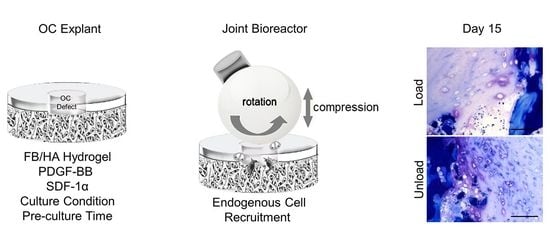
Mechanical Stress Inhibits Early Stages of Endogenous Cell Migration: A Pilot Study in an Ex Vivo Osteochondral Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Osteochondral Tissue Harvest and Culture
2.2. Fibrin-HA Hydrogel Preparation and Incorporation of PDGF-BB or SDF1α
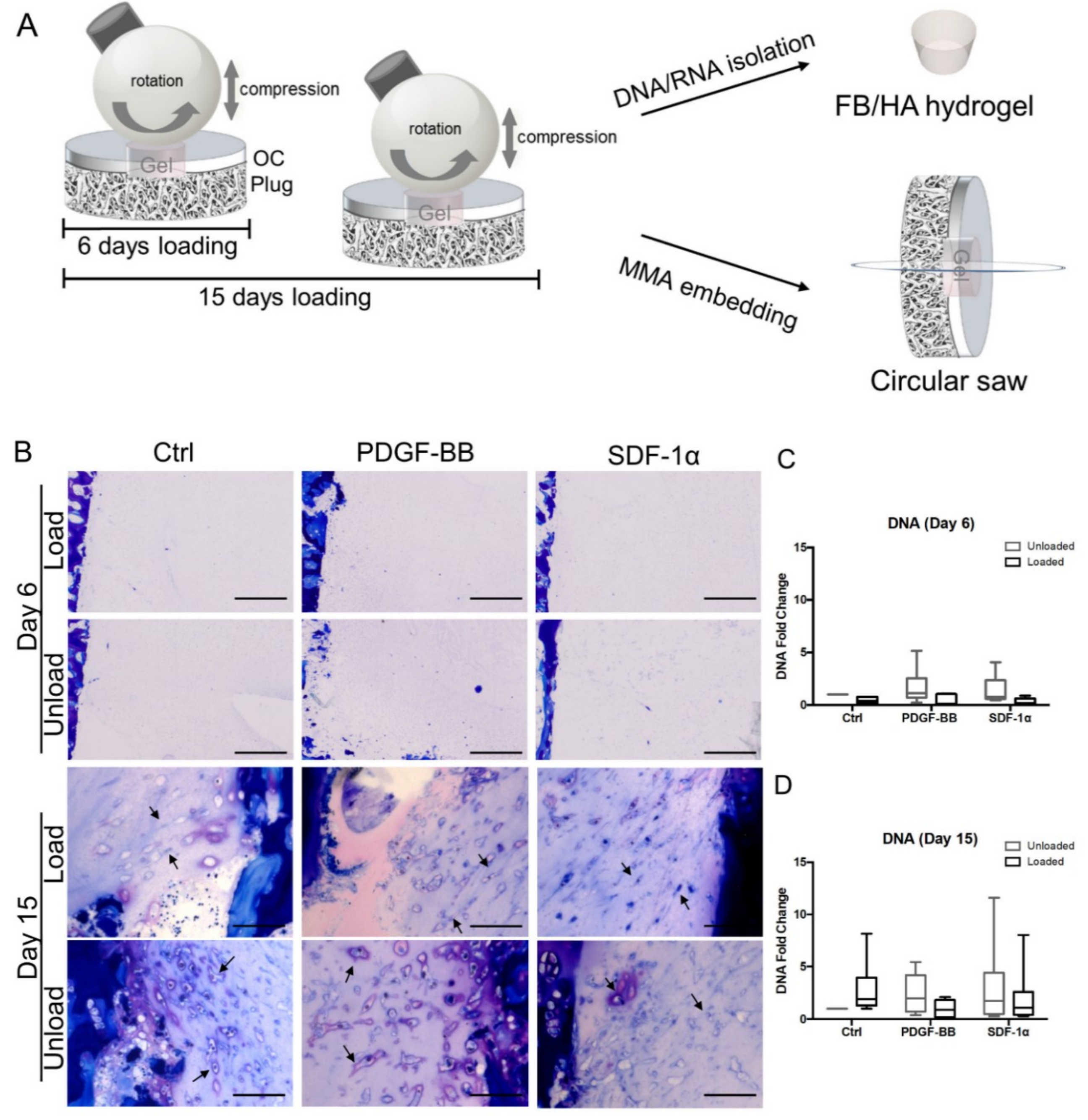
