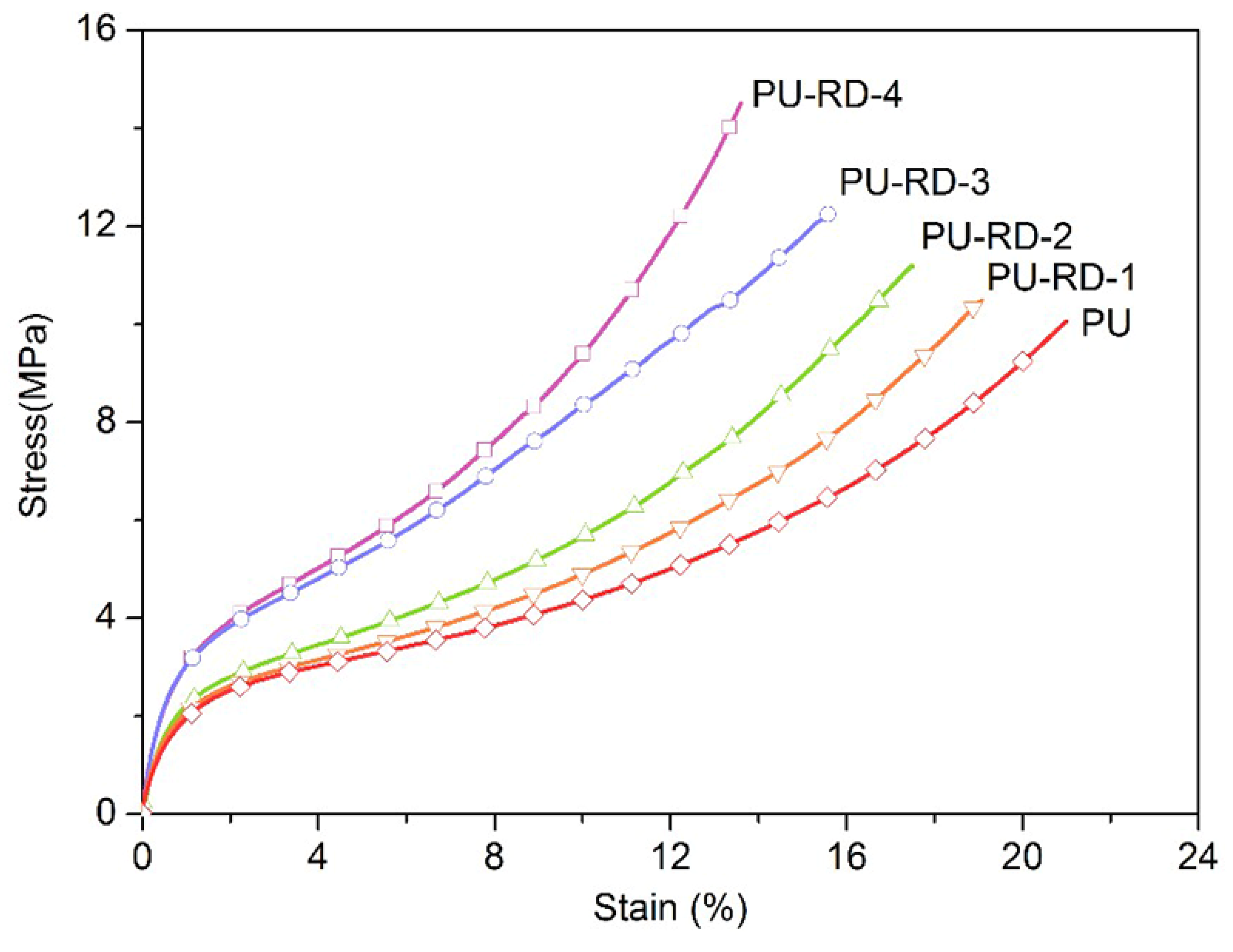
3.3. Mechanical Properties
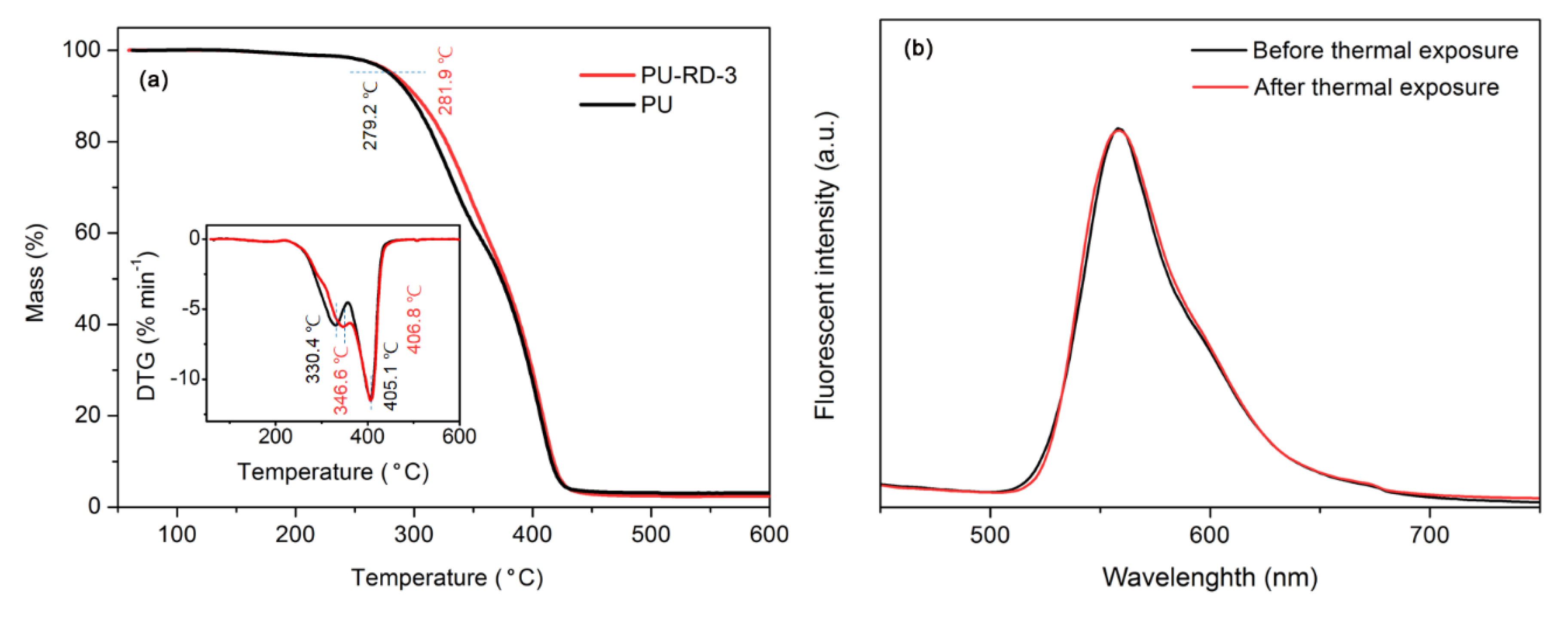
3.4. Thermal Stability
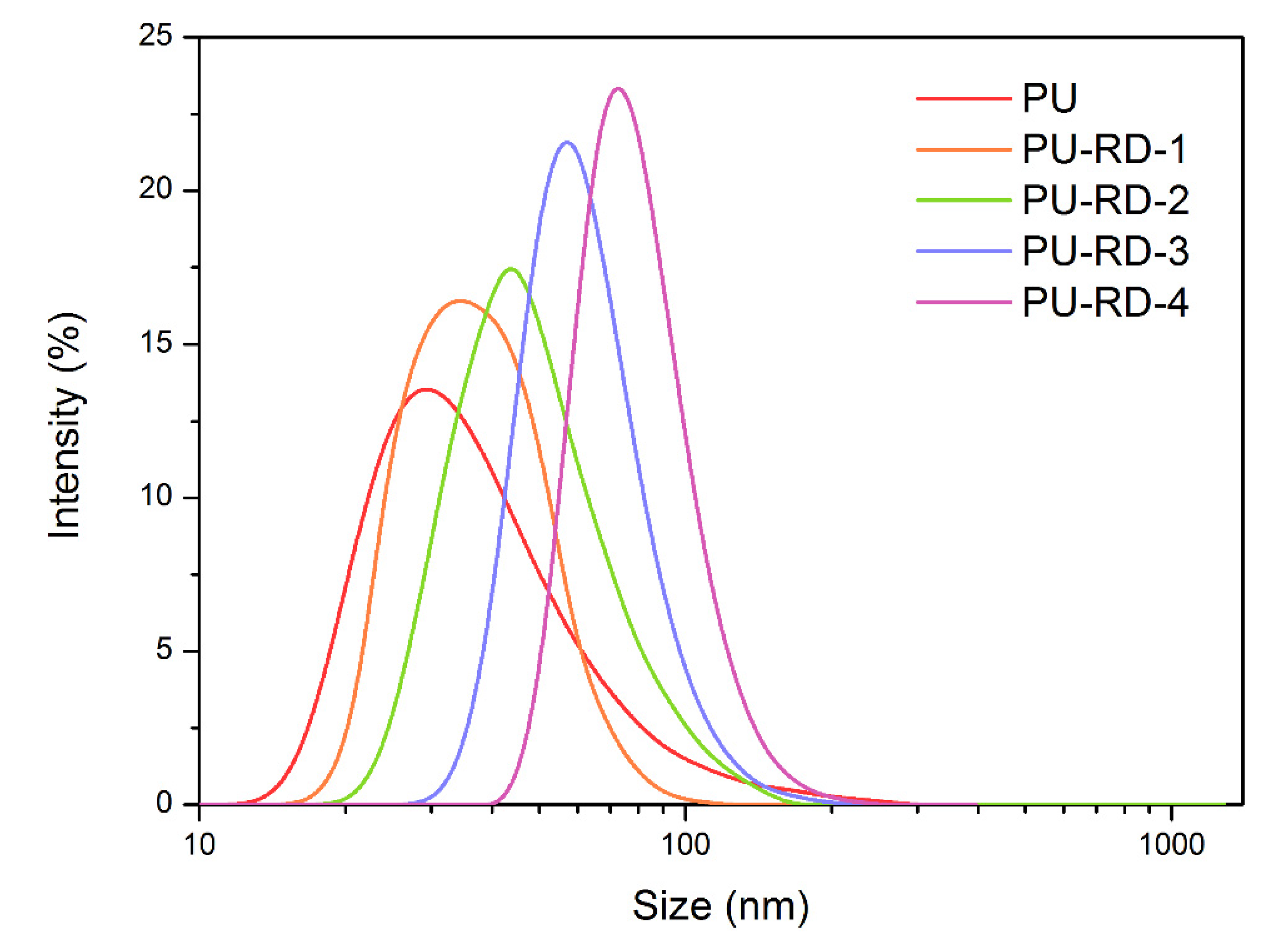
3.5. Emulsion Particle Size Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cao, H.; Li, B.; Jiang, X.; Zhu, X.; Kong, X.Z. Fluorescent linear polyurea based on toluene diisocyanate: Easy preparation, broad emission and potential applications. Chem. Eng. J. 2020, 399, 125867. [Google Scholar] [CrossRef] [PubMed]
- Rene, W.; Lenoble, V.; Chioukh, M.; Branger, C. A turn-on fluorescent ion-imprinted polymer for selective and reliable optosensing of lead in real water samples. Sens. Actuators B Chem. 2020, 319, 128252. [Google Scholar] [CrossRef]
- Li, D.; Wu, L.; Qu, F.; Ribadeneyra, M.C.; Tu, G.; Gautrot, J.E. Core-independent approach for polymer brush-functionalised nanomaterials with a fluorescent tag for RNA delivery. Chem. Commun. 2019, 55, 14166–14169. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Wang, J.-P.; Song, Y.; Qi, T.; Li, G.L. Bioinspired Healable Mechanochromic Function from Fluorescent Polyurethane Composite Film. Chemistryopen 2020, 9, 272–276. [Google Scholar] [CrossRef]
- Ghosh, S.; Manna, R.; Dey, S. Polyurethane network using 1-naphthylamine embedded epoxy-based polymer: Ferric ion selective fluorescent probe. Polym. Bull. 2019, 76, 205–213. [Google Scholar] [CrossRef]
- Liu, H.; Hu, T.; Wang, D.; Shi, J.; Zhang, J.; Wang, J.-X.; Pu, Y.; Chen, J.-F. Preparation of fluorescent waterborne polyurethane nanodispersion by high-gravity miniemulsion polymerization for multifunctional applications. Chem. Eng. Process. Process. Intensif. 2019, 136, 36–43. [Google Scholar] [CrossRef]
- Li, M.; Qiang, X.; Xu, W.; Zhang, H. Synthesis, characterization and application of AFC-based waterborne polyurethane. Prog. Org. Coat. 2015, 84, 35–41. [Google Scholar] [CrossRef]
