Ciprofloxacin-Collagen-Based Materials with Potential Oral Surgical Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
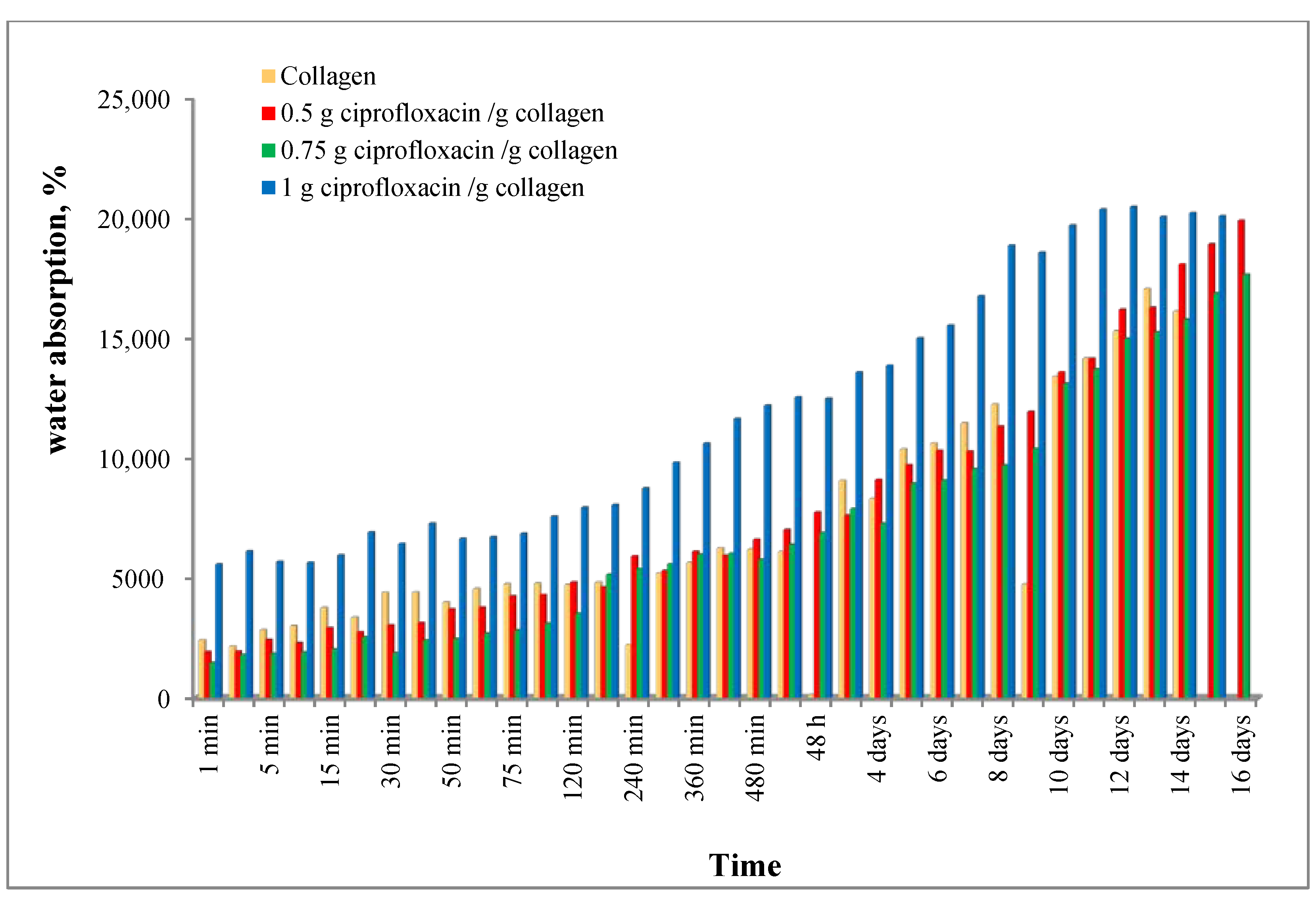
2.2. Water Absorption
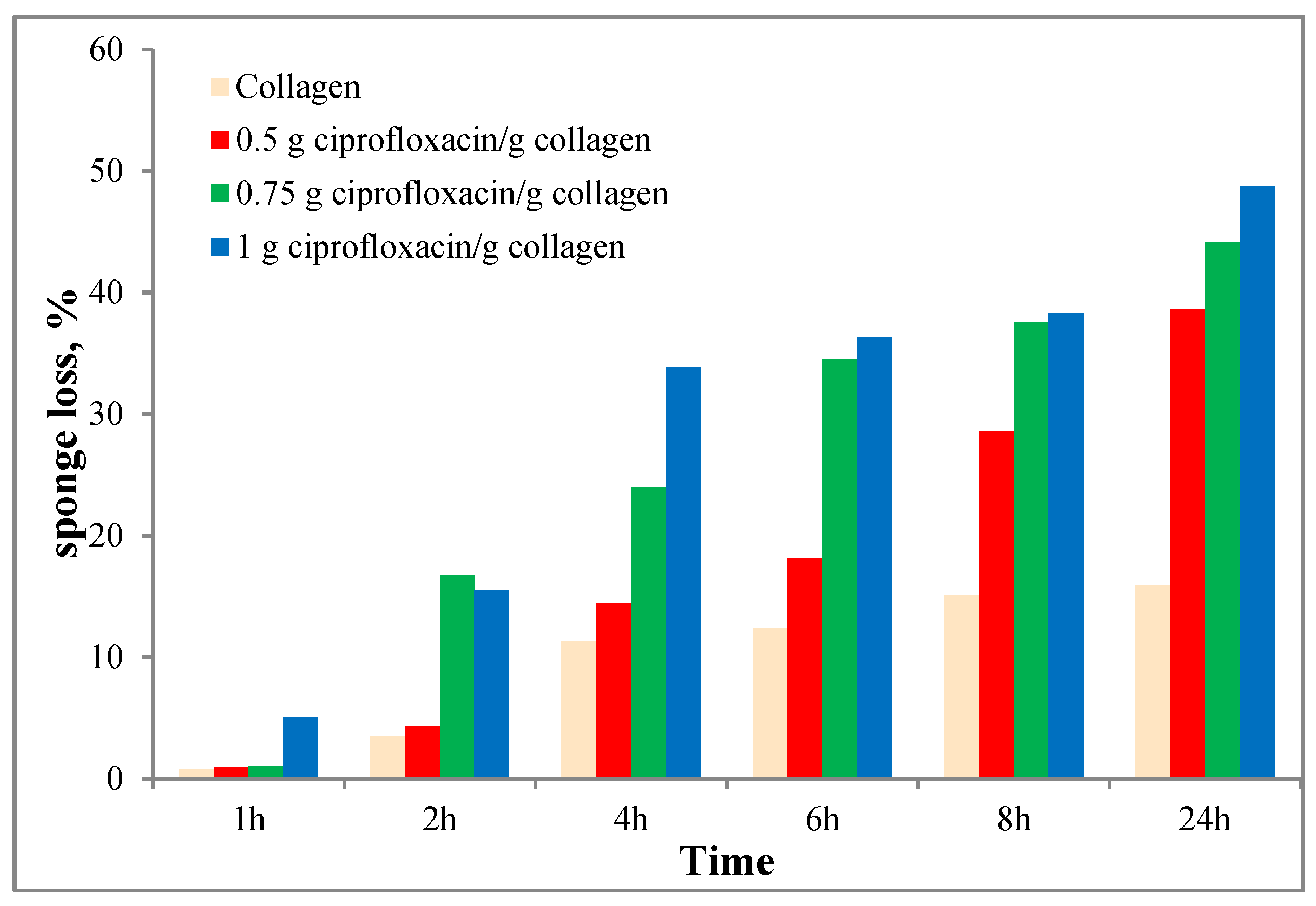
2.3. Enzymatic Degradation
2.4. In Vitro Ciprofloxacin Release
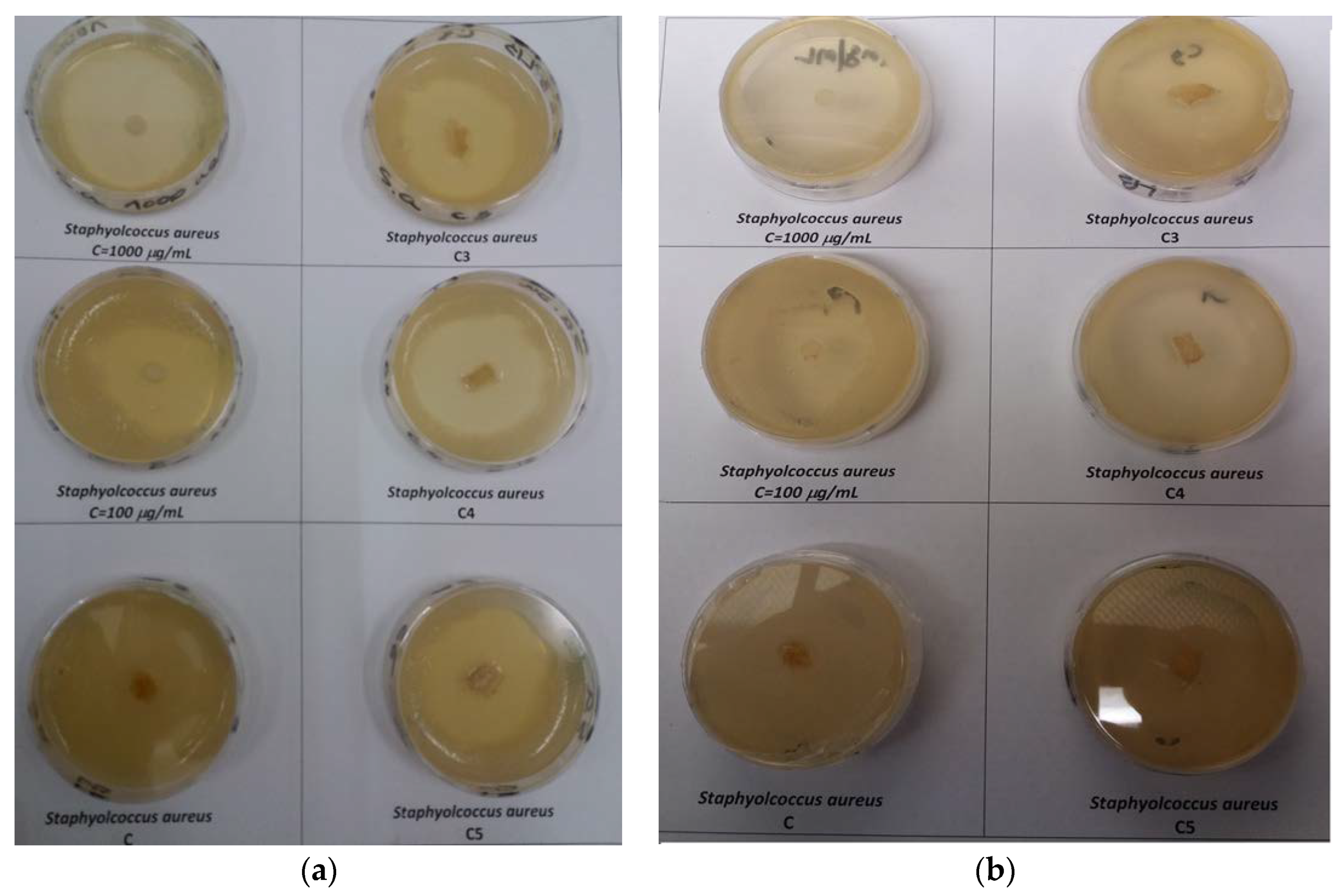
2.5. In Vitro Biological Effects of Ciprofloxacin-Collagen Sponges on Some Pathogen Microorganism
2.6. Cytotoxic Study of Collagen-Based Materials on Normal Cells—In Vitro Tests
2.7. Statistical Analysis
3. Results and Discussion
3.1. Water Absorption
3.2. Enzymatic Degradation
3.3. In Vitro Ciprofloxacin Release
3.4. In Vitro Biological Effects of Ciprofloxacin–Collagen Sponges on Some Pathogen Microorganism
3.5. Cytotoxic Study of Collagen-Based Materials on Normal Cells—In Vitro Tests
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kang, S.Y.; Teknos, T.N.; Old, M.O. Reconstruction of Buccal Defects. In Head and Neck Cancer–Management and Reconstruction, 2nd ed.; Genden, E.M., Ed.; Thieme Publishers: New York, NY, USA, 2020; pp. 43–51. [Google Scholar]
- Booth, P.W. Reconstructive surgery—Harvesting, skin mucosa and cartilage. In Operative Oral and Maxillofacial Surgery, 2nd ed.; Langdon, J.D., Patel, M.F., Ord, R.A., Brennan, P.A., Eds.; Hodder Arnold, Hachette: London, UK, 2009; pp. 173–186. [Google Scholar]
- Cordeiro, P.G.; Matros, E.; Chiarini, L.; Anesi, A.; Negrello, S.; De Santis, G.; Nocini, P.F. Atlas of Mandibular and Maxillary Reconstruction with the Fibula Flap; De Santis, G., Cordeiro, P.G., Chiarini, L., Eds.; Springer: Cham, Switzerland, 2019; pp. 21–52. [Google Scholar]
- Common Free Flaps for Head and Neck Reconstruction. Microsurgical Reconstruction of the Head and Neck; Neligan, P.C., Wei, F.-C., Eds.; Thieme Medical Publishers, Inc.: New York, NY, USA, 2009; pp. 255–415. [Google Scholar]
