Investigating the Mechanisms of AquaporinZ Reconstitution through Polymeric Vesicle Composition for a Biomimetic Membrane
Abstract
1. Introduction
2. Methods
2.1. Chemicals and Materials
2.2. Copolymer Characterization
2.3. Vesicle Fabrication and AqpZ Reconstitution
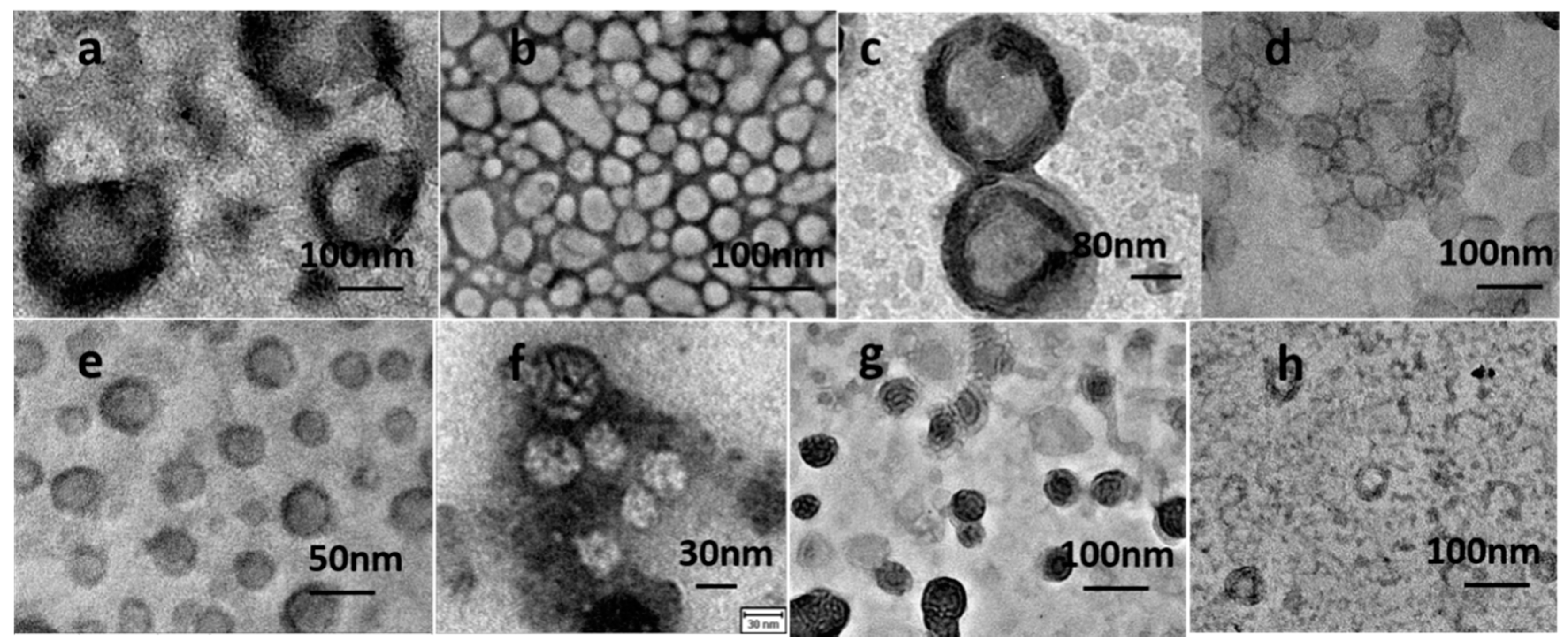
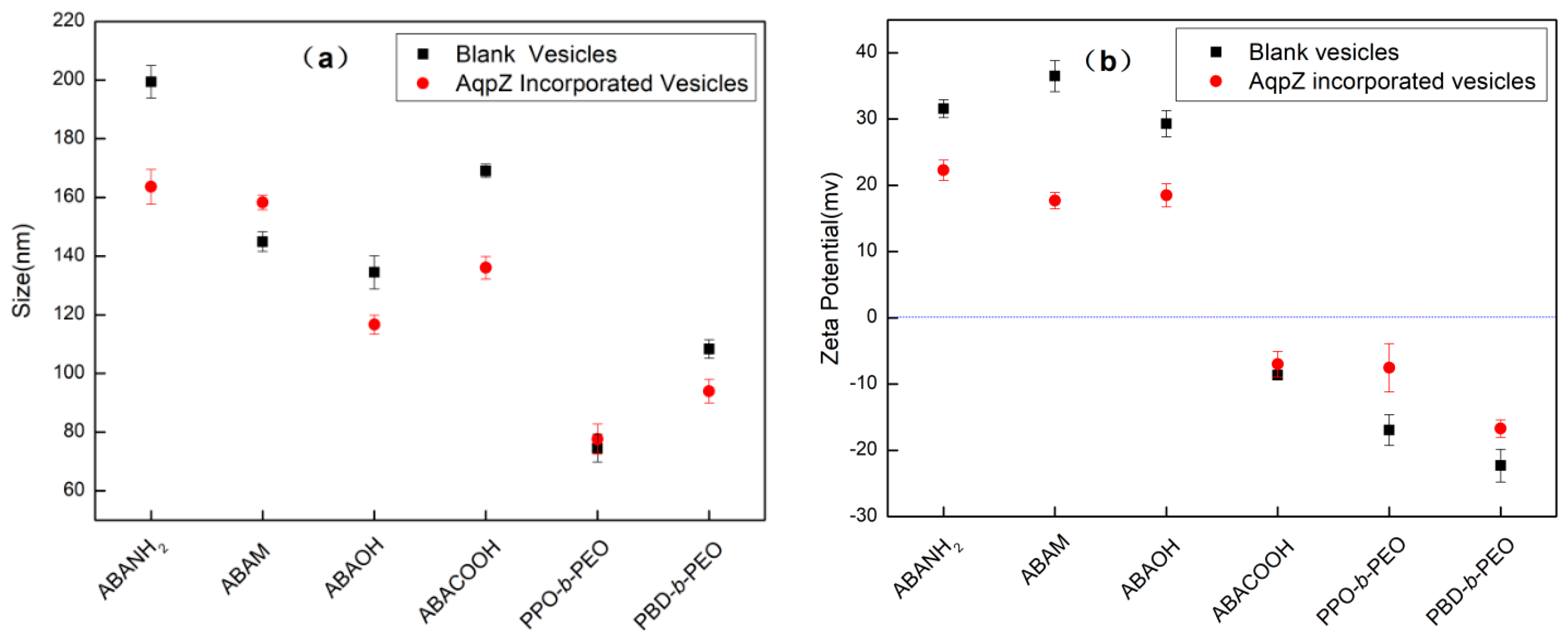
2.4. Vesicle Characterization by DLS and FETEM
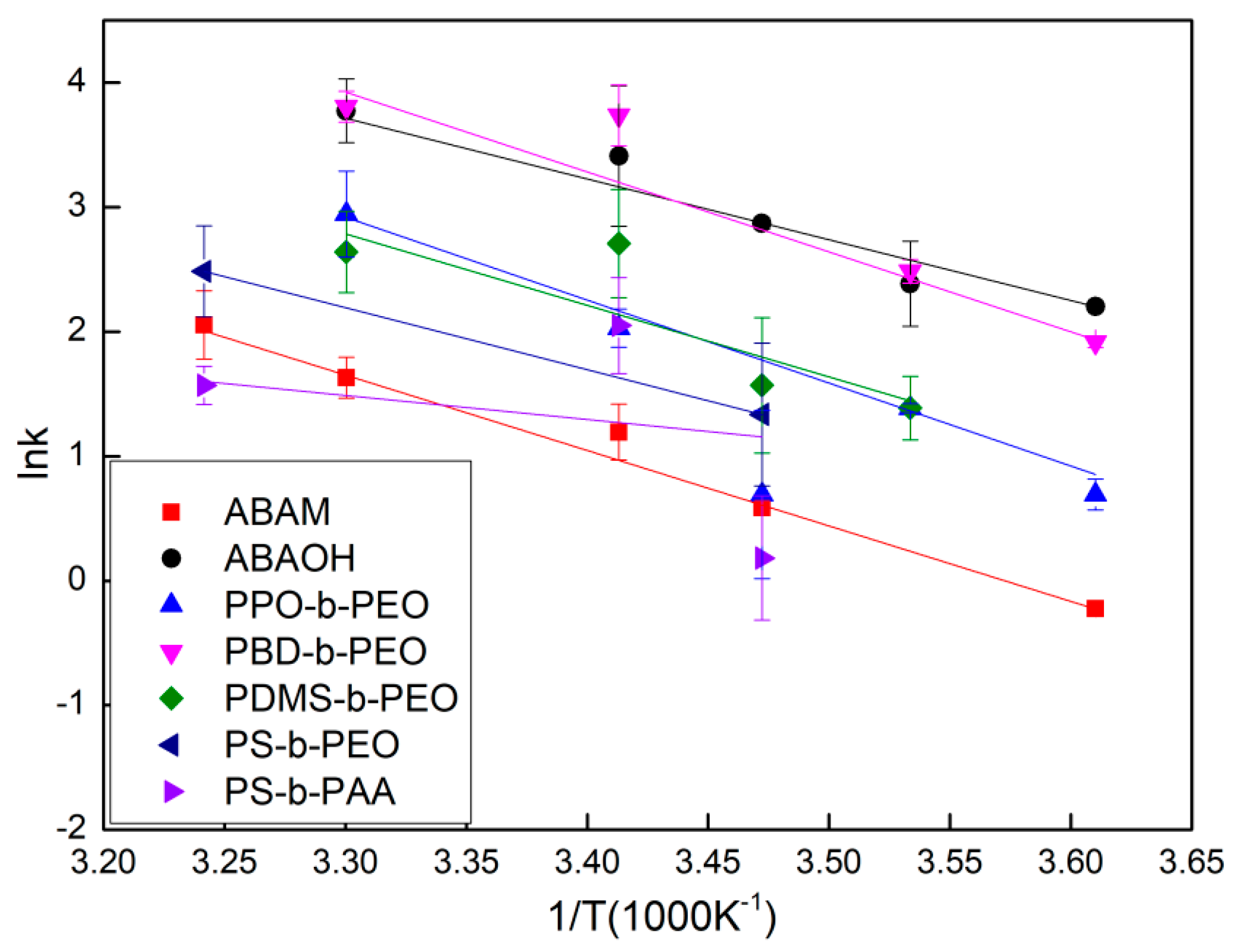
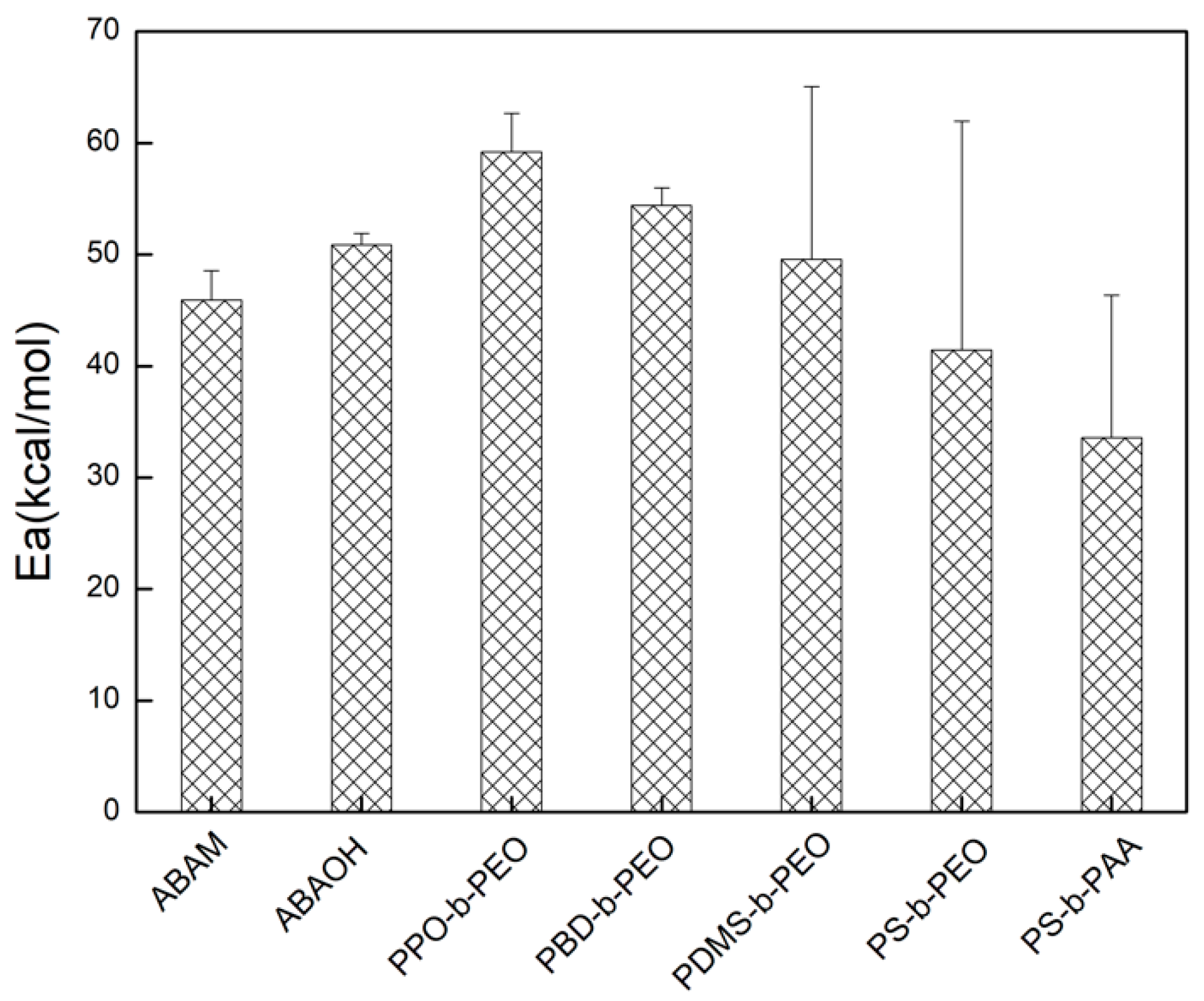
2.5. Determination of the Arrhenius Activation Energy Using Stopped-Flow Spectrometry
2.6. Membrane Rigidity and Thermal Expansivity
3. Results and Discussion
3.1. Copolymer and Vesicle Characterization
3.2. Arrhenius Activation Energy
3.3. Membrane Rigidity and Thermal Expansivity
3.4. Thermal Behavior of Detergent and Effect of the Chemical Composition by DSC
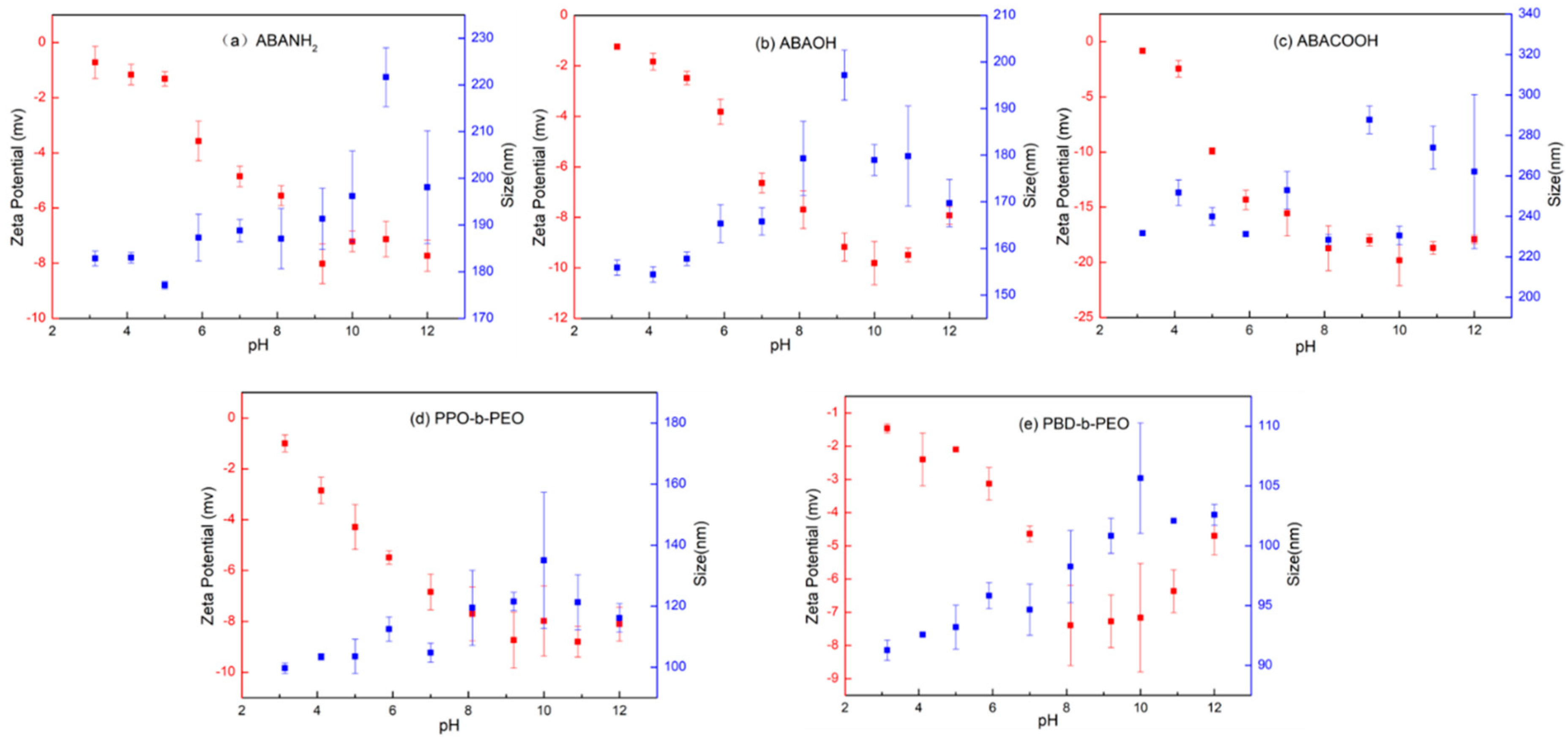
3.5. Effect of pH on Vesicular Size and Stability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Pendergast, M.M.; Hoek, E.M.V. A review of water treatment membrane nanotechnologies. Energy Environ. Sci. 2011, 4, 1946. [Google Scholar] [CrossRef]
- Greenlee, L.F.; Lawler, D.F.; Freeman, B.D.; Marrot, B.; Moulin, P. Reverse osmosis desalination: Water sources, technology, and today’s challenges. Water Res. 2009, 43, 2317–2348. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, Y.J.; Nam, K.T. Protein/peptide based nanomaterials for energy application. Curr. Opin. Biotechnol. 