- Lian, F.; Wang, C.; Wu, Q.; Yang, M.; Wang, Z.; Zhang, C. In situ synthesis of stretchable and highly stable multi-color carbon-dots/polyurethane composite films for light-emitting devices. RSC Adv. 2020, 10, 1281–1286. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, X.-Y.; Dai, J.; Li, J. Synthesis and Fluorescent Performance of Fluorescein-functionalized Waterborne Polyurethane. J. Macromol. Sci. Part A 2012, 49, 890–896. [Google Scholar] [CrossRef]
- Akindoyo, J.O.; Beg, D.H.; Ghazali, S.; Islam, M.R.; Jeyaratnam, N.; Yuvaraj, A.R. Polyurethane types, synthesis and applications—A review. RSC Adv. 2016, 6, 114453–114482. [Google Scholar] [CrossRef]
- Somisetti, V.; Narayan, R.; Raju, K.V.S.N. Multifunctional polyurethane coatings derived from phosphated cardanol and undecylenic acid based polyols. Prog. Org. Coat. 2019, 134, 91–102. [Google Scholar] [CrossRef]
- Yang, Y.; Cao, X.; Luo, H.; Cai, X. Thermal stability and decomposition behaviors of segmented copolymer poly(urethane-urea-amide). J. Polym. Res. 2018, 25, 242. [Google Scholar] [CrossRef]
- Gomez, I.J.; Wu, J.; Roper, J.; Beckham, H.; Meredith, J.C.; Roper, I.J.A. High Throughput Screening of Mechanical Properties and Scratch Resistance of Tricomponent Polyurethane Coatings. ACS Appl. Polym. Mater. 2019, 1, 3064–3073. [Google Scholar] [CrossRef]
- Dong, F.; Maganty, S.; Meschter, S.J.; Cho, J. Effects of curing conditions on structural evolution and mechanical properties of UV-curable polyurethane acrylate coatings. Prog. Org. Coat. Int. Rev. J. 2018, 114, 58–67. [Google Scholar] [CrossRef]
- Liu, J.; Jiao, X.; Cheng, F.; Fan, Y.; Wu, Y.; Yang, X. Fabrication and performance of UV cured transparent silicone modified polyurethane–acrylate coatings with high hardness, good thermal stability and adhesion. Prog. Org. Coat. 2020, 144, 105673. [Google Scholar] [CrossRef]