- Herford, A.S.; Ghali, G.E. Local and Regional Flaps. In Peterson’s Principles of Oral and Maxillo Facial Surgery, 3rd ed.; Miloro, M., Ghali, G.E., Larsen, P.E., Waite, P.D., Eds.; Peoples’s Medical Publishing House: Shelton, CT, USA, 2011; pp. 877–892. [Google Scholar]
- Fernandes, R. Local and Regional Flaps in Head and Neck Surgery Reconstruction—A Practical Approach, 1st ed.; John Wiley and Sons: Hoboken, NJ, USA, 2015; pp. 133–139. [Google Scholar]
- Van der Waal, I. Atlas of Oral Diseases; Springer: Berlin/Heidelberg, Germany, 2016; pp. 17–18. [Google Scholar]
- Fry, E.D. The Prevention of Surgical Site Infection in Elective Colon Surgery. Scientifica 2013, 2013, 896297. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Guidelines for the Prevention of Surgical Site Infection; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Eshghpour, M.; Khajavi, A.; Bagheri, M.; Banihashemi, E. Value of Prophylactic Postoperative Antibiotic Therapy after Bimaxillary Orthognathic Surgery: A Clinical Trial. Iran. J. Otorhinolaryngol. 2014, 26, 207–210. [Google Scholar] [PubMed]
- Purghel, F.; Badea, R.; Ciuvica, R.; Anastasiu, A. The use of antibiotics in traumatology and orthopaedic surgery. Med. J. Clin. Med. 2006, 1, 58–65. [Google Scholar]
- Agarwal, B.B. Prophylactic antibiotics in surgery. J. Int. Med Sci. Acad. 2013, 26, 207. [Google Scholar]
- McHugh, S.M.; Collins, C.J.; Corrigan, M.A.; Hill, A.D.K.; Humpreys, H. The role of topical antibiotics used as prophylaxis in surgical site infection prevention. J. Antimicrob. Chemother. 2011, 66, 693–701. [Google Scholar] [CrossRef]
- Fleischman, A.N.; Austin, M.S. Local intra-wound administration of powdered antibiotics in orthopaedic surgery. J. Bone Jt. Infect. 2017, 2, 23–28. [Google Scholar] [CrossRef]
- Morgenstern, M.; Vallejo, A.; McNally, M.A.; Moriarty, T.F.; Ferguson, J.Y.; Nijs, S.; Metsemakers, W.J. The effect of local antibiotic prophylaxis when treating open limb fractures. Bone Jt. Res. 2018, 7, 447–456. [Google Scholar] [CrossRef]
