Effect of Thermal Annealing on Conformation of MEH-PPV Chains in Polymer Matrix: Coexistence of H- and J-Aggregates
Abstract
1. Introduction
2. Experimental Details
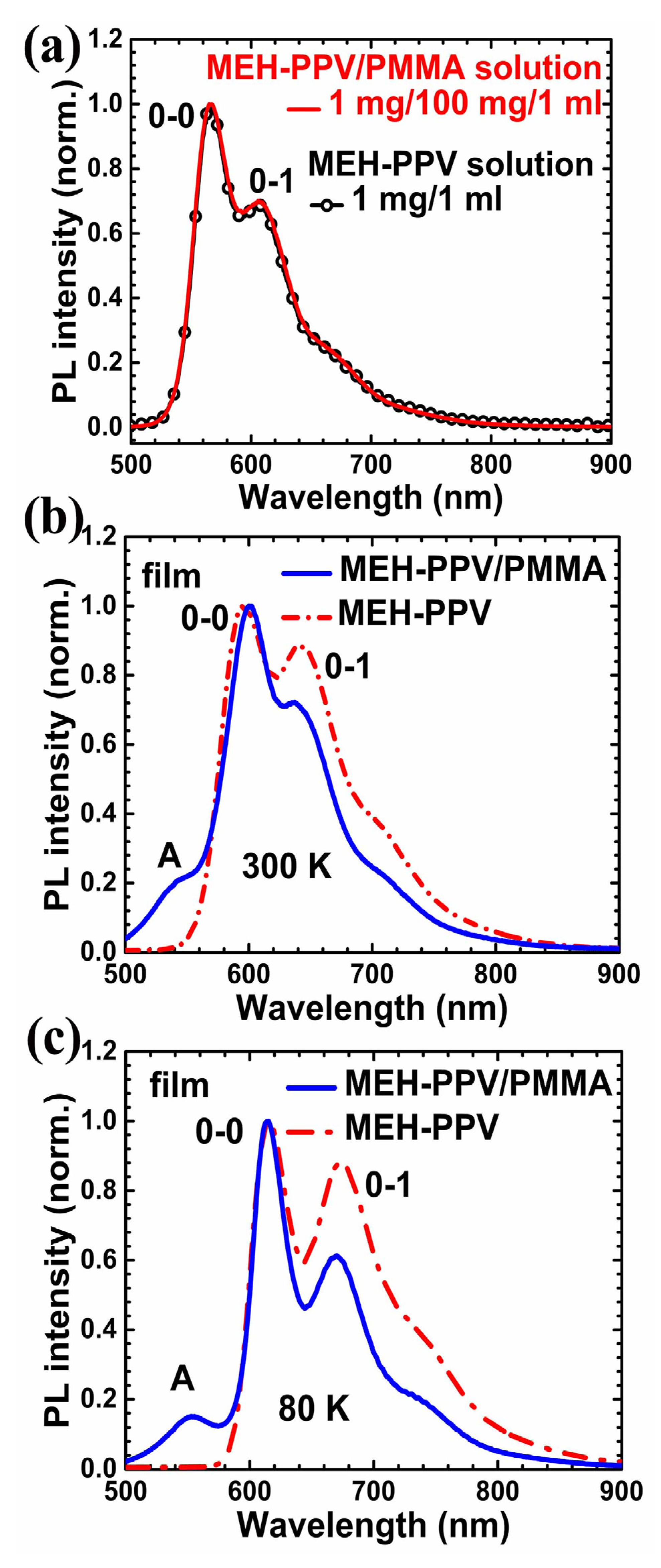
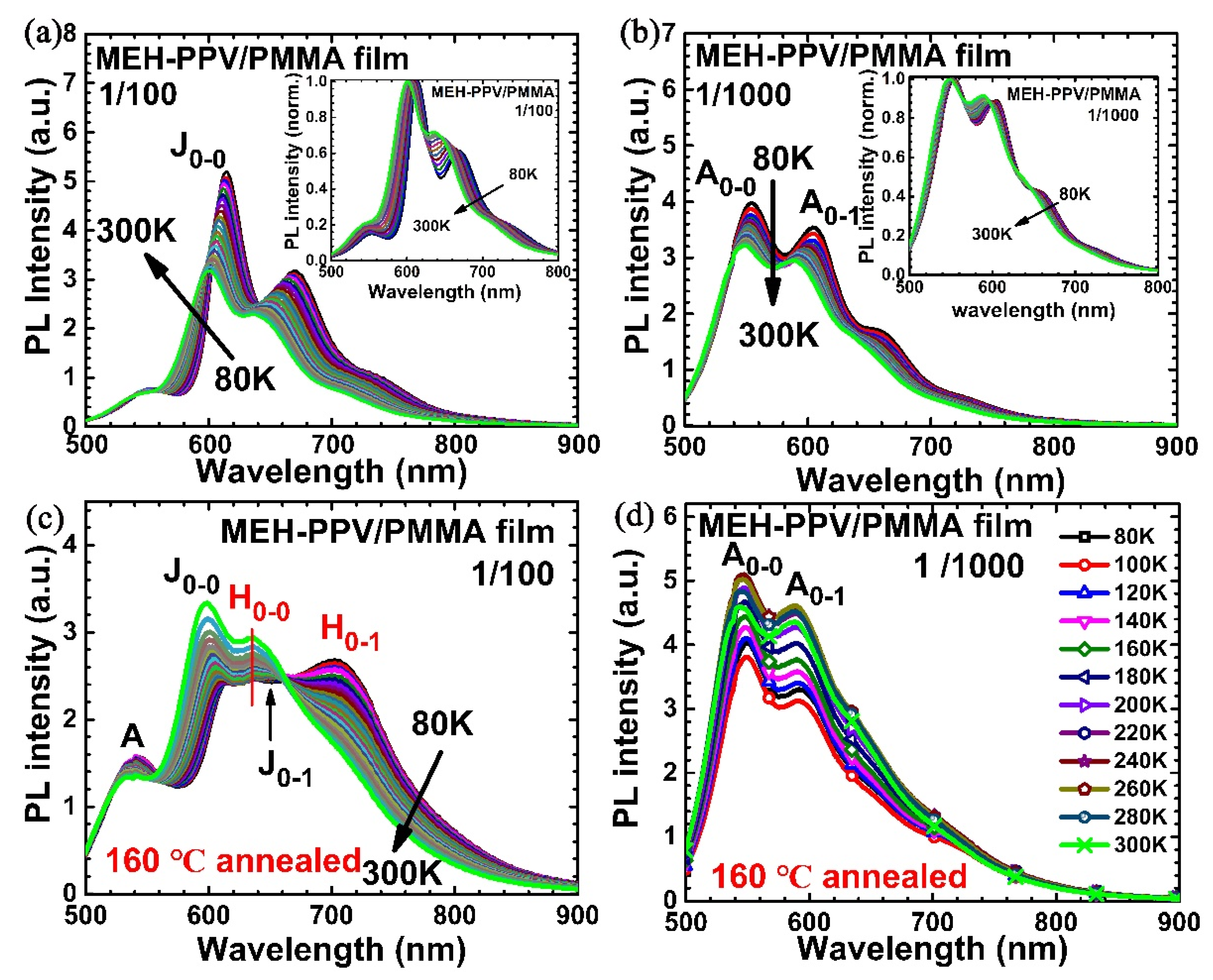
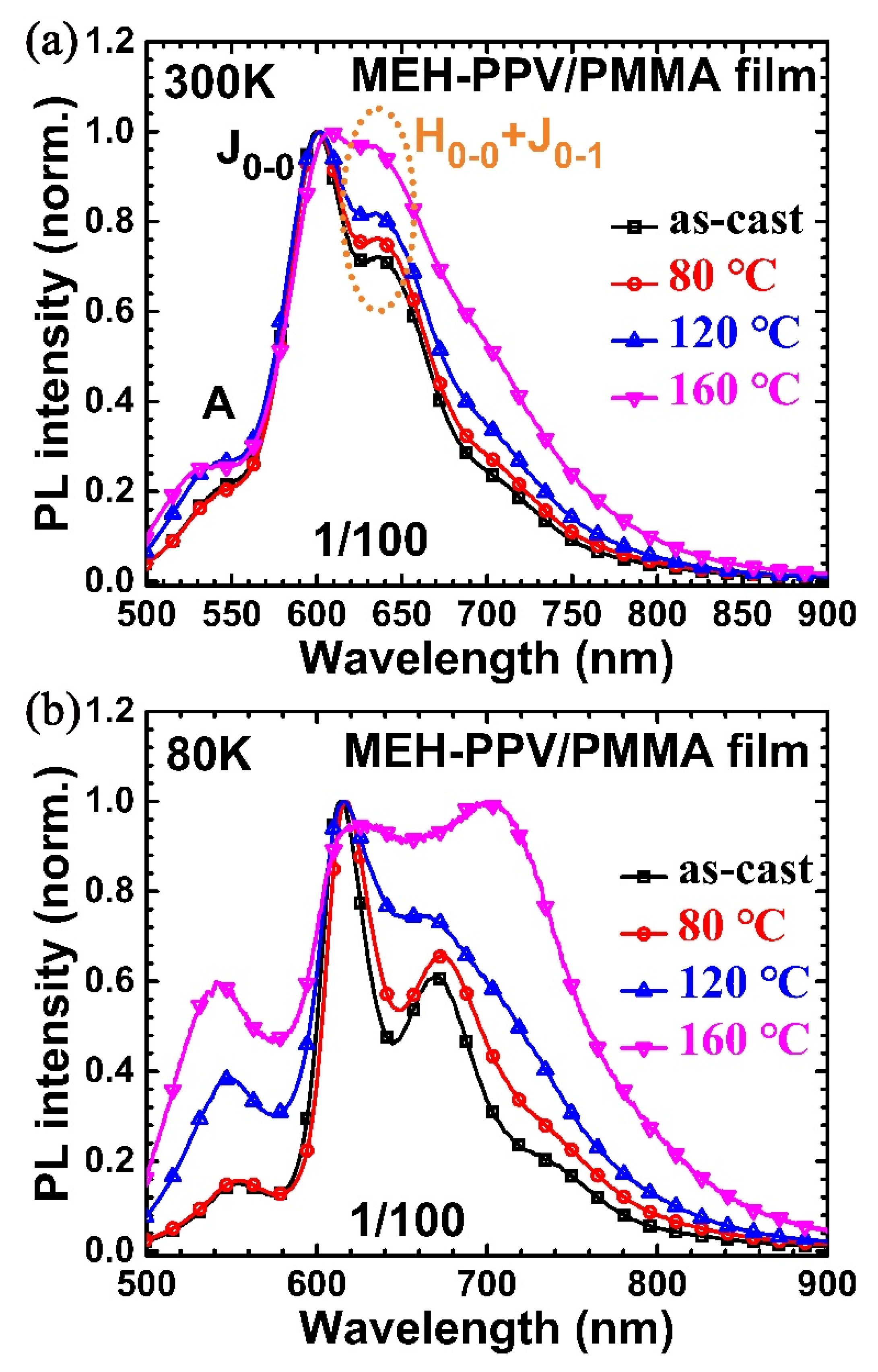
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Friend, R.H.; Gymer, R.W.; Holmes, A.B.; Burroughes, J.H.; Marks, R.N.; Taliani, C.; Bradley, D.D.C.; Dos Santos, D.A.; Bredas, J.L.; Logdlund, M.; et al. Electroluminescence in conjugated polymers. Nature 1999, 397, 121–128. [Google Scholar] [CrossRef]
- Yao, E.-P.; Yang, Z.; Meng, L.; Sun, P.; Dong, S.; Yang, Y.; Yang, Y. High-Brightness Blue and White LEDs based on Inorganic Perovskite Nanocrystals and their Composites. Adv. Mater. 2017, 29, 1606859. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Bazan, G.C. Homogeneous fluorescence-based DNA detection with water-soluble conjugated polymers. Chem. Mater. 2004, 16, 4467–4476. [Google Scholar] [CrossRef]
- Pushpavanam, K.; Narayanan, E.; Rege, K. Molecular and Nanoscale Sensors for Detecting Ionizing Radiation in Radiotherapy. Chemnanomat 2016, 2, 385–395. [Google Scholar] [CrossRef]
- Yu, G.; Gao, J.; Hummelen, J.C.; Wudl, F.; Heeger, A.J. Polymer photovoltaic cells: Enhanced efficiencies via a network of internal donor-acceptor heterojunctions. Science 1995, 270, 1789–1791. [Google Scholar] [CrossRef]
- Le Corre, V.M.; Chatri, A.R.; Doumon, N.Y.; Koster, L.J.A. Charge Carrier Extraction in Organic Solar Cells Governed by Steady-State Mobilities. Adv. Energy Mater. 2017, 7, 1701138. [Google Scholar] [CrossRef]
- Li, M.; Balawi, A.H.; Leenaers, P.J.; Ning, L.; Heintges, G.H.L.; Marszalek, T.; Pisula, W.; Wienk, M.M.; Meskers, S.C.J.; Yi, Y.; et al. Impact of polymorphism on the optoelectronic properties of a low-bandgap semiconducting polymer. Nat. Commun. 2019, 10, 1–11. [Google Scholar] [CrossRef]
