1. Introduction
Aramids are polyamids which exhibit aromatic groups in the macromolecular backbone (aromatic polyamids = aramids). The most important species are poly(meta-phenylenterephthalamid) (m-aramid) and poly(para-phenylenterephthalamid) (p-aramid). Both m-aramid and p-aramid are commercialized by the leading producers Teijin (Arnhem, The Netherlands) and DuPont (Wilmington, DE, USA) under the trade names Teijin-conex
® and Twaron
® and Nomex
® and Kevlar
®, respectively. Aramid fibers excel by their outstanding mechanical and thermal properties. They are high strength, resistant against aggressive chemicals and all common acids and bases, extremely heat resistant and noncombustible. Technically they are used, e.g., in bulletproof vests, heat resistant clothing for firefighters and soldiers or as reinforcement fibers in composites used in airplanes and cars [
1,
2].
For most applications of m-aramids, customers request the dyeing of the material. However, the conventional dyeing of aramids is an extremely difficult, time-consuming and costly process. In particular, it needs polluting chemicals, the so-called carriers, which allow the dyestuff to penetrate the fiber matrix. Such compounds, e.g., benzyl alcohol, benzaldehyde, acetophenone and, in some countries, even chloro-organic compounds, are highly controversial in view of workplace exposure and environmental impact [
3,
4]. Therefore, strict regulations for the use and the appropriate disposal of the dyeing liquors exist in many countries. To meet these requirements, the dyeing companies already face a huge technical and ecological effort, which significantly raises the overall costs for aramid manufacturing. In the medium term, governments, e.g., in the European Union, plan the further decrease in limit values or even an entire ban on such carriers, which—in default of alternatives—could endanger many jobs in this textile sector.
Thus, textile industries around the world have, for many years, been looking for alternative dyeing techniques for aramid fibers—in particular to avoid the discussed use of the problematic carriers.
In the past, scientists have reported the use of ionic liquids (IL) in various textile-related processes, e.g., for textile finishing, textile functionalization and for the spinning of textile fibers, as well as for the dyeing of textiles [
5]. Ionic liquids—often referred to as “green solvents”—are salts with a melting point lower than 100 °C. ILs excel by their extremely low vapor pressure, high thermo-stability and nonflammability, which makes them easy to handle in typical textile finishing processes. Moreover, ILs have high and temperature-related dielectric constants, therefore exhibiting outstanding solvent power for various textile-related substances, such as cellulose, keratin and silicones. Like common salts, ILs consist of cations and anions, e.g., imidazolium, pyridinium, phosphonium plus ammonium (cations) and alkyl sulfates, hexafluoro phosphate and tetrafluoro borate plus halogenides (anions). These two components can be varied in a broad range, yielding designed properties. For instance, melting point, viscosity, density and hydrophobicity can be adjusted for specific applications. In the non-textile sector, ionic liquids have already reached the market. Examples are solvents (catalysis, organic syntheses, polymerizations, synthesis of nanoparticles), electrolytes (batteries, fuel cells, sensors), separation techniques (membranes, gas separation, extractions), analytics (gas chromatography, protein crystallization), lubricants and fuel additives [
6,
7,
8].
We published our first study on the IL-based dyeing of common fiber types, such as cotton and polyester, in 2006 [
9,
10]. Yuan et al. (2010) used 1-butyl-3-methylimidazolium chloride to increase the dye uptake in wool colorization [
11]. Additionally, 1-(2-hydroxyethyl)-3-methylimidazolium chloride, 2-hydroxidiethanolamine and a glycerine-based eutectic solvent were employed to increase, e.g., the color strength of polyester textiles [
12,
13,
14]. In our own study, we have successfully demonstrated that polyethylene terephthalate (PET) fibers can be dyed in manifold shades with commercial disperse dyestuffs in various commercial ILs far above 100 °C, using open non-pressurized systems. Compared to conventionally dyed PET, the fibrous material shows no significant differences in terms of mechanical properties and fastness to rubbing, washing and light. In addition, we have successfully demonstrated that the typical auxiliaries of commercial dyestuffs are not necessary within the IL dyeing procedure. Furthermore, we have shown that PET/cotton blends can generally be dyed in one step using both needed dyestuff types in one dyeing liquor (mixture of reactive dyes for cotton and disperse dyes for PET) [
15].
In widening our general IL dyeing concept, here, we present a study on the dyeing of the high-performance m-aramid fiber with ionic liquids in the absence of any polluting carrier. In the first step, the dyeing quality and the influence of fiber crystallinity were studied for different classes of dyestuffs. For two chosen systems, the relevant physical chemical dyeing parameters, such as tensile strength, elongation and fastness to washing, rubbing and light, were investigated systematically.