- Heal, C.F.; Banks, J.L.; Lepper, P.D.; Kontopantelis, E.; van Driel, M.L. Topical antibiotics for preventing surgical site infection in wounds healing by primary intention. Cochrane Database Syst. Rev. 2016, 11, CD011426. [Google Scholar] [CrossRef]
- Najjar, P.A.; Smink, D.S. Prophylactic antibiotics and prevention of surgical site infections. Surg. Clin. N. Am. 2015, 95, 269–283. [Google Scholar] [CrossRef]
- Bonello, D.; Starface, Y. Surgical antibiotic prophylaxis: Adherence to hospital’s guidelines. Malta Med. J. 2016, 28, 3–10. [Google Scholar]
- Suhaeri, M.; Noh, M.H.; Moon, J.H.; Kim, I.G.; Oh, S.J.; Ha, S.S.; Lee, J.H.; Park, K. Novel skin patch combining human fibroblast-derived matrix and ciprofloxacin for infected wound healing. Theranostics 2018, 8, 5025–5038. [Google Scholar] [CrossRef] [PubMed]
- Puoci, F.; Piangiolino, C.; Givigliano, F.; Parisi, O.I.; Cassano, R.; Trombino, S.; Curcio, M.; Iemma, F.; Cirillo, G.; Spizzirri, U.G.; et al. Ciprofloxacin-collagen conjugate in the wound healing treatment. J. Funct. Biomater. 2012, 3, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Sripriya, R.; Kumar, M.S.; Ahmed, M.R.; Sehgal, P.K. Collagen bilayer dressing with ciprofloxacin, an effective system for infected wound healing. J. Biomater. Sci. Polymer Edn. 2007, 18, 335–351. [Google Scholar] [CrossRef] [PubMed]
- Lukitowati, F.; Abbas, B.; Erizal, T.; Redja, I.W.; Febryani, H.A. Preparation and characterization of collagen-ciprofloxacin HCL membranes produced using gamma irradiation as a candidate for wound dressing. IOP Conf. Ser. J. Phys. Conf. Ser. 2020, 1436, 012025. [Google Scholar] [CrossRef]
- Shanmugam, K.; Subha, V.; Renganathan, S. Type 1 collagen scaffold functionalized with ciprofloxacin loaded gelatin microspheres—Fabrication, In vitro & In vivo evaluation, histological and biochemical analysis. MOJ Drug Des Develop Ther. 2019, 3, 1–10. [Google Scholar]