- Brown, P.J.; Thomas, D.S.; Kohler, A.; Wilson, J.S.; Kim, J.S.; Ramsdale, C.M.; Sirringhaus, H.; Friend, R.H. Effect of interchain interactions on the absorption and emission of poly(3-hexylthiophene). Phys. Rev. B 2003, 67, 064203. [Google Scholar] [CrossRef]
- Hu, Z.; Tenery, D.; Bonner, M.S.; Gesquiere, A.J. Correlation between spectroscopic and morphological properties of composite P3 HT/PCBM nanoparticles studied by single particle spectroscopy. J. Lumin. 2010, 130, 771–780. [Google Scholar] [CrossRef]
- Kasha, M. Energy transfer mechanisms and the molecular exciton model for molecular aggregates. Radiat. Res. 1963, 20, 55–70. [Google Scholar] [CrossRef]
- Hochstrasser, R.M.; Kasha, M. Application of the exciton model to mono-molecular lamellar systems. Photochem. Photobiol. 1964, 3, 317–331. [Google Scholar] [CrossRef]
- Ananthakrishnan, N.; Padmanaban, G.; Ramakrishnan, S.; Reynolds, J.R. Tuning polymer light-emitting device emission colors in ternary blends composed of conjugated and nonconjugated polymers. Macromolecules 2005, 38, 7660–7669. [Google Scholar] [CrossRef]
- Granstrom, M.; Inganas, O. White light emission from a polymer blend light emitting diode. Appl. Phys. Lett. 1996, 68, 147–149. [Google Scholar] [CrossRef]
- He, G.F.; Li, Y.F.; Liu, J.; Yang, Y. Enhanced electroluminescence using polystyrene as a matrix. Appl. Phys. Lett. 2002, 80, 4247–4249. [Google Scholar] [CrossRef]
- Su, C.-Y.; Hua, C.-C. Aggregation properties of MEH-PPV/PMMA blends in solution and thin film. J. Polym. Res. 2017, 24, 12. [Google Scholar] [CrossRef]
- Bout, D.A.V.; Wai-Tak, Y.; Dehong, H.; Dian-Kui, F.; Swager, T.M.; Barbara, P.F. Discrete intensity jumps and intramolecular electronic energy transfer in the spectroscopy of single conjugated polymer molecules. Science 1997, 277, 1074–1077. [Google Scholar] [CrossRef]
- Huser, T.; Yan, M.; Rothberg, L.J. Single chain spectroscopy of conformational dependence of conjugated polymer photophysics. Proc. Natl. Acad. Sci. USA 2000, 97, 11187–11191. [Google Scholar] [CrossRef]