- Zhu, J.; Wu, Z.; Xiong, D.; Pan, L.; Liu, Y.-J. Preparation and properties of a novel low crystallinity cross-linked network waterborne polyurethane for water-based ink. Prog. Org. Coat. 2019, 133, 161–168. [Google Scholar] [CrossRef]
- Trzebiatowska, P.J.; Echart, A.S.; Calvo-Correas, T.; Eceiza, A.; Datta, J. The changes of crosslink density of polyurethanes synthesised with using recycled component. Chemical structure and mechanical properties investigations. Prog. Org. Coat. 2018, 115, 41–48. [Google Scholar] [CrossRef]
- Xing, C.; Wang, L.; Xian, L.; Wang, Y.; Zhang, L.; Xi, K.; Zhang, Q.; Jia, X. Enhanced Thermal Ageing Stability of Mechanophore in Polyurethane Network by Introducing Polyhedral Oligomeric Silsesquioxanes (POSS). Macromol. Chem. Phys. 2018, 219, 1800042. [Google Scholar] [CrossRef]
- Pan, L.; Xiong, Z.; Song, L.; Ban, J.-F.; Lu, S. Synthesis and characterization of sisal fibre polyurethane network cross-linked with triple-shape memory properties. New J. Chem. 2018, 42, 7130–7137. [Google Scholar] [CrossRef]
- Qian, Y.; An, X.; Huang, X.; Pan, X.; Zhu, J.; Zhu, X. Recyclable Self-Healing Polyurethane Cross-Linked by Alkyl Diselenide with Enhanced Mechanical Properties. Polymers 2019, 11, 773. [Google Scholar] [CrossRef]
- Hermida-Merino, D.; O’Driscoll, B.; Hart, L.R.; Harris, P.J.F.; Colquhoun, H.M.; Slark, A.T.; Prisacariu, C.; Hamley, I.W.; Hayes, W. Enhancement of microphase ordering and mechanical properties of supramolecular hydrogen-bonded polyurethane networks. Polym. Chem. 2018, 9, 3406–3414. [Google Scholar] [CrossRef]