- Arafat, M.T.; Tronci, G.; Wood, D.J.; Russell, S.J. In-situ crosslinked wet spun collagen triple helices with nanoscale-regulated ciprofloxacin release capability. Mater. Lett. 2019, 255, 126550. [Google Scholar] [CrossRef]
- Bisaccia, D.R.; Aicale, R.; Tarantino, D.; Peretti, G.M.; Maffulli, N. Biological and chemical changes in fluoroquinolone-associated tendinopathies: A systematic review. Br. Med. Bull. 2019, 130, 39–49. [Google Scholar] [CrossRef]
- Tsai, W.-C.; Hsu, C.-C.; Chen, C.P.C.; Chang, H.-N.; Wong, A.M.K.; Lin, M.-S.; Pang, J.-H.S. Ciprofloxacin Up-Regulates Tendon Cells to Express Matrix Metalloproteinase-2 with Degradation of Type I Collagen. J. Orthop. Res. 2011, 29, 67–73. [Google Scholar] [CrossRef]
- Daneman, N.; Lu, H.; Redelmeier, D.A. Fluoroquinolones and collagen associated severe adverse events: A longitudinal cohort study. BMJ Open 2015, 5, e010077. [Google Scholar] [CrossRef]
- Radu, N.; Voicescu, M.; Radu, E.; Tanasescu, C. Biomaterial with antioxidant and antifungal activities, obtained from Romanian indigenous plants. Mol. Cryst. Liq. Cryst. 2017, 655, 243–249. [Google Scholar] [CrossRef]
- Radu, N.; Roman, V.; Tanasescu, C. Biomaterials obtained from probiotic consortia of microorganisms. Potential applications in regenerative medicine. Mol. Cryst. Liq. Cryst. 2016, 628, 115–123. [Google Scholar] [CrossRef]
- Meyer, M. Processing of collagen based biomaterials and the resulting materials properties. BioMed Eng. Online 2019, 18, 24. [Google Scholar] [CrossRef] [PubMed]
- Copes, F.; Pien, N.; Van Vlierberghe, S.; Boccafoschi, F.; Mantovani, D. Collagen-Based Tissue Engineering Strategies for Vascular Medicine. Front. Bioeng. Biotechnol. 2019, 7, 7. [Google Scholar] [CrossRef] [PubMed]
- Shekhter, A.B.; Fayzullin, A.L.; Vukolova, M.N.; Rudenko, T.G.; Osipycheva, V.D.; Litvitsky, P.F. Medical Applications of Collagen and Collagen-Based Materials. Curr. Med. Chem. 2019, 26, 506–516. [Google Scholar] [CrossRef]