2. Materials and Methods
2.1. Materials
The experiments were carried out using the amorphous m-aramid fabric Nomex Comfort (surface weight 245 g/m2) and the crystalline m-aramid fabric Nomex 450 (surface weight 140 g/m2). For the comparison of the light fastness, we used conventionally dyed Nomex Comfort as a reference material (surface weight 245 g/m2, dyed blue, black, orange with the use of carriers). All textiles were purchased from IBENA Textilwerke GmbH (Bocholt, Germany). For the dyeing experiments, we used several different ionic liquids. Most of the discussed experiments were carried out with 1-ethyl-3-methylimidazolium ethyl sulfate (EMIM-EtSO4, Sigma-Aldrich, St. Louis, MO, USA). Commercial cationic dyestuffs were used for the dyeing of the aramid fibers. Doracryl® Red GL, Yellow XGRL, Blue GL and Orange R were purchased from M. Dohmen GmbH (Moenchengladbach, Germany). The acid dyestuff Nigrosin (Acid Black 2) was obtained from Sigma-Aldrich. The pure fluorescent dyestuffs for PET Maxilon® Flavine crude were purchased from Huntsman (Langweid a.L., Germany).
2.2. General Dyeing Procedure
Three hundred micrograms of the respective dyestuffs were dissolved in 15 g EMIM-EtSO4 (or other IL). A piece of aramid fabric (2 cm × 2 cm) was submersed in the preheated solution and kept there for one hour at the specified temperature without stirring. After the dwell time, the fabric was removed from the dyeing bath and was put into a beaker with water. Afterwards, the fabric was rinsed with running water till the water was clear. Then the fabric was dried overnight at room temperature. All colored textile materials were additionally rinsed with acetone to remove excessive dyestuffs.
2.3. Characterization
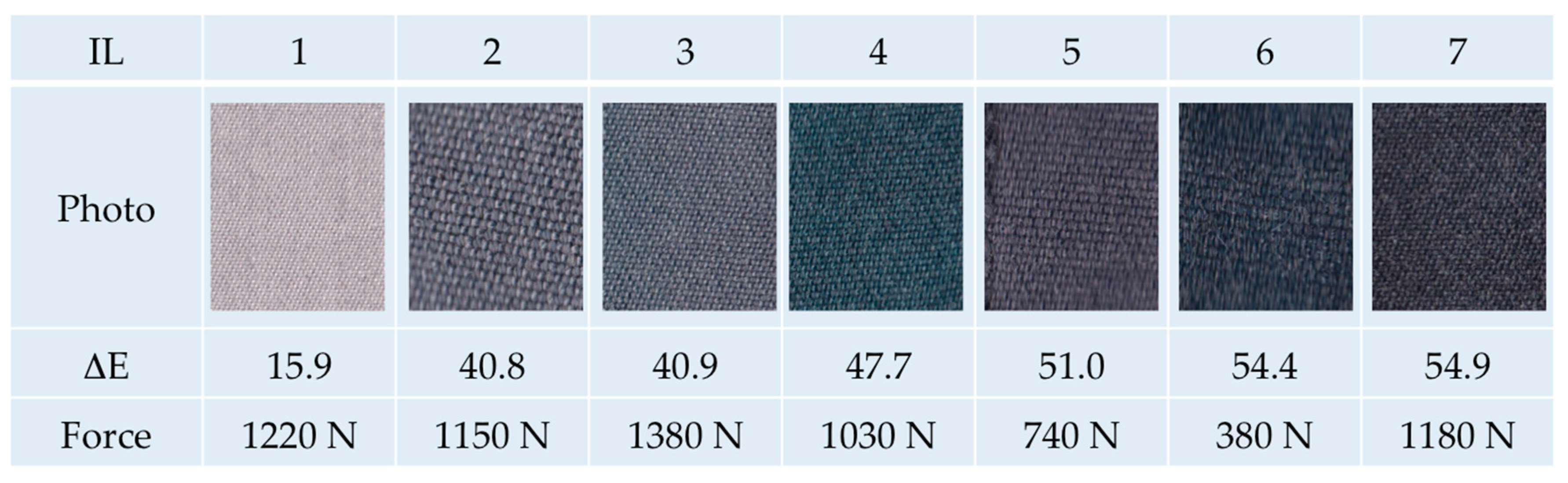
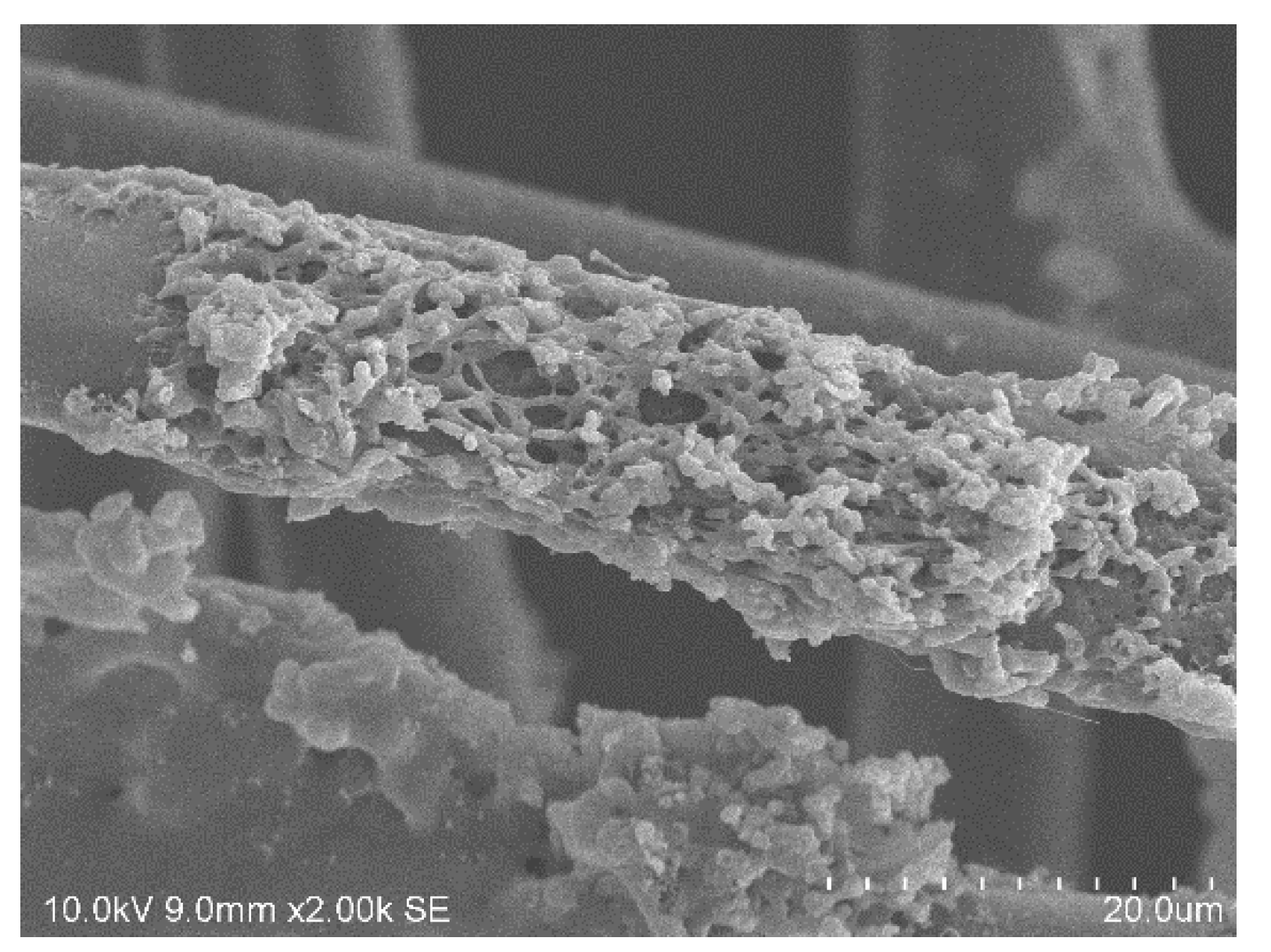
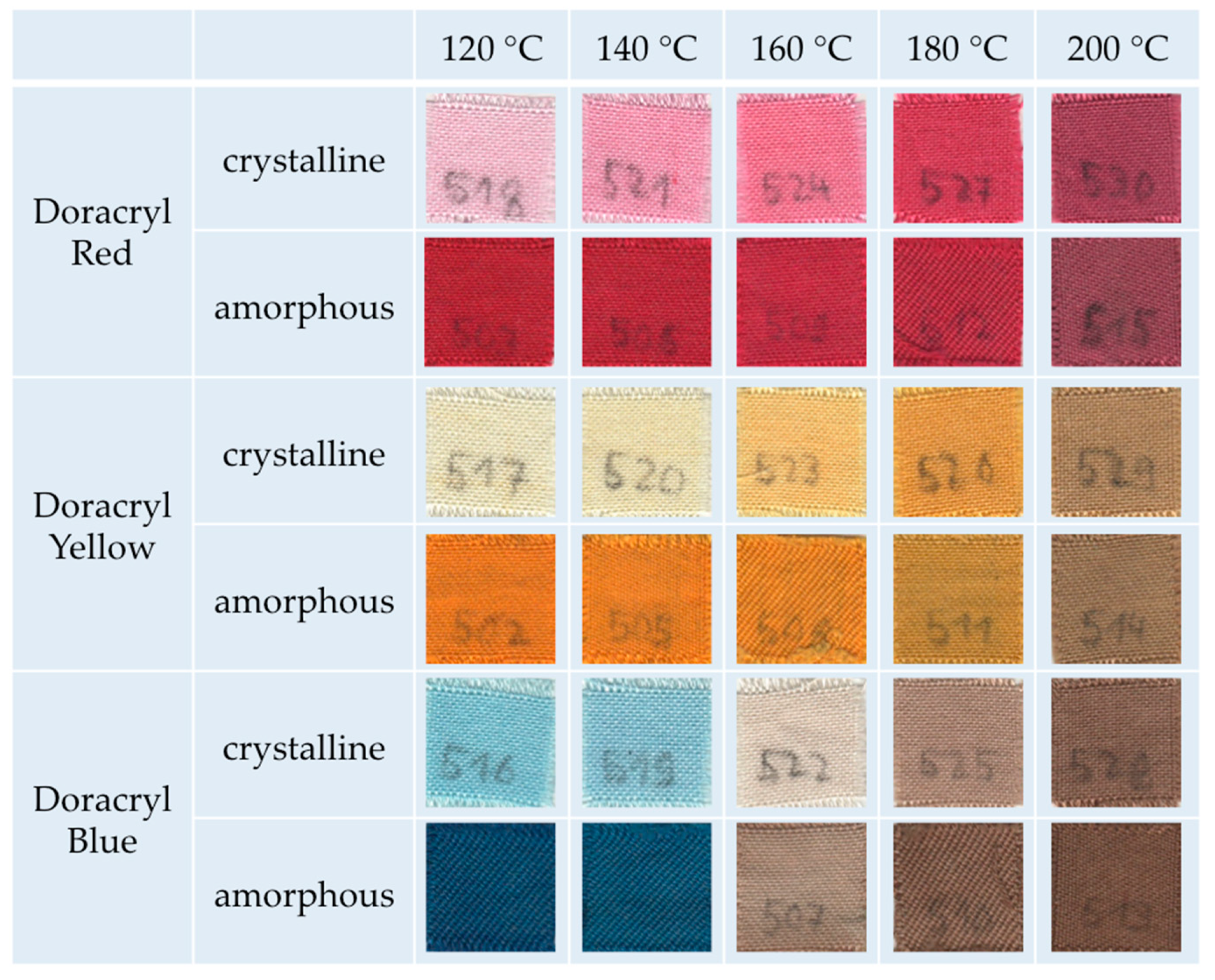
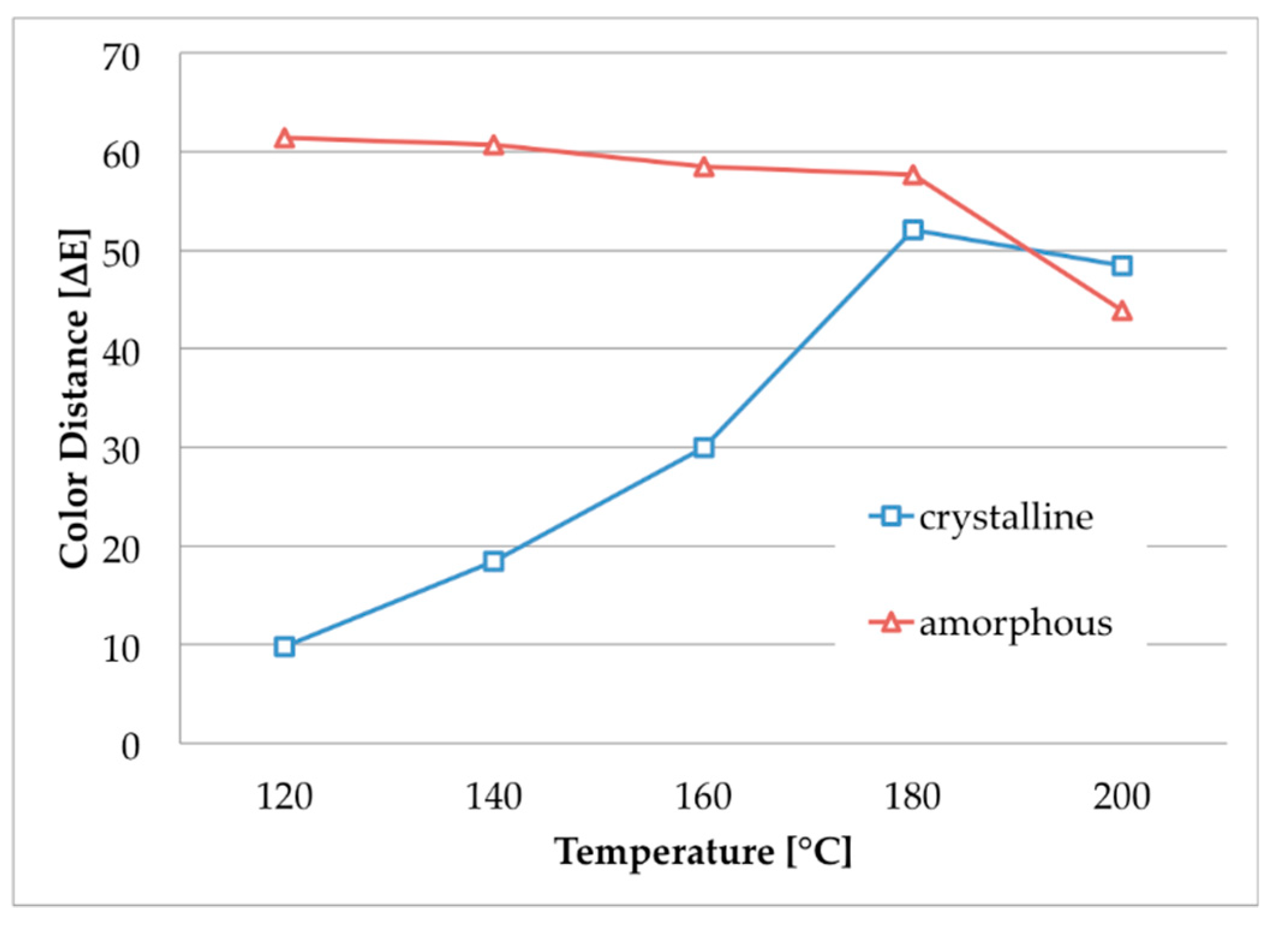
UV–Vis spectra of the samples were evaluated using a Cary 5E spectrophotometer (Varian GmbH, Waldbronn, Germany). The colorimetric measurements were conducted by a Colorlite sph850 (Colorlite GmbH, Katlenburg-Lindau, Germany) using L*a*b* color space. The mentioned color distances ΔE are the differences between the uncolored and the colored textile