2013, 24, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Sengur-Tasdemir, R.; Aydin, S.; Turken, T.; Genceli, E.A.; Koyuncu, I. Biomimetic Approaches for Membrane Technologies. Sep. Purif. Rev. 2015, 45, 122–140. [Google Scholar] [CrossRef]
- Shen, Y.-X.; Saboe, P.O.; Sines, I.T.; Erbakan, M.; Kumar, M. Biomimetic membranes: A review. J. Membr. Sci. 2014, 454, 359–381. [Google Scholar] [CrossRef]
- Binesh, A.R.; Kamali, R. Molecular dynamics insights into human aquaporin 2 water channel. Biophys. Chem. 2015, 207, 107–113. [Google Scholar] [CrossRef]
- Chen, L.Y. Erythritol predicted to inhibit permeation of water and solutes through the conducting pore of P. falciparum aquaporin. Biophys. Chem. 2015, 198, 14–21. [Google Scholar] [CrossRef]
- Borgnia, M.J.; Kozono, D.; Calamita, G.; Agre, P. Functional Reconstitution and Characterization of AqpZ, the E. coli Water Channel Protein. J. Mol. Biol. 1999, 291, 1169–1179. [Google Scholar] [CrossRef]
- Calamita, G. The Escherichia coli aquaporin-Z water channel. Mol. Microbiol. 2000, 37, 254–262. [Google Scholar] [CrossRef]
- Zhang, Y.; Cui, Y.; Chen, L.Y. Mercury inhibits the L170C mutant of aquaporin Z by making waters clog the water channel. Biophys. Chem. 2012, 160, 69–74. [Google Scholar] [CrossRef]