- Arévalo-Alquichire, S.; Morales-Gonzalez, M.; Navas-Gómez, K.; Díaz, L.E.; Gómez-Tejedor, J.; Serrano, M.-A.; Valero, M.F. Influence of Polyol/Crosslinker Blend Composition on Phase Separation and Thermo-Mechanical Properties of Polyurethane Thin Films. Polymers 2020, 12, 666. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.; Huang, C.; Zhuang, H.; Gong, H.; Zhang, C.; Ren, R.; Ma, Y. Degradable polyurethane based on star-shaped polyester polyols (trimethylolpropane and E-caprolactone) for marine antifouling. Prog. Org. Coat. 2015, 87, 161–170. [Google Scholar] [CrossRef]
- Chattopadhyay, D.K.; Sreedhar, B.; Raju, K.V.S.N. Thermal stability of chemically crosslinked moisture-cured polyurethane coatings. J. Appl. Polym. Sci. 2010, 95, 1509–1518. [Google Scholar] [CrossRef]
- Yarmohammadi, M.; Komeili, S.; Shahidzadeh, M. Studying Crosslinker Chemical Structure Effect on the Tuning Properties of HTPB--Based Polyurethane. Propellants Explos. Pyrotech. 2018, 43, 156–161. [Google Scholar] [CrossRef]
- Diao, Q.; Guo, H.; Yang, Z.; Luo, W.; Li, T.; Hou, D. A rhodamine-6G-based “turn-on” fluorescent probe for selective detection of Fe3+ in living cells. Anal. Methods 2019, 11, 794–799. [Google Scholar] [CrossRef]
- Berube, M.-A.; Schorr, D.; Ball, R.J.; Landry, V.; Blanchet, P. Determination of In Situ Esterification Parameters of Citric Acid-Glycerol Based Polymers for Wood Impregnation. J. Polym. Environ. 2018, 26, 970–979. [Google Scholar] [CrossRef]
- Marques, N.D.N.; Balaban, R.D.C.; Halila, S.; Borsali, R. Synthesis and characterization of carboxymethylcellulose grafted with thermoresponsive side chains of high LCST: The high temperature and high salinity self-assembly dependence. Carbohydr. Polym. 2018, 184, 108–117. [Google Scholar] [CrossRef]
- Datta, J.; Głowińska, E. Effect of hydroxylated soybean oil and bio-based propanediol on the structure and thermal properties of synthesized bio-polyurethanes. Ind. Crop. Prod. 2014, 61, 84–91. [Google Scholar] [CrossRef]