- Feist, F.A.; Tommaseo, G.; Basche, T. Observation of very narrow linewidths in the fluorescence excitation spectra of single conjugated polymer chains at 1.2 K. Phys. Rev. Lett. 2007, 98, 208301. [Google Scholar] [CrossRef]
- Feist, F.A.; Basche, T. Fluorescence excitation and emission spectroscopy on single MEH-PPV chains at low temperature. J. Phys. Chem. B 2008, 112, 9700–9708. [Google Scholar] [CrossRef]
- Lin, H.; Tian, Y.; Zapadka, K.; Persson, G.; Thomsson, D.; Mirzov, O.; Larsson, P.-O.; Widengren, J.; Scheblykin, I.G. Fate of Excitations in Conjugated Polymers: Single-Molecule Spectroscopy Reveals Nonemissive “Dark” Regions in MEH-PPV Individual Chains. Nano Lett. 2009, 9, 4456–4461. [Google Scholar] [CrossRef]
- Ostroverkhova, O. Organic Optoelectronic Materials: Mechanisms and Applications. Chem. Rev. 2016, 116, 13279–13412. [Google Scholar] [CrossRef] [PubMed]
- Ebihara, Y.; Vacha, M. Relating conformation and photophysics in single MEH-PPV chains. J. Phys. Chem. B 2008, 112, 12575–12578. [Google Scholar] [CrossRef]
- Lupton, J.M. Single-Molecule Spectroscopy for Plastic Electronics: Materials Analysis from the Bottom-Up. Adv. Mater. 2010, 22, 1689–1721. [Google Scholar] [CrossRef] [PubMed]
- Sartori, S.S.; De Feyter, S.; Hofkens, J.; Van der Auweraer, M.; De Schryver, F.; Brunner, K.; Hofstraat, J.W. Host matrix dependence on the photophysical properties of individual conjugated polymer chains. Macromolecules 2003, 36, 500–507. [Google Scholar] [CrossRef]
- Vogelsang, J.; Brazard, J.; Adachi, T.; Bolinger, J.C.; Barbara, P.F. Watching the Annealing Process One Polymer Chain at a Time. Angew. Chem. Int. Ed. 2011, 50, 2257–2261. [Google Scholar] [CrossRef] [PubMed]
- Lupton, J.M. Chromophores in Conjugated Polymers-All Straight? Chemphyschem 2012, 13, 901–907. [Google Scholar] [CrossRef]