- Kumar, M.; Grzelakowski, M.; Zilles, J.; Clark, M.; Meier, W. Highly permeable polymeric membranes based on the incorporation of the functional water channel protein Aquaporin Z. Proc. Natl. Acad. Sci. USA 2007, 104, 20719–20724. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Habel, J.E.; Shen, Y.X.; Meier, W.P.; Walz, T. High-density reconstitution of functional water channels into vesicular and planar block copolymer membranes. J. Am. Chem. Soc. 2012, 134, 18631–18637. [Google Scholar] [CrossRef] [PubMed]
- Habel, J.; Hansen, M.; Kynde, S.; Larsen, N.; Midtgaard, S.R.; Jensen, G.V.; Bomholt, J.; Ogbonna, A.; Almdal, K.; Schulz, A.; et al. Aquaporin-Based Biomimetic Polymeric Membranes: Approaches and Challenges. Membranes 2015, 5, 307–351. [Google Scholar] [CrossRef] [PubMed]
- Giwa, A.; Hasan, S.W.; Yousuf, A.; Chakraborty, S.; Johnson, D.J.; Hilal, N. Biomimetic membranes: A critical review of recent progress. Desalination 2017, 420, 403–424. [Google Scholar] [CrossRef]
- Sanders, C.R.; Prosser, R.S. Bicelles: A model membrane system for all seasons? Structure 1998, 6, 1227–1234. [Google Scholar] [CrossRef]
- Dvinskikh, S.; Dürr, U.; Yamamoto, K.; Ramamoorthy, A. A High-Resolution Solid-State NMR Approach for the Structural Studies of Bicelles. J. Am. Chem. Soc. 2006, 128, 6326–6327. [Google Scholar] [CrossRef]
- Ravula, T.; Hardin, N.Z.; Ramamoorthy, A. Polymer nanodiscs: Advantages and limitations. Chem. Phys. Lipids 2019, 219, 45–49. [Google Scholar] [CrossRef]
