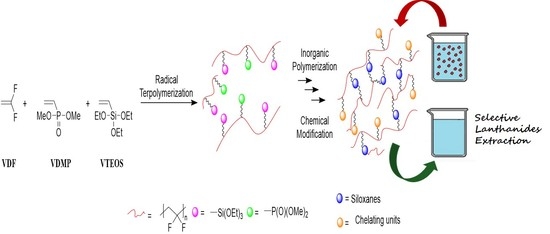
Solid–Liquid Europium Ion Extraction via Phosphonic Acid-Functionalized Polyvinylidene Fluoride Siloxanes
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Characterization
2.2.1. Nuclear Magnetic Resonance (NMR) Spectroscopy
2.2.2. Thermogravimetric Analysis (TGA)
2.2.3. Differential Scanning Calorimetry (DSC)
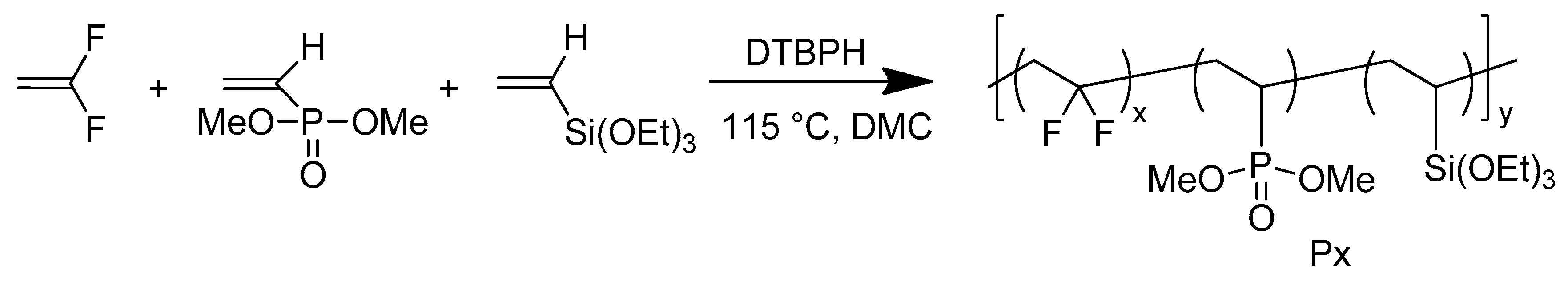
2.3. Radical Terpolymerization of VDF with VTEOS and VDMP
2.4. Crosslinking of poly(VDF-ter-VDMP-ter-VTEOS) Terpolymer
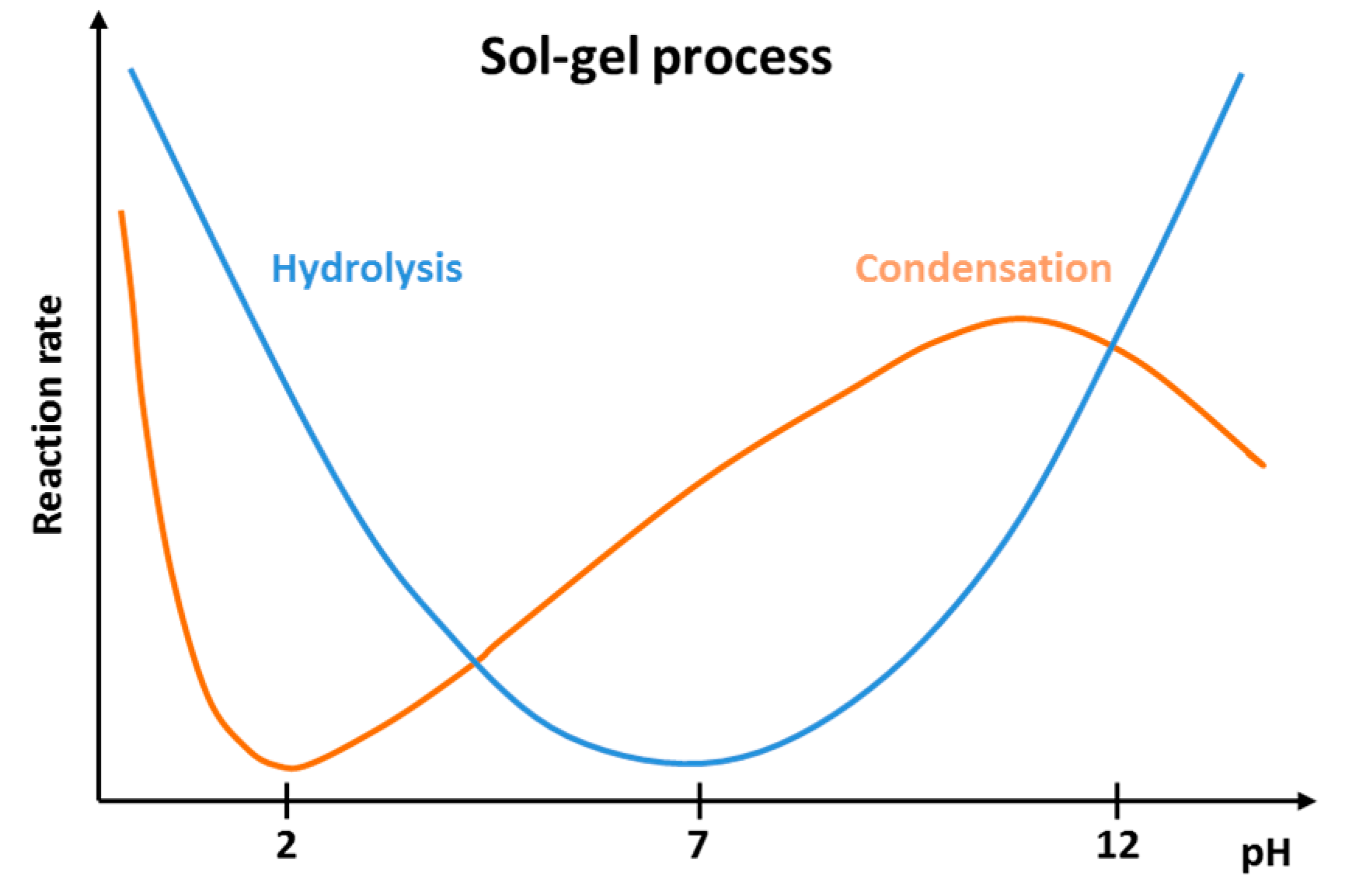
2.5. Hydrolysis of Phosphonate Groups into Phosphonic Acid in poly(VDF-ter-VDMP-ter-VTEOS) Terpolymer
2.6. Complexometric Titration
3. Results and Discussion
3.1. Preparation of poly(VDF-ter-VDMP-ter-VTEOS) Terpolymers
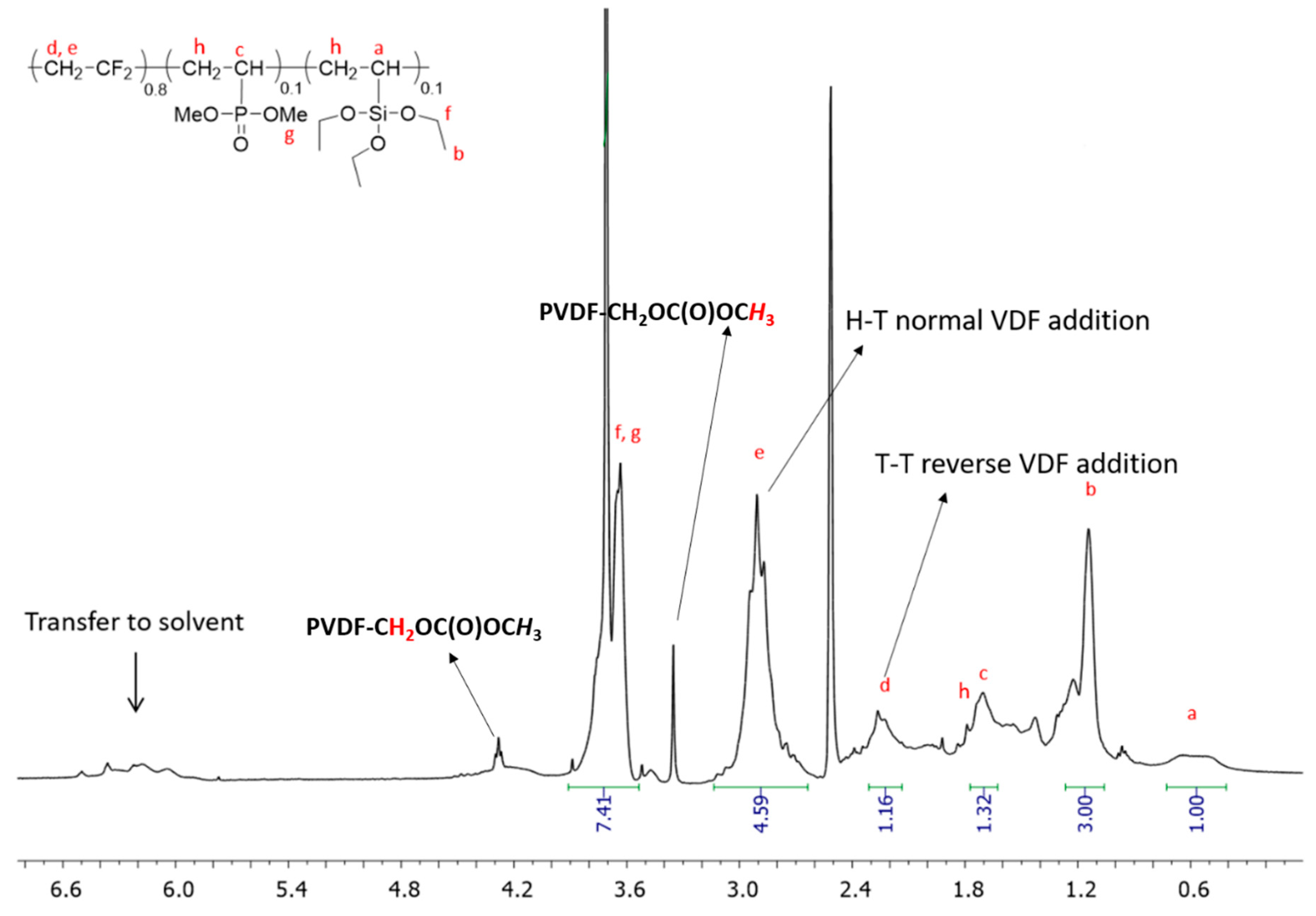
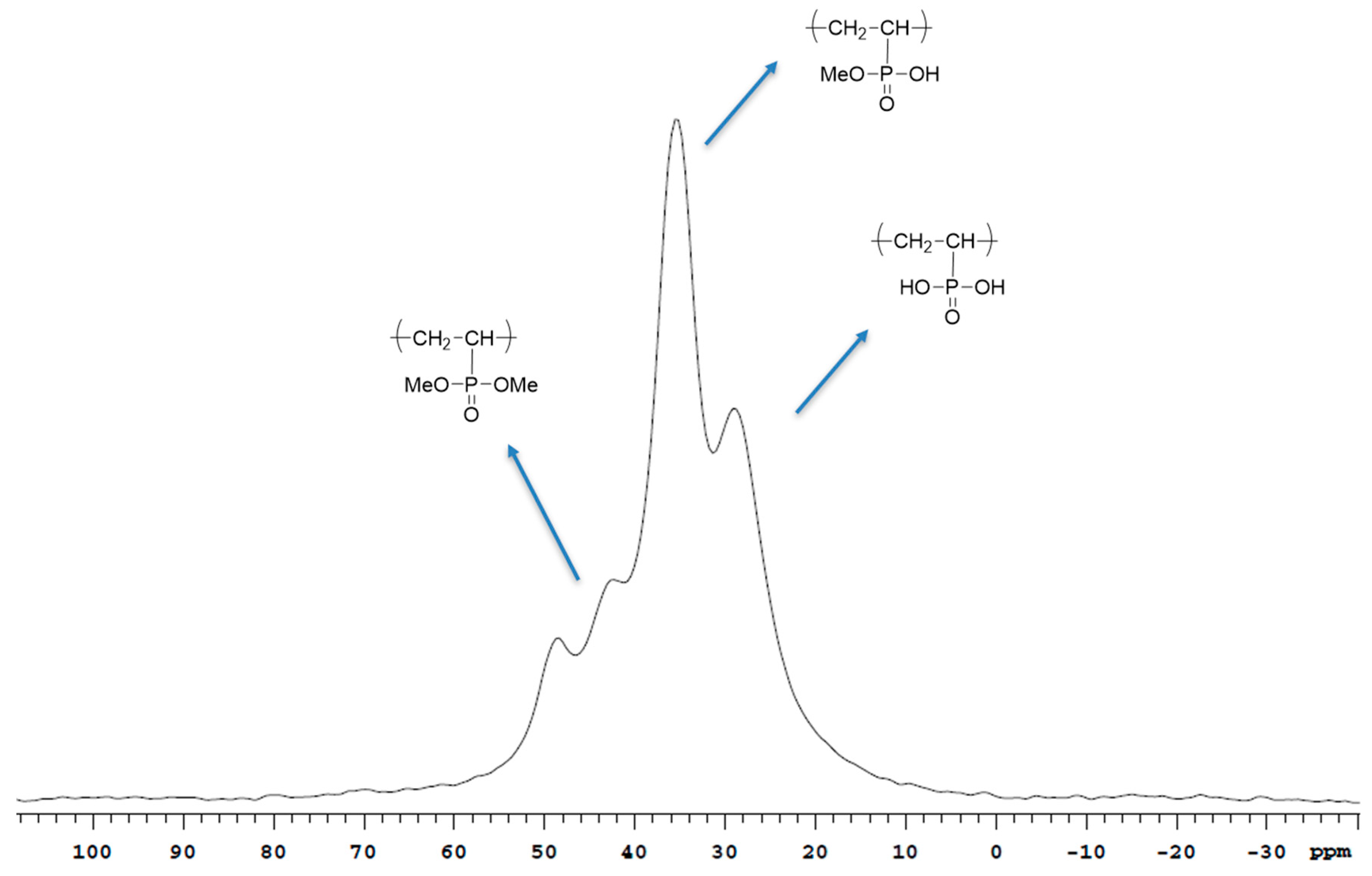
3.2. Characterization of poly(VDF-ter-VTEOS-ter-VDMP) Terpolymers by 1H, 19F, 31P and 29Si NMR Spectroscopies
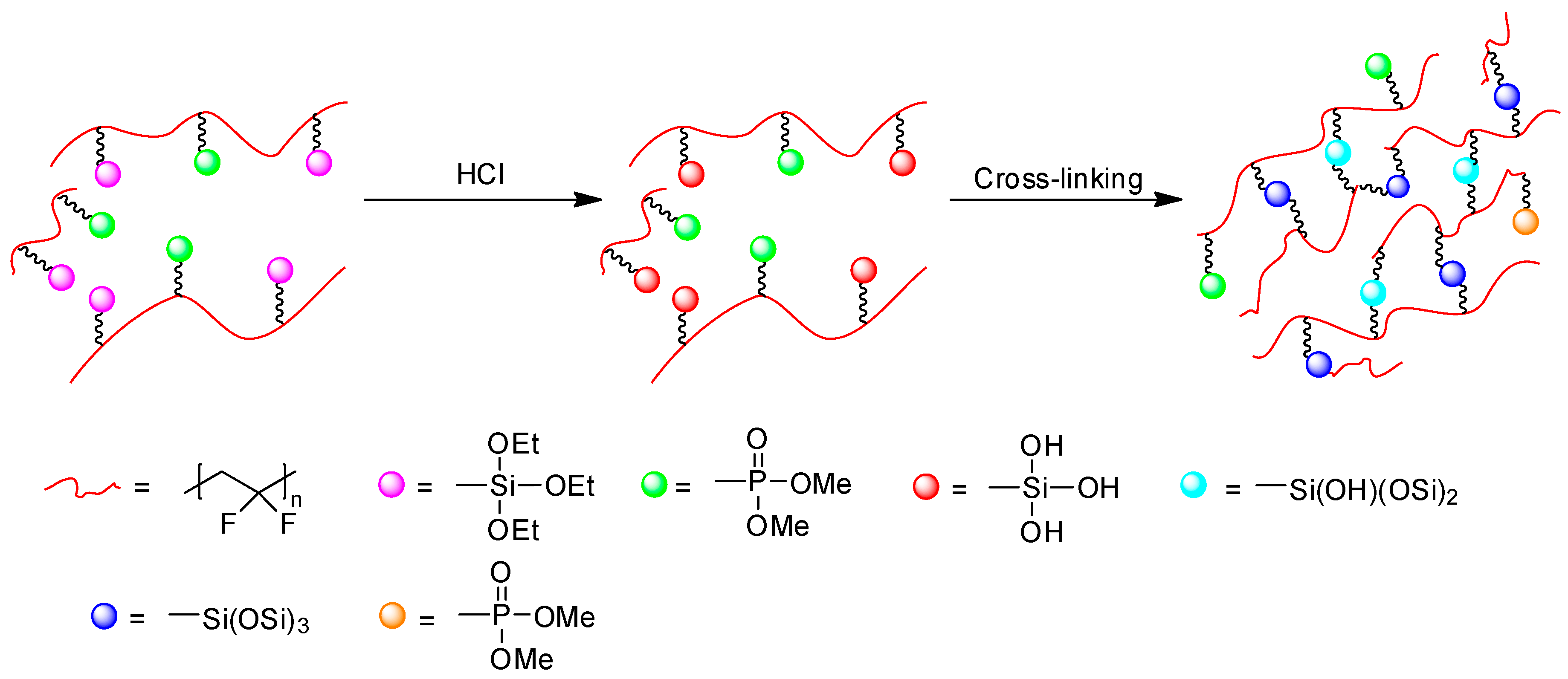
3.3. Crosslinking of poly(VDF-ter-VDMP-ter-VTEOS) Terpolymer
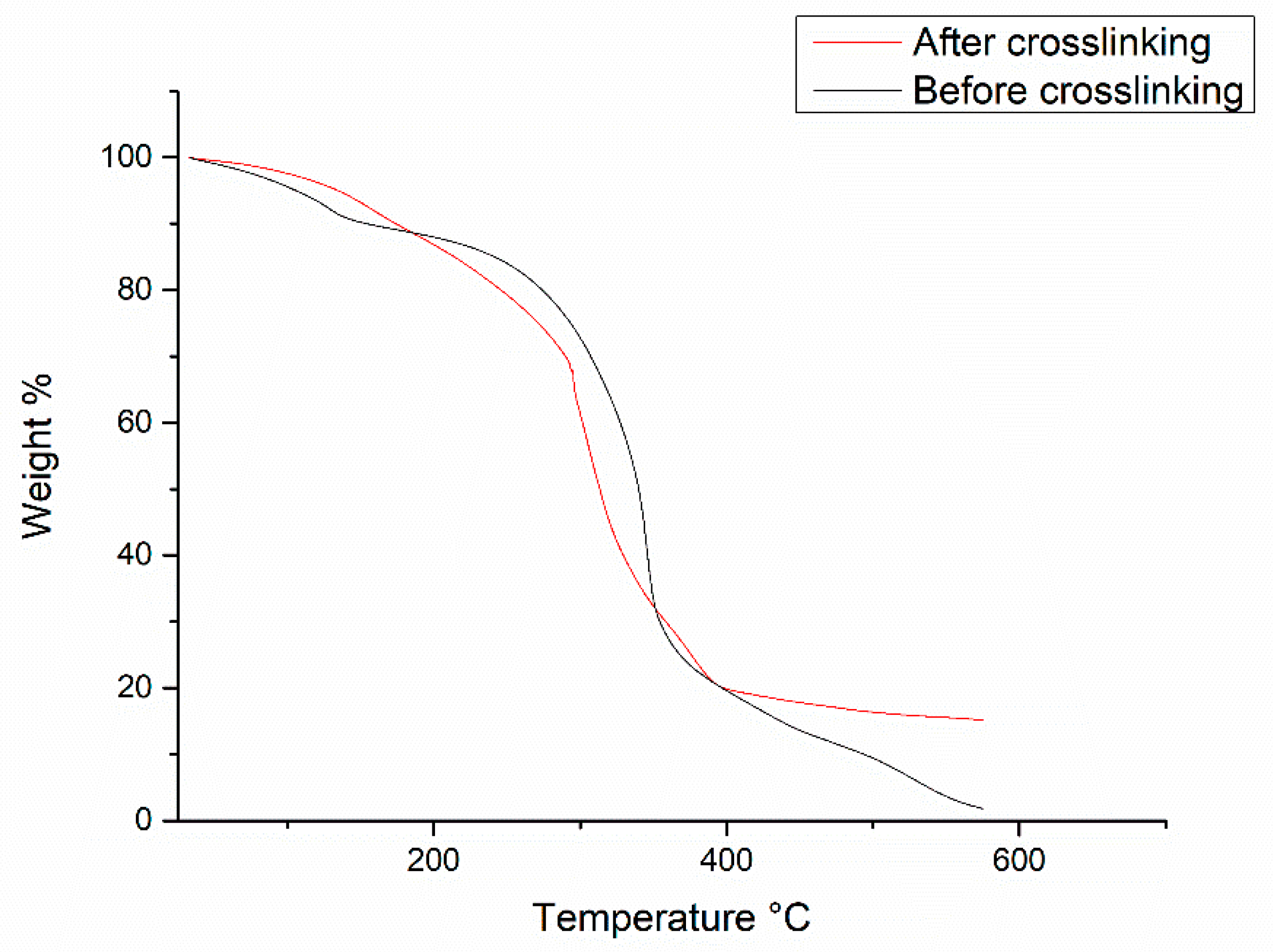
3.4. Thermal Properties of poly(VDF-ter-VTEOS-ter-VDMP) Terpolymer
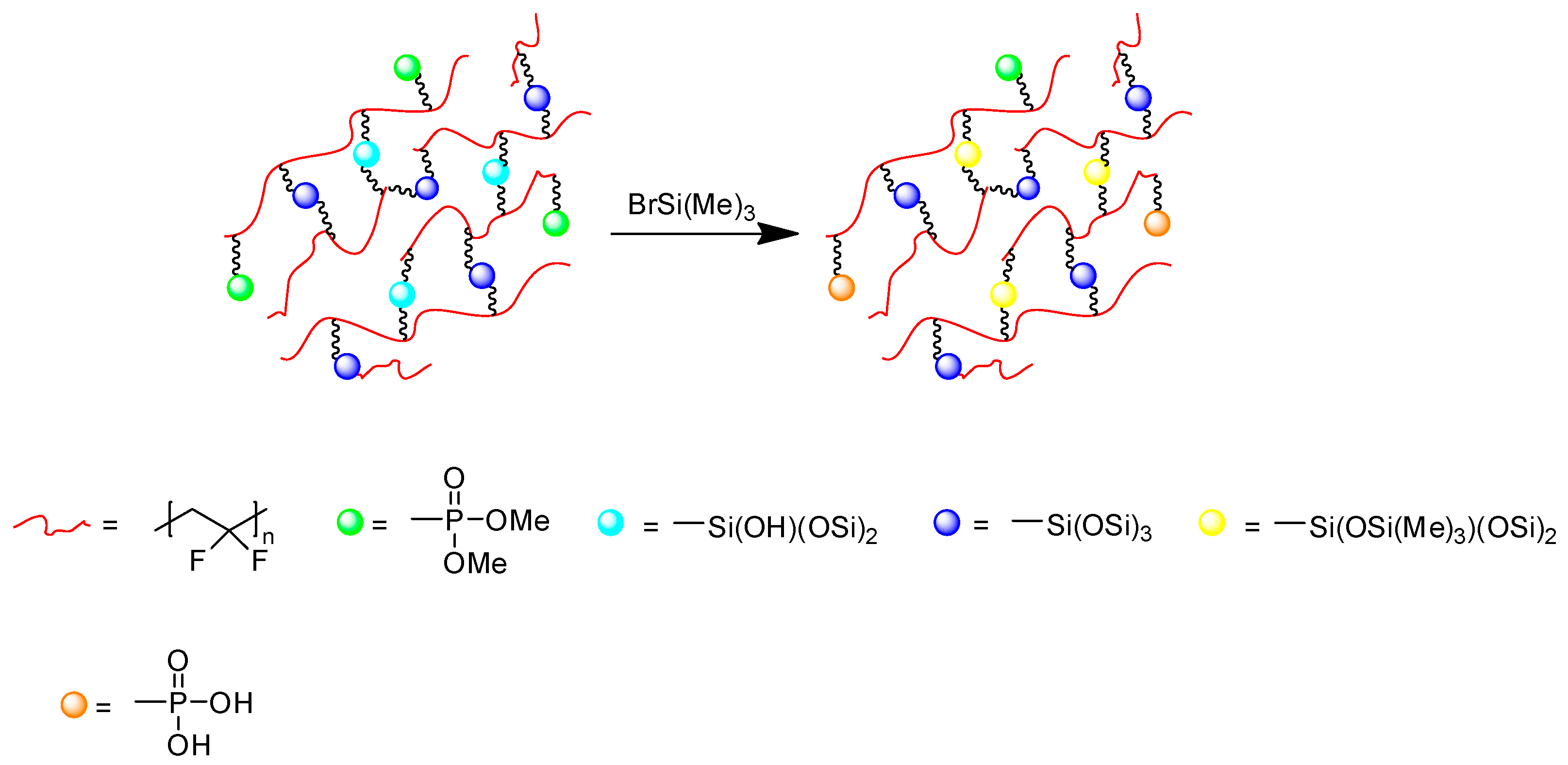
3.5. Hydrolysis of Phosphonate Groups into Phosphonic Acid in poly(VDF-ter-VTEOS-ter-VDMP) Terpolymer
3.6. Eu(III) Uptake from Aqueous Medium
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chipasa, K.B. Accumulation and fate of selected heavy metals in a biological wastewater treatment system. Waste Manag. 2003, 23, 135–143. [Google Scholar] [CrossRef]
- Dean, J.G.; Bosqui, F.L.; Lanouette, K.H. Removing heavy metals from waste water. Environ. Sci. Technol. 1972, 6, 518–522. [Google Scholar] [CrossRef]
- Boullanger, A.; Gracy, G.; Bibent, N.; Devautour-Vinot, S.; Clément, S.; Mehdi, A. From an Octakis(3-cyanopropyl)silsesquioxane building block to a highly COOH-functionalized hybrid organic–inorganic material. Eur. J. Inorg. Chem. 2012, 2012, 143–150. [Google Scholar] [CrossRef]
- Besson, E.; Mehdi, A.; Van der Lee, A.; Chollet, H.; Reye, C.; Guilard, R.; Corriu, R.J. Selective lanthanides sequestration based on a self-assembled organosilica. Eur. Chem. J. 2010, 16, 10226–10233. [Google Scholar] [CrossRef] [PubMed]
- Besson, E.; Mehdi, A.; Reyé, C.; Gaveau, P.; Corriu, R.J.P. Self-assembly of layered organosilicas based on weak intermolecular interactions. Dalton Trans. 2010, 39, 7534–7539. [Google Scholar] [CrossRef]
- Beauvais, R.A.; Alexandratos, S.D. Polymer-supported reagents for the selective complexation of metal ions: An overview. React. Funct. Polym. 1998, 36, 113–123. [Google Scholar] [CrossRef]
- Feng, D.; Aldrich, C.; Tan, H. Treatment of acid mine water by use of heavy metal precipitation and ion exchange. Miner. Eng. 2000, 13, 623–642. [Google Scholar] [CrossRef]
- Sirkar, K.K. Membranes, phase interfaces, and separations: Novel techniques and membranes—An overview. Ind. Eng. Chem. Res. 2008, 47, 5250–5266. [Google Scholar] [CrossRef]
- Rubio, J.; Souza, M.L.; Smith, R.W. Overview of flotation as a wastewater treatment technique. Miner. Eng. 2002, 15, 139–155. [Google Scholar] [CrossRef]
- Chen, G. Electrochemical technologies in wastewater treatment. Sep. Purif. Technol. 2004, 38, 11–41. [Google Scholar] [CrossRef]
- Srivastava, N.K.; Majumder, C.B. Novel biofiltration methods for the treatment of heavy metals from industrial wastewater. J. Hazard. Mater. 2008, 151, 1–8. [Google Scholar] [CrossRef]