materials. ΔE is calculated as . To evaluate fiber cross sections of the dyed textile material, single fiber filaments were removed from the fabric and embedded in instant adhesive between two polypropylene plates. Thin slices of the cured sample were cut off and put on object plates. The samples were examined by microscopy using a VHX digital microscope (Keyence Deutschland GmbH, Neu-Isenburg, Germany). Scanning electron microscopy of the fibers was conducted with a SEM S-3400 N II (Hitachi High-Technologies Europe GmbH, Krefeld, Germany). Mechanical properties of the m-aramid fabric were characterized by the maximum force and elongation at maximum force measured according to DIN EN ISO 13934-1 using a ZMART pro Typ 1455 (Zwick GmbH & Co KG, Ulm, Germany) (standard atmosphere: 20 °C, 65% rel. humidity, clamping length: 200 mm, fabric width: 30 mm, draw rate: 100 mm/min, preload force: 5 N). The fastness to washing (scale 1–5, best achievable level 5) was conducted according to DIN EN ISO 105-C06 using a Linitest plus (Atlas Material Testing Technology GmbH, Linsengericht-Altenhasslau, Germany). The light fastness (scale 1–8, best achievable level 8) was evaluated according to DIN EN ISO 105-B02 using a Xenotest 150 S (Atlas Material Testing Technology GmbH, Altenhasslau, Germany). The fastness to rubbing (scale 1–5, best achievable level 5) was conducted according to EN ISO 105-X12 using a Crockmeter (Crockmaster, James Heal, UK) (standard atmosphere: 20 °C, 65% rel. humidity, conditioning: 4 h, cone for rubbing: cylindrical (16 mm), weight of 9 N, absorption of water: 95–100%).
4. Discussion
High-performance textiles made of aramid fibers supply a huge and steadily growing market of important safety products and light-weight materials, where light but strong and thermo-stable materials are desired. A long-standing problem in this field, however, is the conventional dyeing procedure of aramid fibers, which does not meet modern requirements for employment protection and pollution control. With our new operational dyeing technique using ILs, we have successfully demonstrated that the coloration of m-aramids is possible in the absence of any carriers, the widely used but most problematic auxiliaries in aramid dyeing.