- Nasr, M.L.; Baptista, D.; Strauss, M.; Sun, Z.J.; Grigoriu, S.; Huser, S.; Pluckthun, A.; Hagn, F.; Walz, T.; Hogle, J.M.; et al. Covalently circularized nanodiscs for studying membrane proteins and viral entry. Nat. Methods 2017, 14, 49–52. [Google Scholar] [CrossRef]
- Veena Pata, N.D. The Effect of Chain Length on Protein Solubilization in Polymer-Based Vesicles (Polymersomes). Biophys. J. 2003, 85, 2111–2118. [Google Scholar] [CrossRef]
- Shen, W.; Hu, J.; Hu, X. Impact of amphiphilic triblock copolymers on stability and permeability of phospholipid/polymer hybrid vesicles. Chem. Phys. Lett. 2014, 600, 56–61. [Google Scholar] [CrossRef]
- Discher, B.M.; Won, Y.Y.; Ege, D.S.; Lee, J.C.; Bates, F.S.; Discher, D.E.; Hammer, D.A. Polymersomes: Tough Vesicles Made from Diblock Copolymers. Science 1999, 284, 1143–1146. [Google Scholar] [CrossRef] [PubMed]
- Blanazs, A.; Armes, S.P.; Ryan, A.J. Self-Assembled Block Copolymer Aggregates: From Micelles to Vesicles and their Biological Applications. Macromol. Rapid Commun. 2009, 30, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Tanner, P.; Graff, A.; Palivan, C.G.; Meier, W. Mimicking the cell membrane with block copolymer membranes. J. Polym. Sci. Part A Polym. Chem. 2012, 50, 2293–2318. [Google Scholar] [CrossRef]
- Taubert, A.; Napoli, A.; Meier, W. Self-assembly of reactive amphiphilic block copolymers as mimetics for biological membranes. Curr. Opin. Chem. Biol. 2004, 8, 598–603. [Google Scholar] [CrossRef]