- Tihan, G.T.; Rău, I.; Zgârian, R.G.; Ungureanu, C.; Barbaresso, R.C.; Albu Kaya, M.G.; Dinu-Pîrvu, C.; Ghica, M.V. Oxytetracycline versus doxycycline collagen sponges designed as potential carrier supports in biomedical applications. Pharmaceutics 2019, 11, 363. [Google Scholar] [CrossRef]
- Barbaresso, R.C.; Rău, I.; Zgârian, R.G.; Meghea, A.; Ghica, M.V. Niflumic acid-collagen delivery systems used as anti-inflammatory drugs and analgesics in dentistry. Comptes Rendus Chim. 2014, 17, 12–17. [Google Scholar] [CrossRef]
- Marin, Ș.; Albu Kaya, M.G.; Ghica, M.V.; Dinu-Pîrvu, C.E.; Popa, L.; Udeanu, D.I.; Mihai, G.; Enăchescu, M. Collagen-polyvinyl alcohol-indomethacin biohybrid matrices as wound dressings. Pharmaceutics 2018, 10, 224. [Google Scholar] [CrossRef]
- Ghica, M.V.; Albu Kaya, M.G.; Dinu-Pîrvu, C.E.; Lupuleasa, D.; Udeanu, D.I. Development, optimization and in vitro/in vivo characterization of collagen-dextran spongious wound dressings loaded with flufenamic acid. Molecules 2017, 22, 1552. [Google Scholar] [CrossRef]
- Vineet, R.V.; Nayak, M.; Kotigadde, S.; Antony, B. Isolation of root canal pathogens from primary endodontic infection and retreatment cases a clinical comparative study. Univ. J. Dent. Sci. 2016, 2, 6–10. [Google Scholar]
- Caramihai, M.; Radu, N.; Bostan, M.; Ion, G.; Chirvase, A.A.; Constantin, M.; Doni, M.; Babeanu, N. Fuzzy predictions regarding in vitro behavior of cells line under cytostatic treatment. In Proceedings of the 7th IEEE International Conference on E-Health and Bioengineering (EHB), Iasi, Romania, 21–23 November 2019; pp. 1–5. [Google Scholar]
- Ramer, R.; Schmied, T.; Wagner, C.; Haustein, M.; Hinz, B. The antiangiogenic action of cisplatin on endothelial cells is mediated through the release of tissue inhibitor of matrix metalloproteinases-1 from lung cancer cells. Oncotarget 2018, 9, 34038–34055. [Google Scholar] [CrossRef]