- Hu, X.; Liu, X.; Liu, M.; Li, G.; Cheng, C. A photochromic waterborne polyurethane-based dye with chemically fixed azobenzene groups. Polym. Bull. 2019, 76, 3437–3450. [Google Scholar] [CrossRef]
- Fujii, K.; Iyi, N.; Sasai, R.; Hayashi, S. Preparation of a Novel Luminous Heterogeneous System: Rhodamine/Coumarin/Phyllosilicate Hybrid and Blue Shift in Fluorescence Emission. Chem. Mater. 2008, 20, 2994–3002. [Google Scholar] [CrossRef]
- Yang, Z.; Wicks, D.A.; Yuan, J.; Pu, H.; Liu, Y. Newly UV-curable polyurethane coatings prepared by multifunctional thiol- and ene-terminated polyurethane aqueous dispersions: Photopolymerization properties. Polymers 2010, 51, 1572–1577. [Google Scholar] [CrossRef]
- Crawford, D.M.; Escarsega, J.A. Dynamic mechanical analysis of novel polyurethane coating for military applications. Thermochim. Acta 2000, 357, 161–168. [Google Scholar] [CrossRef]
- Campanella, A.; Bonnaillie, L.M.; Wool, R.P. Polyurethane foams from soyoil-based polyols. J. Appl. Polym. Sci. 2009, 112, 2567–2578. [Google Scholar] [CrossRef]
- Chiou, B.-S.; Schoen, P.E. Effects of crosslinking on thermal and mechanical properties of polyurethanes. J. Appl. Polym. Sci. 2002, 83, 212–223. [Google Scholar] [CrossRef]
- Kopczyńska, P.; Datta, J.; Trzebiatowska, P.J. Single-phase product obtained via crude glycerine depolymerisation of polyurethane elastomer: Structure characterisation and rheological behaviour. Polym. Int. 2016, 65, 946–954. [Google Scholar] [CrossRef]
- Camino, G.; Lomakin, S.M.; Lazzari, M. Polydimethylsiloxane thermal degradation Part 1. Kinetic aspects. Polymer 2001, 42, 2395–2402. [Google Scholar] [CrossRef]
- Ma, M.; Sun, G. Antimicrobial cationic dyes: Part 2—Thermal and hydrolytic stability. Dyes Pigment 2004, 63, 39–49. [Google Scholar] [CrossRef]
- Mao, H.; Wang, C.; Wang, Y. Synthesis of polymeric dyes based on waterborne polyurethane for improved color stability. New J. Chem. 2015, 39, 3543–3550. [Google Scholar] [CrossRef]