- Kita-Tokarczyk, K.; Grumelard, J.; Haefele, T.; Meier, W. Block copolymer vesicles—Using concepts from polymer chemistry to mimic biomembranes. Polymer 2005, 46, 3540–3563. [Google Scholar] [CrossRef]
- Krishnarjuna, B.; Ravula, T.; Ramamoorthy, A. Detergent-free extraction, reconstitution and characterization of membrane-anchored cytochrome-b5 in native lipids. Chem. Commun. (Camb.) 2020, 56, 6511–6514. [Google Scholar] [CrossRef]
- Ravula, T.; Ramadugu, S.K.; Di Mauro, G.; Ramamoorthy, A. Bioinspired, Size-Tunable Self-Assembly of Polymer–Lipid Bilayer Nanodiscs. Angew. Chem. Int. Ed. 2017, 56, 11466–11470. [Google Scholar] [CrossRef] [PubMed]
- Ravula, T.; Hardin, N.Z.; Ramadugu, S.K.; Cox, S.J.; Ramamoorthy, A. Formation of pH-Resistant Monodispersed Polymer–Lipid Nanodiscs. Angew. Chem. Int. Ed. 2018, 57, 1342–1345. [Google Scholar] [CrossRef]
- Fuwad, A.; Ryu, H.; Malmstadt, N.; Kim, S.M.; Jeon, T.-J. Biomimetic membranes as potential tools for water purification: Preceding and future avenues. Desalination 2019, 458, 97–115. [Google Scholar] [CrossRef]
- Zhao, Y.-F.; Fan, X.; Chen, X.; Wan, X.; Zhou, Q.-F. Synthesis and characterization of diblock copolymers based on crystallizable poly(ε-caprolactone) and mesogen-jacketed liquid crystalline polymer block. Polymer 2005, 46, 5396–5405. [Google Scholar] [CrossRef]