- Camel, V. Solid phase extraction of trace elements. Spectrochim. Acta Part. B At. Spectrosc. 2003, 58, 1177–1233. [Google Scholar] [CrossRef]
- Pustam, A.N.; Alexandratos, S.D. Engineering selectivity into polymer-supported reagents for transition metal ion complex formation. React. Funct. Polym. 2010, 70, 545–554. [Google Scholar] [CrossRef]
- Hosseini, M.S.; Hosseini-Bandegharaei, A.; Raissi, H.; Belador, F. Sorption of Cr(VI) by Amberlite XAD-7 resin impregnated with brilliant green and its determination by quercetin as a selective spectrophotometric reagent. J. Hazard. Mater. 2009, 169, 52–57. [Google Scholar] [CrossRef] [PubMed]
- León-González, M.E.; Pérez-Arribas, L.V. Chemically modified polymeric sorbents for sample preconcentration. J. Chromatogr. A 2000, 902, 3–16. [Google Scholar] [CrossRef]
- Metilda, P.; Sanghamitra, K.; Mary Gladis, J.; Naidu, G.R.K.; Prasada Rao, T. Amberlite XAD-4 functionalized with succinic acid for the solid phase extractive preconcentration and separation of uranium(VI). Talanta 2005, 65, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Dinu, M.V.; Dragan, E.S. Heavy metals adsorption on some iminodiacetate chelating resins as a function of the adsorption parameters. React. Funct. Polym. 2008, 68, 1346–1354. [Google Scholar] [CrossRef]
- Türkmen, D.; Yılmaz, E.; Öztürk, N.; Akgöl, S.; Denizli, A. Poly(hydroxyethyl methacrylate) nanobeads containing imidazole groups for removal of Cu(II) ions. Mater. Sci. Eng. C 2009, 29, 2072–2078. [Google Scholar] [CrossRef]
- Bernard, J.; Branger, C.; Beurroies, I.; Denoyel, R.; Blanc, S.; Margaillan, A. Synthesis of a poly(vinylcatechol-co-divinylbenzene) resin and accessibility to catechol units. Polymer 2010, 51, 2472–2478. [Google Scholar] [CrossRef]
- Köytepe, S.; Erdoğan, S.; Seçkin, T. Synthesis of polyimide from 5,5″-bis(bromomethyl)-2,2′:6′,2″-terpyridine and investigation of the polymer sorption behavior towards some metal ions. J. Hazard. Mater. 2009, 162, 695–702. [Google Scholar] [CrossRef]
- Monge, S.; Canniccioni, B.; David, G.; Robin, J.-J. CHAPTER 1 Polymerization of phosphorus-containing (meth)acrylate monomers. In Phosphorus-Based Polymers: From Synthesis to Applications; The Royal Society of Chemistry: Cambridge, UK, 2014; pp. 1–18. [Google Scholar]
- Maiti, S.; Banerjee, S.; Palit, S.K. Phosphorus-containing polymers. Prog. Polym. Sci. 1993, 18, 227–261. [Google Scholar] [CrossRef]
- Monge, S.; David, G. Phosphorus-Based Polymers: From Synthesis to Applications; The Royal Society of Chemistry: Cambridge, UK, 2014; p. 318. [Google Scholar]
- Zhao, C.S.; Chen, L.; Wang, Y.Z. A phosphorus-containing thermotropic liquid crystalline copolyester with low mesophase temperature and high flame retardance. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 5752–5759. [Google Scholar] [CrossRef]
- Monge, S.; Canniccioni, B.; Graillot, A.; Robin, J.J. Phosphorus-containing polymers: A great opportunity for the biomedical field. Biomacromolecules 2011, 12, 1973–1982. [Google Scholar] [CrossRef] [PubMed]
- Horrocks, R.A.; Zhang, S. Enhancing polymer char formation by reaction with phosphorylated polyols. 1. Cellulose. Polymer 2001, 42, 8025–8033. [Google Scholar] [CrossRef]
- Ananda Kumar, S.; Balakrishnan, T.; Alagar, M.; Denchev, Z. Development and characterization of silicone/phosphorus modified epoxy materials and their application as anticorrosion and antifouling coatings. Prog. Org. Coat. 2006, 55, 207–217. [Google Scholar] [CrossRef] [Green Version]