Our main findings are:
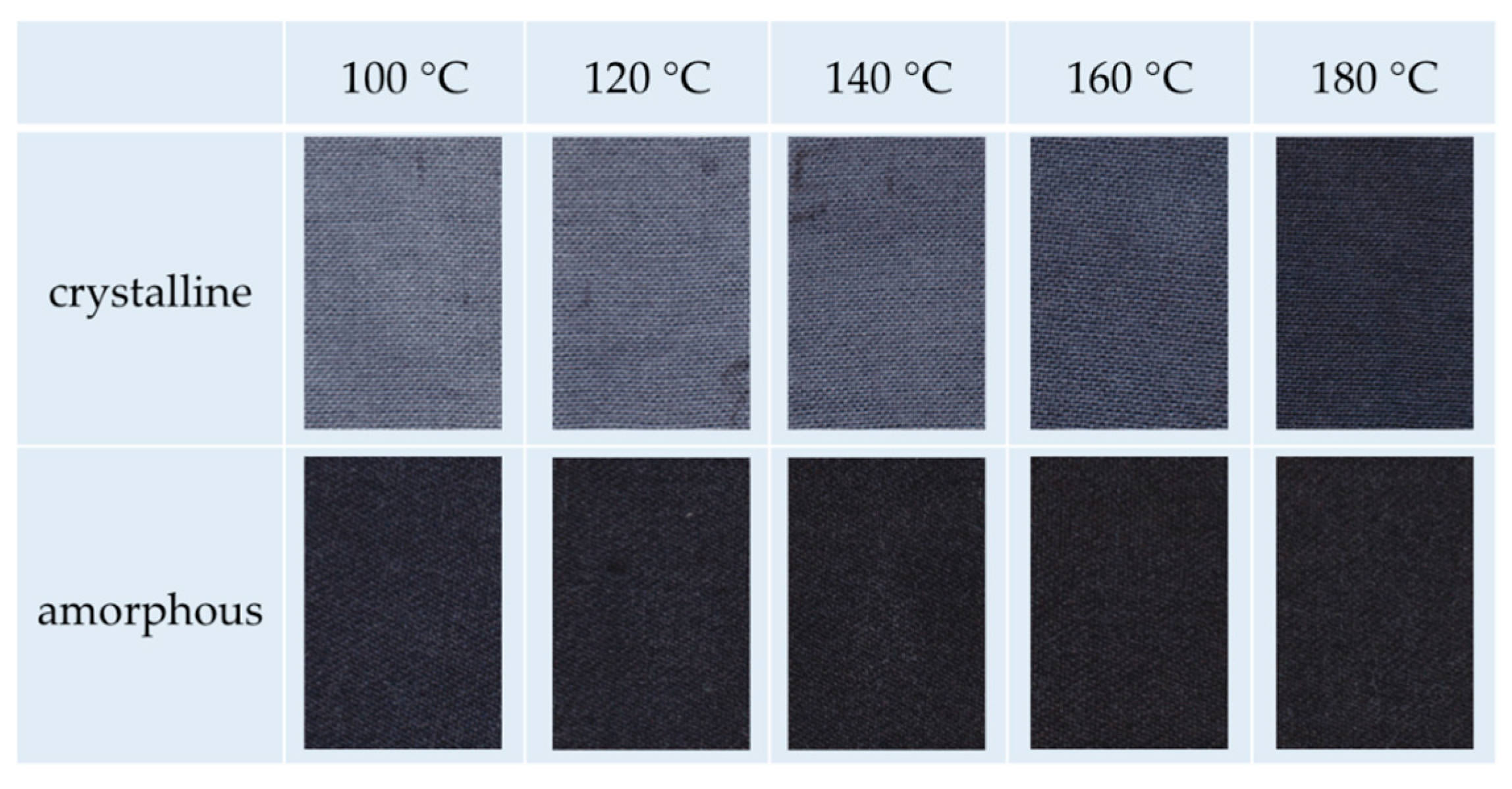
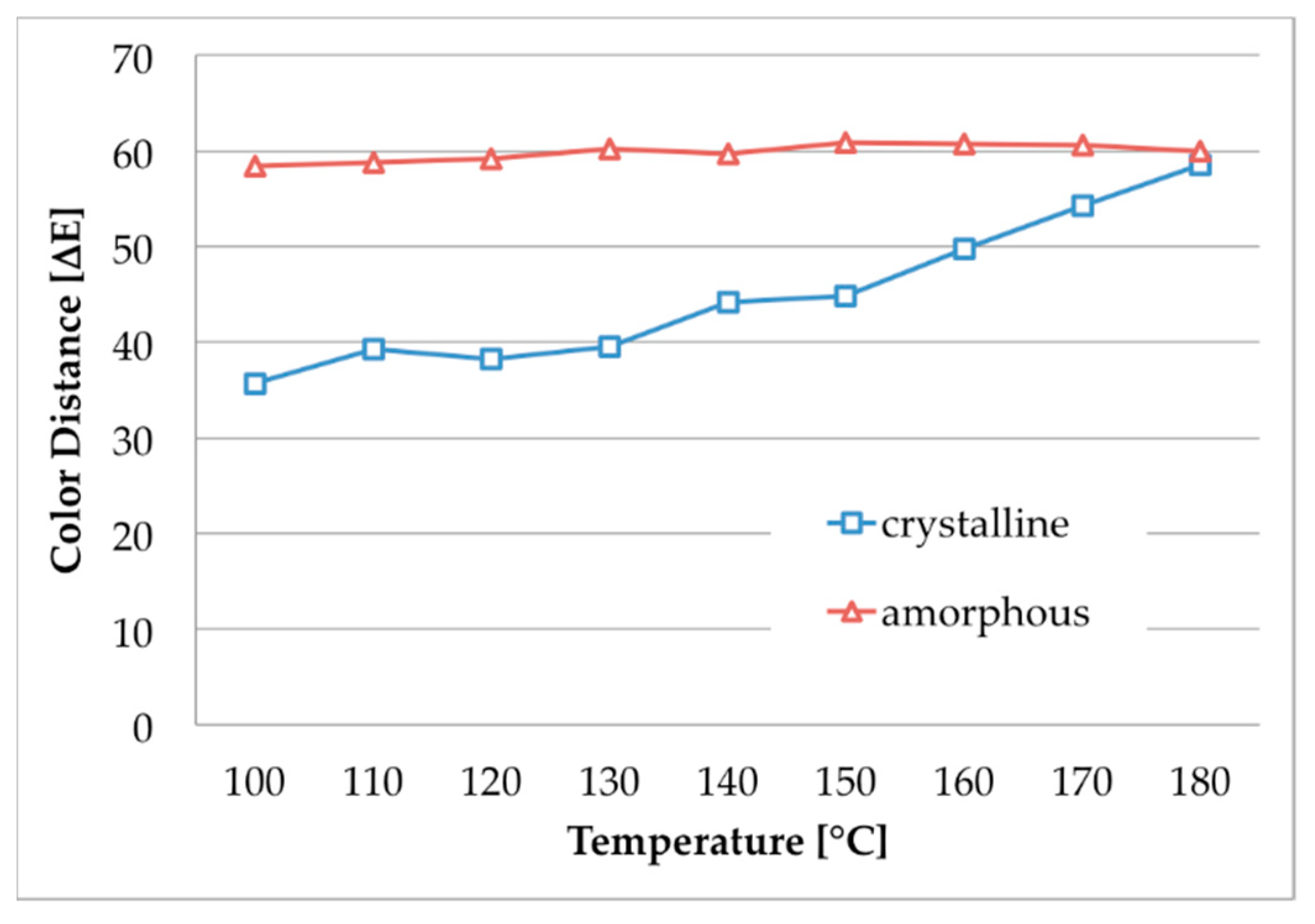
the dyeability of m-aramid fibers strongly depends on their crystallinity;
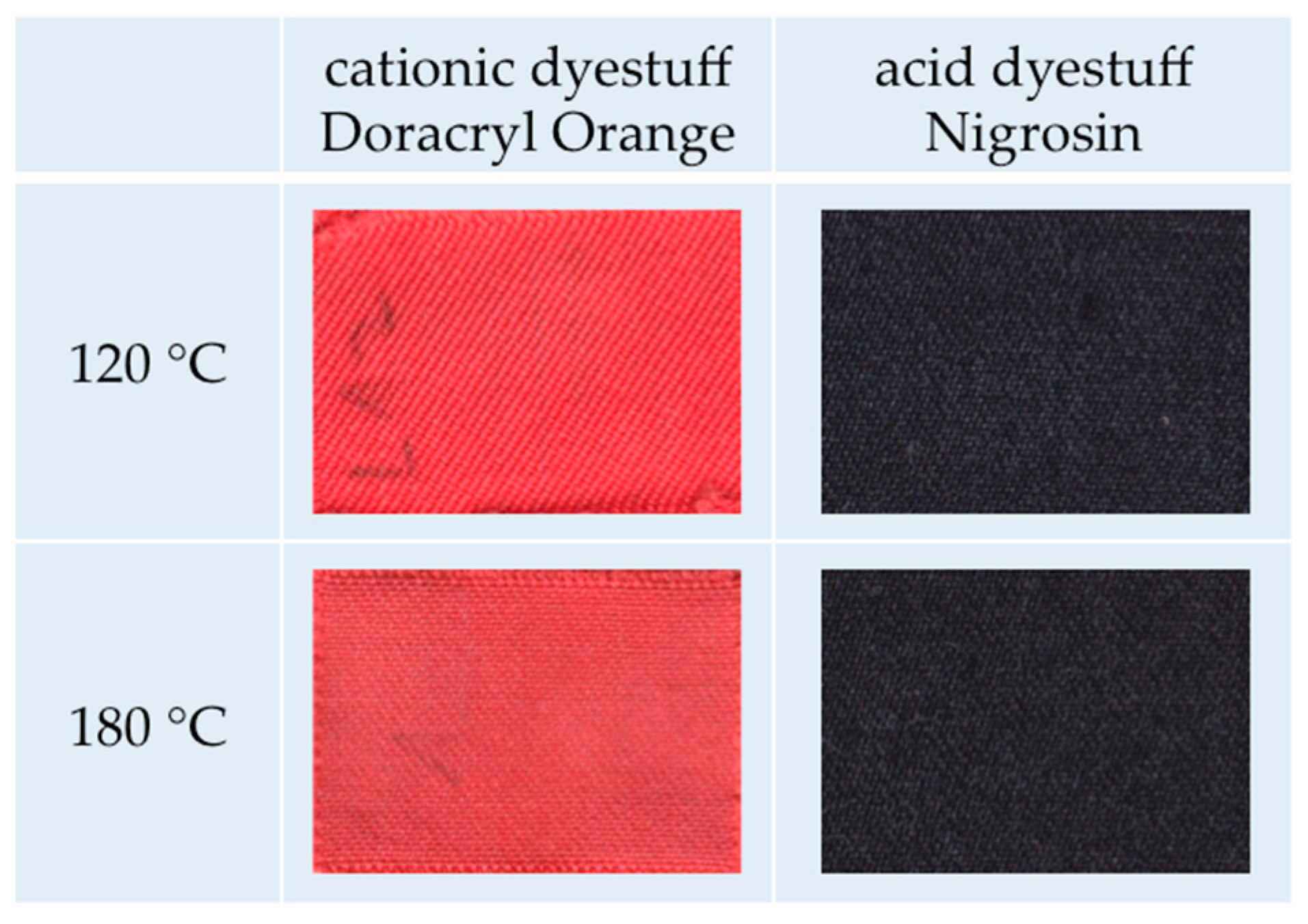
amorphous m-aramid fibers are generally dyeable in ILs with typical cationic dyes;
EMIM-EtSO4 represents the most promising IL, yielding bright and uniform shades;
the recommended temperature is 120–140 °C;
m-aramid fibers with higher crystallinity can be dyed with thermo-stable dyestuffs at higher temperatures, e.g., acid dyes;
no (ill) effect on the mechanical properties;
full fiber penetration;
excellent fastness towards washing, rubbing and light;
no harmful and polluting carriers are needed.
The results are in line with our own findings on the dyeing of other fiber types, such as polyester, cotton and their blends in ILs. In all cases, deep-colored textiles could be obtained with outstanding textile-related