- Ioan, D.C.; Rău, I.; Tihan, G.T.; Zgârian, R.G.; Ghica, M.V.; Albu Kaya, M.G.; Dinu-Pîrvu, E.C. Piroxicam-Collagen Based Sponges For Medical Applications. Int. J. Polym. Sci. 2019. [Google Scholar] [CrossRef]
- Albu, M.G. Collagen Gels and Matrices for Biomedical Applications; Lambert Academic Publishing: Saarbrücken, Germany, 2011; pp. 23–24. [Google Scholar]
- Radu, N.; Ferdes, M.; Rau, I. Cytotoxicity study regarding some products derived from Monascus sp. Mol. Cryst. Liq. Cryst. 2012, 555, 189–194. [Google Scholar] [CrossRef]
- Mihaila, M.; Bostan, M.; Hotnog, D.; Ferdes, M.; Brasoveanu, L.I. Real-time analysis of quercetin, resveratrol and/or doxorubicin effects in MCF-7 cells. Rom. Biotechnol. Lett. 2013, 18, 8106–8114. [Google Scholar]
- Hotnog, D.; Mihaila, M.; Botezatu, A.; Matei, G.G.; Hotnog, C.; Anton, G.; Bostan, M.; Brasoveanu, L.I. Genistein potentiates the apoptotic effect of 5-fluorouracyl in colon cancer cell lines. Rom. Biotechnol. Lett. 2013, 18, 255–265. [Google Scholar]
- Bostan, M.; Petrică-Matei, G.G.; Ion, G.; Radu, N.; Mihăilă, M.; Hainăroşie, R.; Braşoveanu, L.I.; Roman, V.; Constantin, C.; Neagu, M.T. Cisplatin effect on head and neck squamous cell carcinoma cells modulated by ERK1/2 protein kinases. Exp. Ther. Med. J. 2019, 18, 5041–5051. [Google Scholar] [CrossRef] [PubMed]
- Wells, C.M.; Beenken, K.E.; Smeltzer, M.S.; Courtney, H.S.; Jennings, J.A.; Haggard, W.O. Ciprofloxacin and rifampin dual antibiotic-loaded biopolymer chitosan sponge for bacterial inhibition. Mil. Med. 2018, 183 (Suppl. 1), 433–444. [Google Scholar] [CrossRef]
- Aranaz, I.; Harris, R.; Navarro-García, F.; Heras, A.; Acosta, N. Chitosan based films as supports for dual antimicrobial release. Carbohydr. Polym. 2016, 146, 402–410. [Google Scholar] [CrossRef]