- Canadell, J.; Hunt, B.J.; Cook, A.G.; Mantecón, A.; Cádiz, V. Flame retardance and shrinkage reduction of polystyrene modified with acrylate-containing phosphorus and crosslinkable spiro-orthoester moieties. Polym. Degrad. Stab. 2007, 92, 1482–1490. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, Y.; Wen, S.; Wang, S. Poly(vinylphosphonic acid) (PVPA) on titanium alloy acting as effective cartilage-like superlubricity coatings. ACS Appl. Mater. Interfaces 2014, 6, 17571–17578. [Google Scholar] [CrossRef]
- Castelvetro, V.; Aglietto, M.; Ciardelli, F.; Chaintore, O.; Lazzari, M. Design of fluorinated acrylic-based polymers as water repellent, intrinsically photostable coating materials for stone. In ACS Symposium Series 787 on Fluorinated Surfaces, Coatings, and Films; Castner, D.G., Grainger, D.W., Eds.; American Chemical Society: Washington, DC, USA, 2001; pp. 29–142. [Google Scholar]
- Kotov, S.V.; Pedersen, S.D.; Qiu, W.; Qiu, Z.M.; Burton, D.J. Preparation of perfluorocarbon polymers containing phosphonic acid groups. J. Fluor. Chem. 1997, 82, 13–19. [Google Scholar] [CrossRef]
- Tayouo, R.; David, G.; Ameduri, B.; Roziere, J.; Roualdes, S. New fluorinated polymers bearing pendant phosphonic acid groups. Proton conducting membranes for fuel cell. Macromolecules 2010, 43, 5269–5276. [Google Scholar] [CrossRef]
- Perrin, R.; Elomaa, M.; Jannasch, P. Nanostructured proton conducting polystyrene-poly(vinylphosphonic acid) block copolymers prepared via sequential anionic polymerizations. Macromolecules 2009, 42, 5146–5154. [Google Scholar] [CrossRef]
- Yamabe, M.; Akiyama, K.; Akatsuka, Y.; Kato, M. Novel phosphonated perfluorocarbon polymers. Eur. Polym. J. 2000, 36, 1035–1041. [Google Scholar] [CrossRef]
- Zaki, M.T.; Ismail, M.I.; Rizkalla, E.N. Metal chelates of phosphonate-containing ligands: VII. Analytical applications of 1-hydroxyethane-1,1-diphosphonic acid. Microchem. J. 1984, 30, 6–11. [Google Scholar] [CrossRef]
- Kabanov, V.A.; Efendiev, A.A.; Orujev, D.D. Complex-forming polymeric sorbents with macromolecular arrangement favorable for ion sorption. J. Appl. Polym. Sci. 1979, 24, 259–267. [Google Scholar] [CrossRef]
- Yoshida, M.; Uezu, K.; Goto, M.; Nakashio, F. Metal ion-imprinted resins with novel bifunctional monomer by surface template polymerization. J. Chem. Eng. Jpn. 1996, 29, 174–176. [Google Scholar] [CrossRef] [Green Version]
- Zhu, L.; Zhu, Z.; Zhang, R.; Hong, J.; Qiu, Y. Synthesis and adsorption performance of lead ion-imprinted micro-beads with combination of two functional monomers. J. Environ. Sci. 2011, 23, 1955–1961. [Google Scholar] [CrossRef]
- Scheirs, J. Modern Fluoropolymers: High Performance Polymers for Diverse Applications; Wiley: Chichester, UK, 1997. [Google Scholar]
- Ameduri, B.; Sawada, H. Fluorinated Polymers: Volume 2: Applications; RSC: Cambridge, UK, 2016; Volume 2. [Google Scholar]
- Smith, D.W.; Iacono, S.T.; Iyer, S.S. Handbook of Fluoropolymer Science and Technology; Wiley: New York, NY, USA, 2014. [Google Scholar]
- Hougham, G.; Cassidy, P.E.; Johns, K.; Davidson, J. Fluoropolymers: Synthesis and Applications; Plenum Publishers: New York, NY, USA, 1999; p. 329. [Google Scholar]
- Ameduri, B.; Boutevin, B. Well Architectured Fluoropolymers: Synthesis, Properties and Applications; Elsevier: Amsterdam, The Netherlands, 2004. [Google Scholar]