properties. For both the dyeing of polyester and the dyeing of m-aramids, the dyeing results are comparable or even better than conventionally dyed textiles. Thus, by simply changing the used IL and the fiber-specific dyestuff type, a comparable dyeing procedure was successfully implemented for a high-performance fiber for the first time. Therefore, this study represents a consequent continuation of our previous work, as well as the approaches of other authors.
5. Conclusions
Today, ionic liquids, as so-called green solvents, play an important role in worldwide research and some technical applications are already being put into practice. Several studies are dealing with the use of ILs even in textile-related processes, such as fiber finishing, functionalization or fiber spinning. In particular, the dyeing of synthetic, as well as natural, fibers in ILs is a growing research objective and some remarkable achievements in this field have been obtained. However, ILs are not yet established commercially in the textile dyeing sector since the existing dyeing technologies for, e.g., polyester or cotton, have not been called into question to date. This is different in the case of the high-performance fiber m-aramid, where the conventional dyeing procedure has huge economic and ecological disadvantages. Our new fundamental and application-oriented study adds to the knowledge on the use of ionic liquids in general and could help to overcome one of the most challenging issues in the dyeing of m-aramid fibers, the relinquishment of carrier chemicals, which will probably be banned within the next few years in many countries worldwide. However, further R&D is needed to industrialize our concept. Our future work will focus on, from an economic point of view, the fundamentally needed recovery of the IL itself by, e.g., ultra-nano filtration of the dyeing liquor.