- Hu, D.; Yu, J.; Wong, K.; Bagchi, B.; Rossky, P.J.; Barbara, P.F. Collapse of stiff conjugated polymers with chemical defects into ordered, cylindrical conformations. Nature 2000, 405, 1030–1033. [Google Scholar] [CrossRef]
- Mirzov, O.; Scheblykin, I.G. Photoluminescence spectra of a conjugated polymer: From films and solutions to single molecules. Phys. Chem. Chem. Phys. 2006, 8, 5569–5576. [Google Scholar] [CrossRef]
- Bolognesi, A.; Pasini, M. Semiconducting Polymers: Chemistry, Physics and Engineering, 2nd ed.; Wiley: Weinheim, Germany, 2006. [Google Scholar]
- Hu, X.; Shao, B.; Geberth, G.T.; Bout, D.A.V. Effects of molecular architecture on morphology and photophysics in conjudated polymers: From single molecules to bulk. Chem. Sci. 2018, 9, 1101–1111. [Google Scholar] [CrossRef]
- Ibnaouf, K.H. Optical and amplified spontaneous emission from an efficient conducting copolymer (PFO-co-MEH-PPV) in solution. J. Lumin. 2017, 192, 707–712. [Google Scholar] [CrossRef]
- Nguyen, T.Q.; Doan, V.; Schwartz, B.J. Conjugated polymer aggregates in solution: Control of interchain interactions. J. Chem. Phys. 1999, 110, 4068–4078. [Google Scholar] [CrossRef]
- Lin, H.; Hania, R.P.; Bloem, R.; Mirzov, O.; Thomsson, D.; Scheblykin, I.G. Single chain versus single aggregate spectroscopy of conjugated polymers. Where is the border? Phys. Chem. Chem. Phys. 2010, 12, 11770–11777. [Google Scholar] [CrossRef]
- Vogelsang, J.; Adachi, T.; Brazard, J.; Bout, D.A.V.; Barbara, P.F. Self-assembly of highly ordered conjugated polymer aggregates with long-range energy transfer. Nat. Mater. 2011, 10, 942–946. [Google Scholar] [CrossRef] [PubMed]
- Ou, J.; Yang, Y.; Lin, W.; Yuan, Z.; Gan, L.; Lin, X.; Chen, X.; Chen, Y. Connection between the conformation and emission properties of poly [2-methoxy-5-(2′-ethyl-hexyloxy)-1,4-phenylene vinylene] single molecules during thermal annealing. Appl. Phys. Lett. 2015, 106, 123304. [Google Scholar] [CrossRef]