- Puts, G.J.; Crouse, P.; Ameduri, B.M. Polytetrafluoroethylene: Synthesis and characterization of the original extreme polymer. Chem. Rev. 2019, 119, 1763–1805. [Google Scholar] [CrossRef] [PubMed]
- Ameduri, B. From vinylidene fluoride (VDF) to the applications of VDF-containing polymers and copolymers: Recent developments and future trends. Chem. Rev. 2009, 109, 6632–6686. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Song, L.; Fu, J.; Tang, P.; Liu, F. Adsorption characteristics of Ni(II) onto MA–DTPA/PVDF chelating membrane. J. Hazard. Mater. 2011, 189, 732–740. [Google Scholar] [CrossRef]
- Song, L.; Zhao, X.; Fu, J.; Wang, X.; Sheng, Y.; Liu, X. DFT investigation of Ni(II) adsorption onto MA-DTPA/PVDF chelating membrane in the presence of coexistent cations and organic acids. J. Hazard. Mater. 2012, 199, 433–439. [Google Scholar] [CrossRef]
- Wang, X.; Chen, C.; Liu, H.; Ma, J. Preparation and characterization of PAA/PVDF membrane-immobilized Pd/Fe nanoparticles for dechlorination of trichloroacetic acid. Water Res. 2008, 42, 4656–4664. [Google Scholar] [CrossRef]
- Tavares, C.R.; Vieira, M.; Petrus, J.C.C.; Bortoletto, E.C.; Ceravollo, F. Ultrafiltration/complexation process for metal removal from pulp and paper industry wastewater. Desalination 2002, 144, 261–265. [Google Scholar] [CrossRef]
- Parshetti, G.K.; Doong, R.-A. Dechlorination of trichloroethylene by Ni/Fe nanoparticles immobilized in PEG/PVDF and PEG/nylon 66 membranes. Water Res. 2009, 43, 3086–3094. [Google Scholar] [CrossRef] [PubMed]
- Shojaee, N.; Patton, W.F.; Lim, M.J.; Shepro, D. Pyrogallol red-molybdate: A reversible, metal chelate stain for detection of proteins immobilized on membrane supports. Electrophoresis 1996, 17, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Vieira, M.; Tavares, C.R.; Bergamasco, R.; Petrus, J.C.C. Application of ultrafiltration-complexation process for metal removal from pulp and paper industry wastewater. J. Membr. Sci. 2001, 194, 273–276. [Google Scholar] [CrossRef]
- Wehbi, M.; Bourgeois, D.; Améduri, B. Use of poly(vinylidene fluoride-co-vinyl dimethylphosphonate) copolymers for efficient extraction of valuable metals. Polym. Chem. UK 2019, 10, 4173–4184. [Google Scholar] [CrossRef]
- Wehbi, M.; Mehdi, A.; Negrell, C.; David, G.; Alaaeddine, A.; Améduri, B. Phosphorus-containing fluoropolymers: State of the art and applications. ACS Appl. Mater. Interfaces 2020, 12, 38–59. [Google Scholar] [CrossRef]
- Taguet, A.; Ameduri, B.; Boutevin, B. Crosslinking of vinylidene fluoride-containing fluoropolymers. Adv. Polym. Sci. 2005, 184, 465. [Google Scholar]
- Hao, G.; Zhu, L.; Yang, W.; Chen, Y.; Huang, Q. Investigation of fluorosilicone polyacrylate film forming behavior on steel and PET substrates. J. Fluor. Chem. 2015, 176, 1–8. [Google Scholar] [CrossRef]
- Lee, K.W.; McCarthy, T.J. Synthesis of a polymer surface containing covalently attached triethoxysilane functionality: Adhesion to glass. Macromolecules 1988, 21, 3353–3356. [Google Scholar] [CrossRef]
- Cai, L.; Li, Z. Synthesis and surface properties of novel fluoroalkylsilyl methacrylate copolymers. J. Fluor. Chem. 2015, 178, 187–194. [Google Scholar] [CrossRef]
- Fukui, K.; Iba, C.; Hokoi, S. Moisture behavior inside building materials treated with silane water repellent. Energy Procedia 2017, 132, 735–740. [Google Scholar] [CrossRef]