- Baudry-Simner, P.J.; Singh, A.; Karlowsky, J.A.; Hoban, D.J.; Zhanel, G.G. The Canadian Antimicrobial Resistance Alliance, Mechanisms of reduced susceptibility to ciprofloxacin in Escherichia coli isolates from Canadian hospitals. Can. J. Infect. Dis. Med Microbiol. 2012, 23, e60–e64. [Google Scholar] [CrossRef][Green Version]
- Firsov, A.A.; Vostrov, S.N.; Shevchenko, A.A.; Zinner, S.H.; Cornaglia, G.; Portnoy, Y.A. MIC-based interspecies prediction of the antimicrobial effects of ciprofloxacin on bacteria of different susceptibilities in an in vitro dynamic model. Antimicrob. Agents Chemother. 1998, 42, 2848–2852. [Google Scholar] [CrossRef]
- Gauzit Amiel, A.; Palomino-Durand, C.; Maton, M.; Lopez, M.; Cazaux, F.; Chai, F.; Neut, C.; Foligné, B.; Martel, B.; Blanchemain, N. Designed sponges based on chitosan and cyclodextrin polymer for a local release of ciprofloxacin in diabetic foot infections. Int. J. Pharm. 2020, 587, 119677. [Google Scholar] [CrossRef]
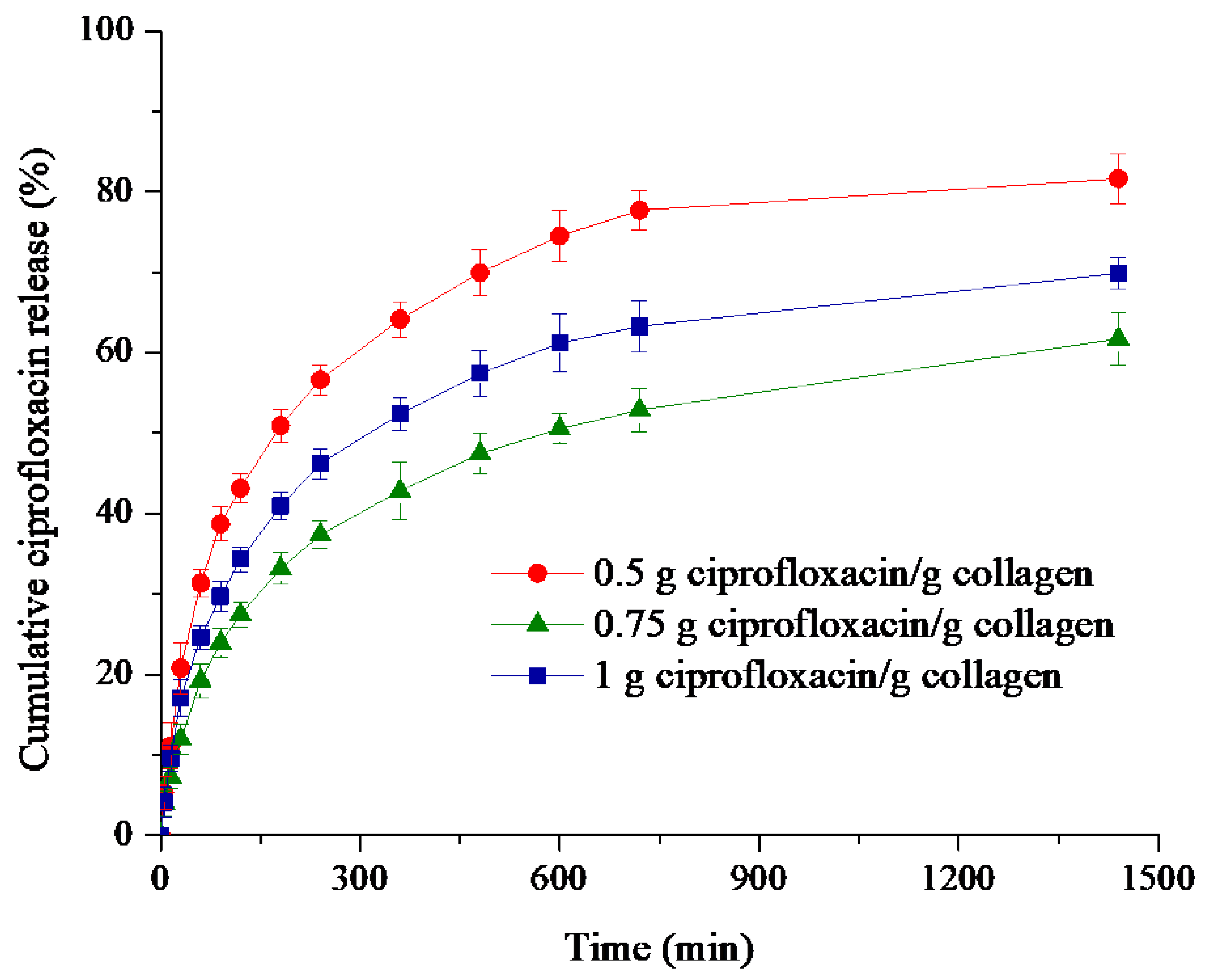
| Ciprofloxacin (CPX)—Collagen (C) Sponges g CPX/g C | Correlation Coefficient, R | Kinetic Constant, k (1/minn) | Release Exponent, n | Drug Released (%) | ||
|---|---|---|---|---|---|---|
| Higuchi Model | Zero-Order Model | Power Law Model | ||||
| 0.5 | 0.9448 | 0.7961 | 0.9762 | 0.084 | 0.33 | 81.63 |
| 0.75 | 0.9716 | 0.8502 | 0.9875 | 0.044 | 0.37 | 61.70 |
| 1 | 0.9572 | 0.8194 | 0.9820 | 0.063 | 0.35 | 69.86 |
| Sample | Inhibition Diameter, mm | |||
|---|---|---|---|---|
| Staphylococcus aureus | Escherichia coli | Candida albicans | Candida parapsilopsis | |
| 100 μg ciprofloxacin/mL | 35.0 ± 1.75 | 0 | 0 | 0 |
| 1000 μg ciprofloxacin/mL | 42.5 ± 2.12 | 44.0 ± 2.20 | 0 | 0 |
| Collagen sponge (C) | 10.5 ± 0.52 | 0 | 0 | 0 |
| Ciprofloxacin–collagen sponge (0.5 g ciprofloxacin/g collagen) (C3) | 37.0 ± 1.85 | 42.0 ± 2.10 | 0 | 0 |
| Ciprofloxacin–collagen sponge (0.75 g ciprofloxacin/g collagen) (C4) | 40.0 ± 2.00 | 42.0 ± 2.10 | 0 | 0 |