- Buzin, A.I.; Pyda, M.; Costanzo, P.; Matyjaszewski, K.; Wunderlich, B. Wunderlich. Calorimetric study of block-copolymers of poly(n-butyl acrylate) and gradient poly(n-butyl acrylate-co-methyl methacrylate). Polymer 2002, 43, 5563–5569. [Google Scholar] [CrossRef]
- Wang, H.; Chung, T.S.; Tong, Y.W.; Jeyaseelan, K.; Armugam, A.; Chen, Z.; Hong, M.; Meier, W. Highly permeable and selective pore-spanning biomimetic membrane embedded with aquaporin Z. Small 2012, 8, 1185–1190. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; He, F.; Wang, B.; Chung, T.-S.; Jeyaseelan, K.; Armugam, A.; Tong, Y.W. An aquaporin-based vesicle-embedded polymeric membrane for low energy water filtration. J. Mater. Chem. A 2013, 1, 7592–7600. [Google Scholar] [CrossRef]
- Gan, H.X.; Zhou, H.; Lin, Q.; Tong, Y.W. Quantification of Aquaporin-Z reconstituted into vesicles for biomimetic membrane fabrication. Sci. Rep. 2017, 7, 11565–11578. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Tong, Y.W. A mechanistic study on amphiphilic block co-polymer poly(butadiene-b-(ethylene oxide)) vesicles reveals the water permeation mechanism through a polymeric bilayer. RSC Adv. 2014, 4, 15304–15313. [Google Scholar] [CrossRef]
- Harris, F.M.; Best, K.B.; Bell, J.D. Use of laurdan fluorescence intensity and polarization to distinguish between changes in membrane fluidity and phospholipid order. Biochim. Biophys. Acta 2002, 1565, 123–128. [Google Scholar] [CrossRef]