- Cai, L.; Dai, L.; Yuan, Y.; Liu, A.; Zhanxiong, L. Synthesis of novel polymethacrylates with siloxyl bridging perfluoroalkyl side-chains for hydrophobic application on cotton fabrics. Appl. Surf. Sci. 2016, 371, 453–467. [Google Scholar] [CrossRef]
- Indulekha, K.; Behera, P.K.; Rajeev, R.S.; Gouri, C.; Ninan, K.N. Polyfluoroalkyl siloxanes with varying trifluoropropyl content: Synthesis, characterization and solvent resistance studies. J. Fluor. Chem. 2017, 200, 24–32. [Google Scholar] [CrossRef]
- Sel, O.; Soulès, A.; Améduri, B.; Boutevin, B.; Laberty-Robert, C.; Gebel, G.; Sanchez, C. Original fuel-cell membranes from crosslinked terpolymers via a “sol-gel” strategy. Adv. Funct. Mater. 2010, 20, 1090–1098. [Google Scholar] [CrossRef]
- Vargo, T.G.; Gardella, J.A.; Meyer, A.E.; Baier, R.E. Hydrogen/liquid vapor radio frequency glow discharge plasma oxidation/hydrolysis of expanded poly (tetrafluoroethylene) (ePTFE) and poly (vinylidene fluoride) (PVDF) surfaces. J. Polym. Sci. A Polym. Chem. 1991, 29, 555–570. [Google Scholar] [CrossRef]
- Hook, D.J.; Vargo, T.G.; Gardella, J.A.; Litwiler, K.S.; Bright, F.V. Silanization of radio frequency glow discharge modified expanded poly(tetrafluoroethylene) using (aminopropyl) triethoxysilane. Langnuir 1991, 7, 142–151. [Google Scholar] [CrossRef]
- Kallury, K.M.R.; Lee, W.E.; Thompson, M. Enhanced stability of urease immobilized onto phospholipid covalently bound to silica, tungsten, and fluoropolymer surfaces. Anal. Chem. 1993, 65, 2459–2467. [Google Scholar] [CrossRef]
- Bekos, E.J.; Ranieri, K.P.; Aebischer, P.; Gardella, J.A.; Bright, F.V. Structural changes of bovine serum albumin upon adsorption to modified fluoropolymer substrates used for neural cell attachment studies. Langmuir 1995, 11, 984–989. [Google Scholar] [CrossRef]
- Asandei, A.; Chen, Y. Room temperature synthesis of vinylidene fluoride/vinylethoxysilane copolymers with under Uv irradiation. Polym. Prepr. 2007, 48, 270–271. [Google Scholar]
- Nagata, N.; Kubota, L.T.; Bueno, M.I.M.S.; Peralta-Zamora, P.G. Adsorption parameters of Cd(II), Pb(II), and Hg(II) on Zirconium(IV) phosphate chemically grafted onto silica gel surface. J. Colloid Interface Sci. 1998, 200, 121–125. [Google Scholar] [CrossRef]
- Florek, J.; Giret, S.; Juère, E.; Larivière, D.; Kleitz, F. Functionalization of mesoporous materials for lanthanide and actinide extraction. Dalton Trans. 2016, 45, 14832–14854. [Google Scholar] [CrossRef] [PubMed]
- Lebed, P.J.; de Souza, K.; Bilodeau, F.; Larivière, D.; Kleitz, F. Phosphonate-functionalized large pore 3-D cubic mesoporous (KIT-6) hybrid as highly efficient actinide extracting agent. Chem. Commun. 2011, 47, 11525–11527. [Google Scholar] [CrossRef] [PubMed]
- Vivero-Escoto, J.L.; Carboni, M.; Abney, C.W.; deKrafft, K.E.; Lin, W. Organo-functionalized mesoporous silicas for efficient uranium extraction. Microporous Mesoporous Mater. 2013, 180, 22–31. [Google Scholar] [CrossRef]
- Yuan, L.-Y.; Liu, Y.-L.; Shi, W.-Q.; Lv, Y.-L.; Lan, J.-H.; Zhao, Y.-L.; Chai, Z.-F. High performance of phosphonate-functionalized mesoporous silica for U(vi) sorption from aqueous solution. Dalton Trans. 2011, 40, 7446–7453. [Google Scholar] [CrossRef]
- Irving, H.; Edgington, D.N. Synergic effects in the solvent extraction of the actinides—IV: Trivalent plutonium, americium and europium. J. Inorg. Nucl. Chem. 1961, 21, 169–180. [Google Scholar] [CrossRef]