- Kwon, Y.; Kaufman, L.J. Nearly Isotropic Conjugated Polymer Aggregates with Efficient Local Exciton Diffusion. J. Phys. Chem. C 2019, 123, 29418–29426. [Google Scholar] [CrossRef]
- Peteanu, L.A.; Sherwood, G.A.; Werner, J.H.; Shreve, A.P.; Smith, T.M. Visualizing Core-Shell Structure in Substituted PPV Oligomer Aggregates Using Fluorescence Lifetime Imaging Microscopy (FLIM). J. Phys. Chem. C 2011, 115, 15607–15616. [Google Scholar] [CrossRef]
- Sherwood, G.A.; Cheng, R.; Smith, T.M.; Werner, J.H.; Shreve, A.P.; Peteanu, L.A.; Wildeman, J. Aggregation Effects on the Emission Spectra and Dynamics of Model Oligomers of MEH-PPV. J. Phys. Chem. C 2009, 113, 18851–18862. [Google Scholar] [CrossRef]
- Nguyen, T.Q.; Wu, J.J.; Doan, V.; Schwartz, B.J.; Tolbert, S.H. Control of energy transfer in oriented conjugated polymer-mesoporous silica composites. Science 2000, 288, 652–656. [Google Scholar] [CrossRef]
- Chen, L.; McBranch, D.W.; Wang, H.L.; Helgeson, R.; Wudl, F.; Whitten, D.G. Highly sensitive biological and chemical sensors based on reversible fluorescence quenching in a conjugated polymer. Proc. Natl. Acad. Sci. USA 1999, 96, 12287–12292. [Google Scholar] [CrossRef]
- Wang, R.; Yang, X.; Hu, S.; Zhang, Y.; Yan, X.; Wang, Y.; Zhang, C.; Sheng, C. Effect of Thermal Annealing on Aggregations in MEH-PPV Films. J. Phys. Chem. C 2019, 123, 11055–11062. [Google Scholar] [CrossRef]
- Yamagata, H.; Spano, F.C. Interplay between intrachain and interchain interactions in semiconducting polymer assemblies: The HJ-aggregate model. J. Chem. Phys. 2012, 136, 184901. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Rothberg, L.J.; Kwock, E.W.; Miller, T.M. Interchain excitations in conjugated polymers. Phys. Rev. Lett. 1995, 75, 1992–1995. [Google Scholar] [CrossRef] [PubMed]
- Iyengar, N.A.; Harrison, B.; Duran, R.S.; Schanze, K.S.; Reynolds, J.R. Morphology evolution in nanoscale light-emitting domains in MEH-PPV/PMMA blends. Macromolecules 2003, 36, 8978–8985. [Google Scholar] [CrossRef]