- Sánchez, S.A.; Tricerri, M.A.; Gunther, G.; Gratton, E. Gunther and E.Gratton. Laurdan Generalized Polarization: From cuvette to microscope. Mod. Res. Edu. Topics Micro. 2007, 2, 1007–1014. [Google Scholar]
- Parasassi, T.; Krasnowska, E.K.; Bagatolli, L.; Gratton, E. Laurdan and Prodan as Polarity-Sensitive Fluorescent Membrane Probes. J. Fluoresc. 1998, 8, 365–373. [Google Scholar] [CrossRef]
- Choi, H.-J.; Montemagno, C. Recent Progress in Advanced Nanobiological Materials for Energy and Environmental Applications. Materials 2013, 6, 5821–5856. [Google Scholar] [CrossRef]
- Ayen, W.Y.; Chintankumar, B.; Jain, J.P.; Kumar, N. Effect of PEG chain length and hydrophilic weight fraction on polymersomes prepared from branched (PEG)3-PLA co-polymers. Polym. Adv. Technol. 2011, 22, 158–165. [Google Scholar] [CrossRef]
- Ravula, T.; Hardin, N.Z.; Ramadugu, S.K.; Ramamoorthy, A. pH Tunable and Divalent Metal Ion Tolerant Polymer Lipid Nanodiscs. Langmuir 2017, 33, 10655–10662. [Google Scholar] [CrossRef] [PubMed]

| Polymers | Molecular Weight Distribution | Vesicle Formation | Hydrophilic Mass Ratio | Price Per Gram (USD) * | Block Tg (°C) |
|---|---|---|---|---|---|
| PMOXA1.3K-b-PDMS5K-b-PMOXA1.3K(ABAM) | 1.30 | √ | 34.21% | 450.00 | -/−127/- |
| PPO5.5K-b-PEO2.7K | 1.07 | √ | 32.93% | 200.00 | −10/−59 |
| PBD5K-b-PEO2.3K | 1.03 | √ | 31.51% | 400.00 | −50/−59 |