- Deshpande, G.; Rezac, M.E. The effect of phenyl content on the degradation of poly(dimethyl diphenyl) siloxane copolymers. Polym. Degrad. Stab. 2001, 74, 363–370. [Google Scholar] [CrossRef]
- Wang, L.; Shen, Y.; Lai, X.; Li, Z. Effect of nanosilica content on properties of polyurethane/silica hybrid emulsion and its films. J. Appl. Polym. Sci. 2010, 119, 3521–3530. [Google Scholar] [CrossRef]





| Tensile Strength (MPa) | Elongation at Break (%) | Tg/°C | |
|---|---|---|---|
| PU | 10.0 | 840.0 | −43.9 |
| PU-RD-1 | 10.5 | 763.9 | −41.3 |
| PU-RD-2 | 11.0 | 711.8 | −39.6 |
| PU-RD-3 | 12.3 | 632.2 | −36.2 |
| PU-RD-4 | 14.5 | 544.1 | −33.4 |
| T5%a/°C | Tmax1b/°C | Tmax2c/°C | |
|---|---|---|---|
| PU | 279.2 | 330.4 | 405.1 |
| PU-RD -3 | 281.9 | 346.6 | 406.8 |
| Average Size (nm) | PDI | |
|---|---|---|
| PU | 48.20 | 0.242 |
| PU-RD-1 | 53.42 | 0.222 |
| PU-RD -2 | 63.83 | 0.195 |
| PU-RD -3 | 69.04 | 0.116 |
| PU-RD -4 | 78.82 | 0.104 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, S.; Chen, Y.; Zhu, Y.; Fan, H. A Fluorescent Polyurethane with Covalently Cross-Linked Rhodamine Derivatives. Polymers 2020, 12, 1989. https://doi.org/10.3390/polym12091989
Tian S, Chen Y, Zhu Y, Fan H. A Fluorescent Polyurethane with Covalently Cross-Linked Rhodamine Derivatives. Polymers. 2020; 12(9):1989. https://doi.org/10.3390/polym12091989
Chicago/Turabian StyleTian, Saiqi, Yinyan Chen, Yifan Zhu, and Haojun Fan. 2020. "A Fluorescent Polyurethane with Covalently Cross-Linked Rhodamine Derivatives" Polymers 12, no. 9: 1989. https://doi.org/10.3390/polym12091989
APA StyleTian, S., Chen, Y., Zhu, Y., & Fan, H. (2020). A Fluorescent Polyurethane with Covalently Cross-Linked Rhodamine Derivatives. Polymers, 12(9), 1989. https://doi.org/10.3390/polym12091989