- Kanemoto, K.; Imanaka, Y.; Akai, I.; Sugisaki, M.; Hashimoto, H.; Karasawa, T. Intrachain photoluminescence dynamics of MEH-PPV in the solid state. J. Phys. Chem. B 2007, 111, 12389–12394. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.Q.; Martini, I.B.; Liu, J.; Schwartz, B.J. Controlling interchain interactions in conjugated polymers: The effects of chain morphology on exciton-exciton annihilation and aggregation in MEH-PPV films. J. Phys. Chem. B 2000, 104, 237–255. [Google Scholar] [CrossRef]
- Eder, T.; Stangl, T.; Gmelch, M.; Remmerssen, K.; Laux, D.; Hoeger, S.; Lupton, J.M.; Vogelsang, J. Switching between H- and J-type electronic coupling in single conjugated polymer aggregates. Nat. Commun. 2017, 8, 1641. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Wang, F.; Niu, M.-S.; Zheng, F.; Bi, P.-Q.; Yang, X.-Y.; Xu, W.-L.; Hao, X.-T. Structural and optical properties of conjugated polymer and carbon-based non-fullerene material blend films for photovoltaic applications. Opt. Mater. Express 2017, 7, 687–697. [Google Scholar] [CrossRef]
- Kong, F.; Wu, X.L.; Huang, G.S.; Yuan, R.K.; Yang, C.Z.; Chu, P.K.; Siu, G.G. Temperature-dependent photoluminescence from MEH-PPV and MEH-OPPV containing oxadiazole in the main chain. Appl. Phys. A 2006, 84, 203–206. [Google Scholar] [CrossRef]
- Drori, T.; Sheng, C.X.; Ndobe, A.; Singh, S.; Holt, J.; Vardeny, Z.V. Below-gap excitation of pi-conjugated polymer-fullerene blends: Implications for bulk organic heterojunction solar cells. Phys. Rev. Lett. 2008, 101, 037401. [Google Scholar] [CrossRef]
- Drori, T.; Gershman, E.; Sheng, C.X.; Eichen, Y.; Vardeny, Z.V.; Ehrenfreund, E. Illumination-induced metastable polaron-supporting state in poly(p-phenylene vinylene) films. Phys. Rev. B 2007, 76, 033203. [Google Scholar] [CrossRef]