| PDMS5K-b-PEO2.1K | 1.20 | √ | 29.58% | 450.00 | −127/−59 |
| PDMS8K-b-PAA5K | 1.20 | × | 38.46% | 250.00 | −127/68 |
| PS5K-b-PEO2K | 1.03 | × | 28.57% | 300.00 | 89/−59 |
| PS5.2K-b-PAA4K | 1.15 | × | 43.48% | 250.00 | 89/68 |
| Samples | Tg | Tm | Tc |
|---|---|---|---|
| ABAM | −89.56 | −45.67 | - |
| ABAOH | −85.79 | −58.83 | - |
| PPO-b-PEO | - | 40.17 | 19.67 |
| PBD-b-PEO | −75.14 | 46.17 | 8.83 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, D.; Zhou, H.; Zhou, S.; Tong, Y.W. Investigating the Mechanisms of AquaporinZ Reconstitution through Polymeric Vesicle Composition for a Biomimetic Membrane. Polymers 2020, 12, 1944. https://doi.org/10.3390/polym12091944
Zhou D, Zhou H, Zhou S, Tong YW. Investigating the Mechanisms of AquaporinZ Reconstitution through Polymeric Vesicle Composition for a Biomimetic Membrane. Polymers. 2020; 12(9):1944. https://doi.org/10.3390/polym12091944
Chicago/Turabian StyleZhou, Danhua, Hu Zhou, Shufeng Zhou, and Yen Wah Tong. 2020. "Investigating the Mechanisms of AquaporinZ Reconstitution through Polymeric Vesicle Composition for a Biomimetic Membrane" Polymers 12, no. 9: 1944. https://doi.org/10.3390/polym12091944
APA StyleZhou, D., Zhou, H., Zhou, S., & Tong, Y. W. (2020). Investigating the Mechanisms of AquaporinZ Reconstitution through Polymeric Vesicle Composition for a Biomimetic Membrane. Polymers, 12(9), 1944. https://doi.org/10.3390/polym12091944