- Shastri, L.V.; Mittal, L.J.; Mittal, J.P. Radiation chemical separation of europium from aqueous lanthanide mixtures. Int. J. Radiat. Appl. Instrum. Part. C. Radiat. Phys. Chem. 1986, 28, 359–361. [Google Scholar] [CrossRef]
- Munshi, K.N.; Dey, A.K. Absorptiometric study of the chelates formed between the lanthanoids and xylenol orange. Microchim. Acta 1968, 56, 1059–1065. [Google Scholar] [CrossRef]
- Safronikhin, A.; Ehrlich, H.; Shcherba, T.; Kuzmina, N.; Lisichkin, G. Formation of complexes on the surface of nanosized europium fluoride. Colloids Surf. A Physicochem. Eng. Asp. 2011, 377, 367–373. [Google Scholar] [CrossRef]
- Gupta, B.L. Absorption characteristics of xylenol orange. Talanta 1974, 21, 683–684. [Google Scholar] [CrossRef]
- Nakagawa, K.; Ishida, Y. Estimation of amorphous specific volume of poly(vinylidene fluoride) as a function of temperature. Kolloid-Z. Z. Polym. 1973, 251, 103–107. [Google Scholar] [CrossRef]
- Nakagawa, K.; Ishida, Y. Annealing effects in poly(vinylidene fluoride) as revealed by specific volume measurements, differential scanning calorimetry, and electron microscopy. J. Polym. Sci. Part B Polym. Phys. 1973, 11, 2153–2171. [Google Scholar] [CrossRef]
- Pianca, M.; Barchiesi, E.; Esposto, G.; Radice, S. End groups in fluoropolymers. J. Fluor. Chem. 1999, 95, 71–84. [Google Scholar] [CrossRef]
- McKenna, C.E.; Schmidhuser, J. Functional selectivity in phosphonate ester dealkylation with bromotrimethylsilane. J. Chem. Soc. Chem. Commun. 1979, 17, 739. [Google Scholar] [CrossRef]
- Das, M.; Shu, C.-M. A green approach towards adoption of chemical reaction model on 2,5-dimethyl-2,5-di-(tert-butylperoxy)hexane decomposition by differential isoconversional kinetic analysis. J. Hazard. Mater. 2016, 301, 222–232. [Google Scholar] [CrossRef]
- Asandei, A.D. Photomediated controlled radical polymerization and block copolymerization of vinylidene fluoride. Chem. Rev. 2016, 116, 2244–2274. [Google Scholar] [CrossRef] [PubMed]
- Asandei, A.D.; Adebolu, O.I.; Simpson, C.P. Mild-temperature Mn2(CO)10-photomediated controlled radical polymerization of vinylidene fluoride and synthesis of well-defined poly(vinylidene fluoride) block copolymers. J. Am. Chem. Soc. 2012, 134, 6080–6083. [Google Scholar] [CrossRef]
- Goldbach, J.T.; Amin-Sanayei, R.; He, W.; Henry, J.; Kosar, W.; Lefebvre, A.; O’brien, G.; Vaessen, D.; Wood, K.; Zerafati, S. Commercial synthesis and applications of poly(vinylidene fluoride). In Fluorinated Polymer Volume 2: Applications; Ameduri, B., Sawada, H., Eds.; Royal Society of Chemistry: Cambridge, UK, 2016. [Google Scholar]
- Zhu, L.; Wang, Q. Novel ferroelectric polymers for high energy density and low loss dielectrics. Macromolecules 2012, 45, 2937–2954. [Google Scholar] [CrossRef]
- Cai, T.; Neoh, K.-G.; Kang, E.T. Functionalized and functionalizable fluoropolymer membranes. In Handbook Fluoropolymers Science & Technology; Smith, D.W., Iacono, S.T., Iyers, S.S., Eds.; Wiley: New York, NY, USA, 2014; pp. 149–181. [Google Scholar]
- Ameduri, B.; Bauduin, G.; Boutevin, B.; Kostov, G.; Petrova, P. Synthesis and polymerization of fluorinated monomers bearing a reactive lateral group. 9. bulk copolymerization of vinylidene fluoride with 4,5,5-trifluoro-4-ene pentyl acetate. Macromolecules 1999, 32, 4544–4550. [Google Scholar] [CrossRef]