- Wei, X.; Vardeny, Z.V.; Sariciftci, N.; Heeger, A. Absorption-detected magnetic-resonance studies of photoexcitations in conjugated-polymer/C60 composites. Phys. Rev. B 1996, 53, 2187. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, R.; Li, Y.; Wang, Z.; Hu, S.; Yan, X.; Zhai, Y.; Zhang, C.; Sheng, C. Optical Properties of Two-Dimensional Perovskite Films of (C6H5C2H4NH3)2[PbI4] and (C6H5C2H4NH3)2 (CH3NH3)2[Pb3I10]. J. Phys. Chem. Lett. 2019, 10, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Feist, F.A.; Tommaseo, G.; Basché, T. Single-molecule spectroscopy of MEH-PPV polymer molecules in different host matrices. J. Phys. Chem. C 2009, 113, 11484–11490. [Google Scholar] [CrossRef]
- Nakamura, T.; Sharma, D.K.; Hirata, S.; Vacha, M. Intrachain aggregates as the origin of green emission in polyfluorene studied on ensemble and single-chain level. J. Phys. Chem. C 2018, 122, 8137–8146. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, S.; Liao, Y.; Zhang, Y.; Yan, X.; Zhao, Z.; Chen, W.; Zhang, X.; Liu, H.; Li, H.; Li, L.; et al. Effect of Thermal Annealing on Conformation of MEH-PPV Chains in Polymer Matrix: Coexistence of H- and J-Aggregates. Polymers 2020, 12, 1771. https://doi.org/10.3390/polym12081771
Hu S, Liao Y, Zhang Y, Yan X, Zhao Z, Chen W, Zhang X, Liu H, Li H, Li L, et al. Effect of Thermal Annealing on Conformation of MEH-PPV Chains in Polymer Matrix: Coexistence of H- and J-Aggregates. Polymers. 2020; 12(8):1771. https://doi.org/10.3390/polym12081771
Chicago/Turabian StyleHu, Shu, Yang Liao, Yang Zhang, Xiaoliang Yan, Zhenlu Zhao, Weiqiang Chen, Xin Zhang, Hongxing Liu, Heng Li, Li Li, and et al. 2020. "Effect of Thermal Annealing on Conformation of MEH-PPV Chains in Polymer Matrix: Coexistence of H- and J-Aggregates" Polymers 12, no. 8: 1771. https://doi.org/10.3390/polym12081771
APA StyleHu, S., Liao, Y., Zhang, Y., Yan, X., Zhao, Z., Chen, W., Zhang, X., Liu, H., Li, H., Li, L., Sun, M., & Sheng, C. (2020). Effect of Thermal Annealing on Conformation of MEH-PPV Chains in Polymer Matrix: Coexistence of H- and J-Aggregates. Polymers, 12(8), 1771. https://doi.org/10.3390/polym12081771