| Ciprofloxacin–collagen sponge (1 g ciprofloxacin/g collagen) (C5) | 38.5 ± 1.92 | 44.5 ± 2.22 | 0 | 0 |
| Sample | PI | ||
|---|---|---|---|
| 6 h | 24 h | 48 h | |
| 100 μg ciprofloxacin/mL | 1.17 ± 0.0234 | 1.21 ± 0.0182 | 1.08 ± 0.0216 |
| Collagen sponge | 1.34 ± 0.0268 | 1.29 ± 0.0194 | 1.23 ± 0.0246 |
| Ciprofloxacin-collagen sponge (0.5 g ciprofloxacin/g collagen) | 1.46 ± 0.0292 | 1.48 ± 0.0222 | 0.94 ± 0.0188 |
| Ciprofloxacin-collagen sponge (0.75 g ciprofloxacin/g collagen) | 1.56 ± 0.0312 | 1.36 ± 0.0204 | 1.04 ± 0.208 |
| Ciprofloxacin-collagen sponge (1 g ciprofloxacin/g collagen) | 1.24 ± 0.0248 | 1.16 ± 0.0174 | 0.77 ± 0.0154 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ioan, D.-C.; Rău, I.; Albu Kaya, M.G.; Radu, N.; Bostan, M.; Zgârian, R.G.; Tihan, G.T.; Dinu-Pîrvu, C.-E.; Lupuliasa, A.; Ghica, M.V. Ciprofloxacin-Collagen-Based Materials with Potential Oral Surgical Applications. Polymers 2020, 12, 1915. https://doi.org/10.3390/polym12091915
Ioan D-C, Rău I, Albu Kaya MG, Radu N, Bostan M, Zgârian RG, Tihan GT, Dinu-Pîrvu C-E, Lupuliasa A, Ghica MV. Ciprofloxacin-Collagen-Based Materials with Potential Oral Surgical Applications. Polymers. 2020; 12(9):1915. https://doi.org/10.3390/polym12091915
Chicago/Turabian StyleIoan, Daniel-Cristian, Ileana Rău, Mădălina Georgiana Albu Kaya, Nicoleta Radu, Marinela Bostan, Roxana Gabriela Zgârian, Graţiela Teodora Tihan, Cristina-Elena Dinu-Pîrvu, Alina Lupuliasa, and Mihaela Violeta Ghica. 2020. "Ciprofloxacin-Collagen-Based Materials with Potential Oral Surgical Applications" Polymers 12, no. 9: 1915. https://doi.org/10.3390/polym12091915
APA StyleIoan, D.-C., Rău, I., Albu Kaya, M. G., Radu, N., Bostan, M., Zgârian, R. G., Tihan, G. T., Dinu-Pîrvu, C.-E., Lupuliasa, A., & Ghica, M. V. (2020). Ciprofloxacin-Collagen-Based Materials with Potential Oral Surgical Applications. Polymers, 12(9), 1915. https://doi.org/10.3390/polym12091915