- Boschet, F.; Cracowski, J.-M.; Montembault, V.; Ameduri, B. Radical copolymerization of α,β-difluoroacrylic acid with vinylidene fluoride. Macromolecules 2010, 43, 4879–4888. [Google Scholar] [CrossRef]
- Guiot, J.; Ameduri, B.; Boutevin, B.; Lannuzel, T. Original crosslinking of poly(vinylidene fluoride) via trialkoxysilane-containing cure-site monomers. J. Polym. Sci. Part A Polym. Chem. 2006, 44, 3896–3910. [Google Scholar] [CrossRef]
- Ameduri, B.; Boutevin, B.; Kostov, G.K.; Petrova, P. Synthesis and polymerization of fluorinated monomers bearing a reactive lateral group. Part 10. Copolymerization of vinylidene fluoride (VDF) with 5-thioacetoxy-1,1,2-trifluoropentene for the obtaining of a novel PVDF containing Mercaptan side-groups. Des. Monomers Polym. 1999, 2, 267–285. [Google Scholar] [CrossRef]
- Twum, E.B.; Gao, C.; Li, X.; McCord, E.F.; Fox, P.A.; Lyons, D.F.; Rinaldi, P.L. Characterization of the chain-ends and branching structures in polyvinylidene fluoride with multidimensional NMR. Macromolecules 2012, 45, 5501–5512. [Google Scholar] [CrossRef]
- Duc, M.; Ameduri, B.; Boutevin, B.; Kharroubi, M.; Sage, J.-M. Telomerization of vinylidene fluoride with methanol. Elucidation of the reaction process and mechanism by a structural analysis of the telomers. Macromol. Chem. Phys. 1998, 199, 1271–1289. [Google Scholar] [CrossRef]
- Pope, E.J.A.; Mackenzie, J.D. Sol-gel processing of silica: II. The role of the catalyst. J. Non-Cryst. Solids 1986, 87, 185–198. [Google Scholar] [CrossRef]
- Clark, J.H. Fluoride ion as a base in organic synthesis. Chem. Rev. 1980, 80, 429–452. [Google Scholar] [CrossRef]
- Dias, A.J.; McCarthy, T.J. Dehydrofluorination of poly(vinylidene fluoride) in dimethylformamide solution: Synthesis of an operationally soluble semiconducting polymer. J. Polym. Sci. Polym. Chem. Ed. 1985, 23, 1057–1061. [Google Scholar] [CrossRef]
- Cihlář, J. Hydrolysis and polycondensation of ethyl silicates. 1. Effect of pH and catalyst on the hydrolysis and polycondensation of tetraethoxysilane (TEOS). Colloids Surf. A Physicochem. Eng. Asp. 1993, 70, 239–251. [Google Scholar] [CrossRef]
- Brinker, C.J.; Scherer, S.W. Sol-Gel Science: The Physics and Chemistry of Sol-Gel Processing; Academic Press: New York, NY, USA, 1990. [Google Scholar]
- Zulfiqar, S.; Rizvi, M.; Munir, A. Thermal degradation of chlorotrifluoroethylene-styrene copolymers. Polym. Degrad. Stab. 1994, 44, 21–25. [Google Scholar] [CrossRef]
- Zulfiqar, S.; Zulfiqar, M.; Rizvi, M.; Munir, A.; McNeill, I.C. Study of the thermal degradation of polychlorotrifluoroethylene, poly(vinylidene fluoride) and copolymers of chlorotrifluoroethylene and vinylidene fluoride. Polym. Degrad. Stab. 1994, 43, 423–430. [Google Scholar] [CrossRef]
- Zulfiqar, S.; Rizvi, M.; Munir, A.; Ghaffar, A.; McNeill, I.C. Thermal degradation studies of copolymers of chlorotrifluoroethylene and methyl methacrylate. Polym. Degrad. Stab. 1996, 52, 341–348. [Google Scholar] [CrossRef]
- Luca, V.; Tejada, J.J.; Vega, D.; Arrachart, G.; Rey, C. Zirconium(IV)–benzene phosphonate coordination polymers: Lanthanide and actinide extraction and thermal properties. Inorg. Chem. 2016, 55, 7928–7943. [Google Scholar] [CrossRef]
- Powell, K.R.; McCleskey, T.M.; Tumas, W.; DeSimone, J.M. Polymers with multiple ligand sites for metal extractions in dense-phase carbon dioxide. Ind. Eng. Chem. Res. 2001, 40, 1301–1305. [Google Scholar] [CrossRef]
- Jenkins, A.L.; Uy, O.M.; Murray, G.M. Polymer-based lanthanide luminescent sensor for detection of the hydrolysis product of the nerve agent soman in water. Anal. Chem. 1999, 71, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Cabrera-Martín, A.; Peral-Fernández, J.L.; Vicente-Péez, S.; Burriel-Martí, F. Reaction of mercury(II) and xylenol orange—I: Effect of the buffering media. Talanta 1969, 16, 1023–1036. [Google Scholar] [CrossRef]
- Atanassova, M.; Kurteva, V.; Billard, I. Coordination chemistry of Europium(III) ion towards acylpyrazolone ligands. Anal. Sci. 2015, 31, 917–922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perić, J.; Trgo, M.; Vukojević Medvidović, N. Removal of zinc, copper and lead by natural zeolite—A comparison of adsorption isotherms. Water Res. 2004, 38, 1893–1899. [Google Scholar] [CrossRef]
- Moussa, M.; Ndiaye, M.M.; Pinta, T.; Pichon, V.; Vercouter, T.; Delaunay, N. Selective solid phase extraction of lanthanides from tap and river waters with ion imprinted polymers. Anal. Chim. Acta 2017, 963, 44–52. [Google Scholar] [CrossRef]
- Shirvani-Arani, S.; Ahmadi, S.J.; Bahrami-Samani, A.; Ghannadi-Maragheh, M. Synthesis of nano-pore samarium (III)-imprinted polymer for preconcentrative separation of samarium ions from other lanthanide ions via solid phase extraction. Anal. Chim. Acta 2008, 623, 82–88. [Google Scholar] [CrossRef]



| Feed (%) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Entry | VDF | VTEOS | VDMP | Initiator | Solvent | T(°C) | Yield (%) | ΔP (bar) |
| P1 | 0 | 50 | 50 | A | DMC | 57 | 0 | 0 |
| P2 | 0 | 50 | 50 | B | DMC | 75 | 0 | 0 |
| P3 | 0 | 50 | 50 | C | DMC | 115 | 0 | 0 |
| P4 | 90 | 10 | 0 | A | DMC | 57 | 0 | 0 |
| P5 | 90 | 10 | 0 | B | DMC | 75 | 0 | 0 |
| P6 | 90 | 10 | 0 | A | PFB | 57 | 0 | 0 |
| P7 | 90 | 10 | 0 | C | PFB | 135 | 62 | 25 |
| P8 | 80 | 20 | 0 | C | PFB | 135 | 56 | 22 |
| P9 | 50 | 50 | 0 | C | PFB | 135 | 49 | 23 |
| P10 | 90 | 10 | 0 | C | DMC | 115 | 75 | 26 |
| P11 | 90 | 0 | 10 | A | DMC | 57 | 0 | 0 |
| P12 | 90 | 0 | 10 | B | DMC | 75 | 0 | 0 |
| P13 | 90 | 0 | 10 | C | DMC | 115 | 80 | 24 |
| P14 | 70 | 0 | 30 | C | DMC | 115 | 81 | 28 |
| P15 | 50 | 0 | 50 | C | DMC | 115 | 79 | 27 |
| P16 | 80 | 10 | 10 | A | DMC | 115 | 0 | 0 |
| P17 | 80 | 10 | 10 | B | DMC | 115 | 0 | 0 |
| P18 | 85 | 5 | 10 | C | DMC | 115 | 78 | 27 |
| P19 | 80 | 10 | 10 | C | DMC | 115 | 77 | 28 |
| P20 | 70 | 20 | 10 | C | DMC | 115 | 80 | 25 |
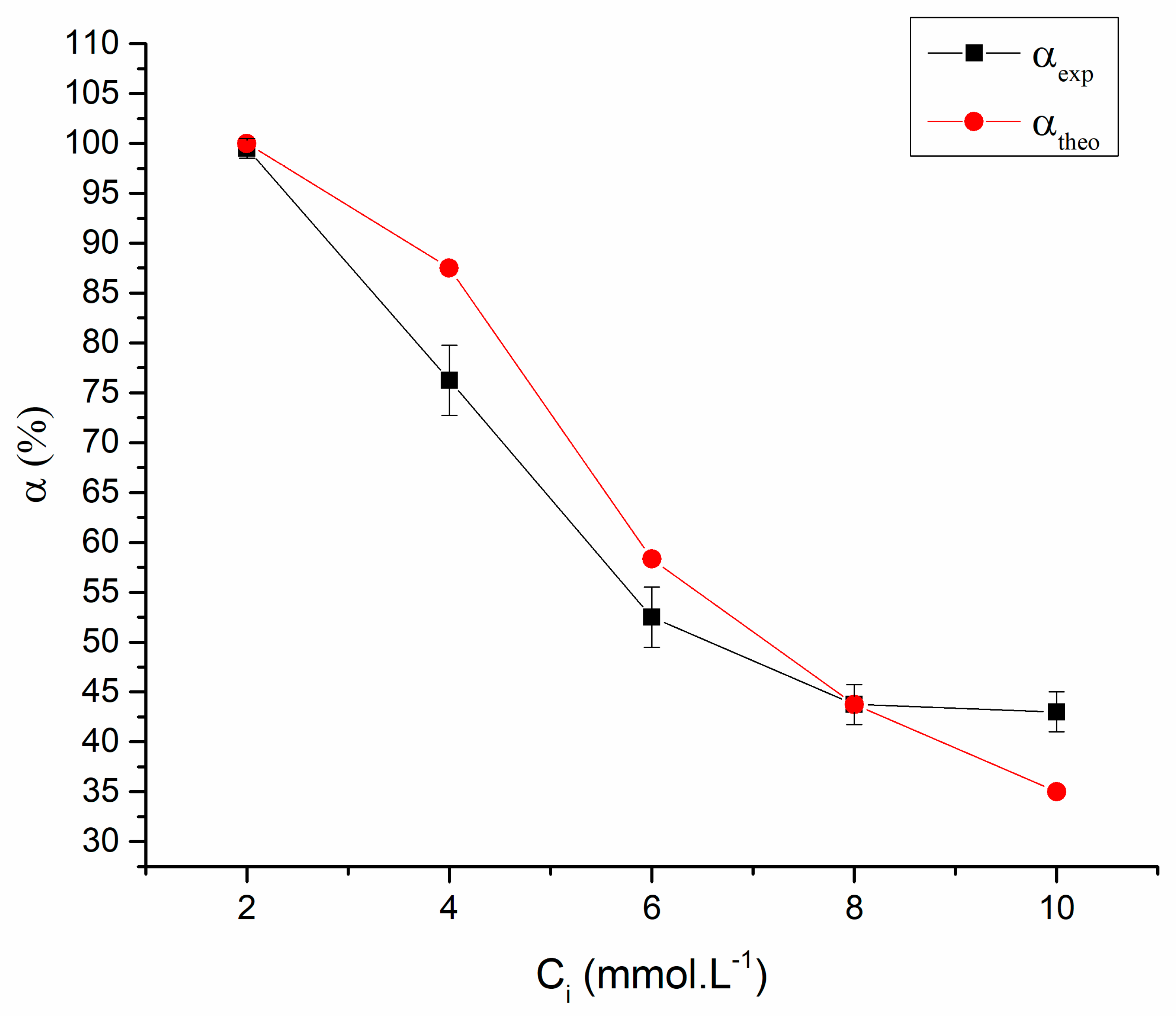
| Solution Concentration (mmol·L−1) | αtheo% | α% |
|---|---|---|
| 2 | 100.0 | 99.5 |
| 4 | 87.5 | 76.2 |
| 6 | 58.3 | 52.2 |
| 8 | 43.7 | 43.7 |
| 10 | 35.0 | 43.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wehbi, M.; Mehdi, A.; Alaaeddine, A.; Jaber, N.; Ameduri, B. Solid–Liquid Europium Ion Extraction via Phosphonic Acid-Functionalized Polyvinylidene Fluoride Siloxanes. Polymers 2020, 12, 1955. https://doi.org/10.3390/polym12091955
Wehbi M, Mehdi A, Alaaeddine A, Jaber N, Ameduri B. Solid–Liquid Europium Ion Extraction via Phosphonic Acid-Functionalized Polyvinylidene Fluoride Siloxanes. Polymers. 2020; 12(9):1955. https://doi.org/10.3390/polym12091955
Chicago/Turabian StyleWehbi, Mohammad, Ahmad Mehdi, Ali Alaaeddine, Nada Jaber, and Bruno Ameduri. 2020. "Solid–Liquid Europium Ion Extraction via Phosphonic Acid-Functionalized Polyvinylidene Fluoride Siloxanes" Polymers 12, no. 9: 1955. https://doi.org/10.3390/polym12091955