2.3. Ex Vivo Osteochondral Defect Model for Endogenous Cell Recruitment under Mechanical Loading
2.4. Histology
2.5. RNA Extraction and Gene Expression Analysis
2.6. DNA Content Measurement
2.7. Statistical Analysis
3. Results
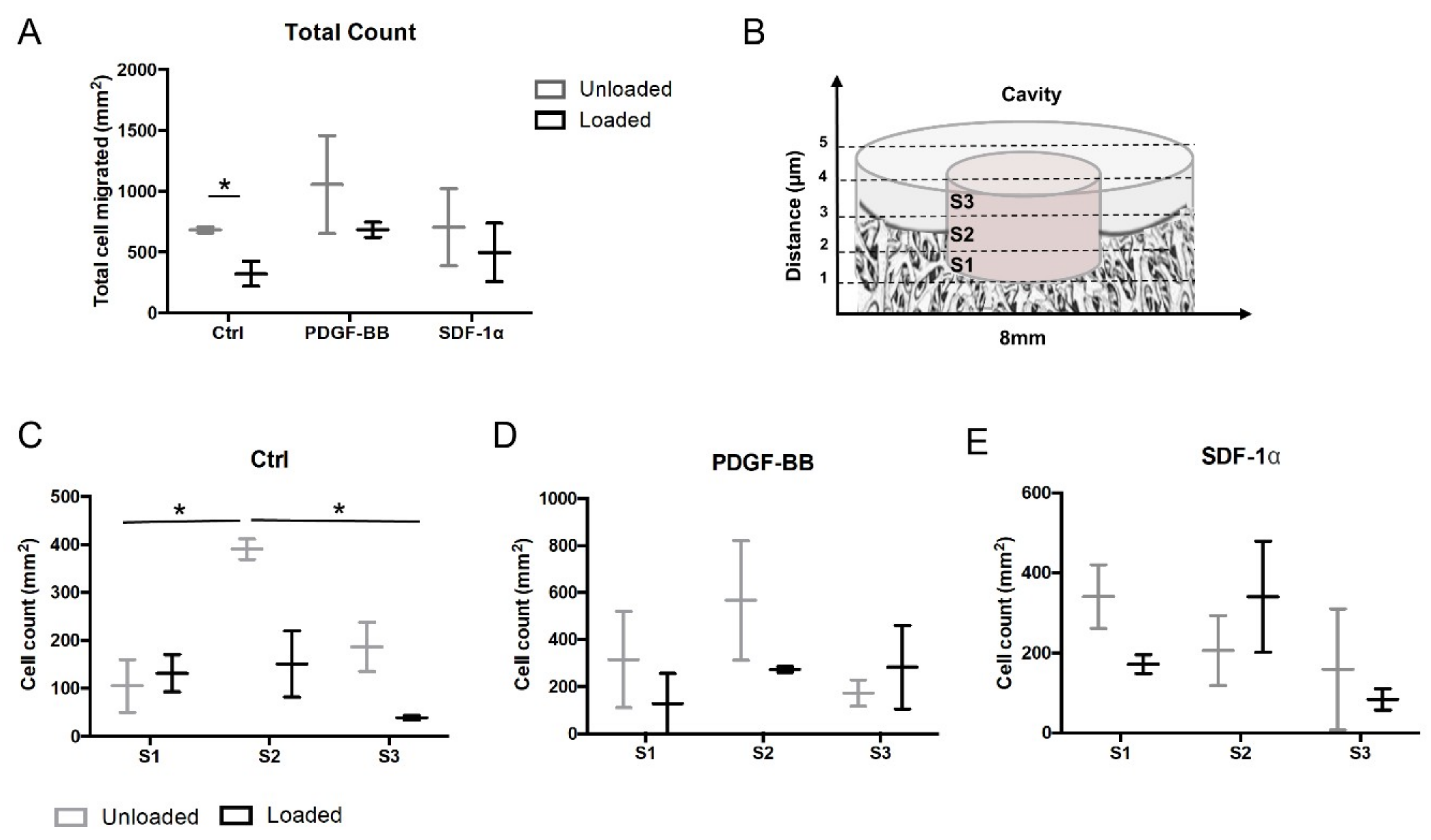
3.1. Mechanical Stimuli Affect Early Cell Migration in an Ex Vivo Osteochondral Culture Model
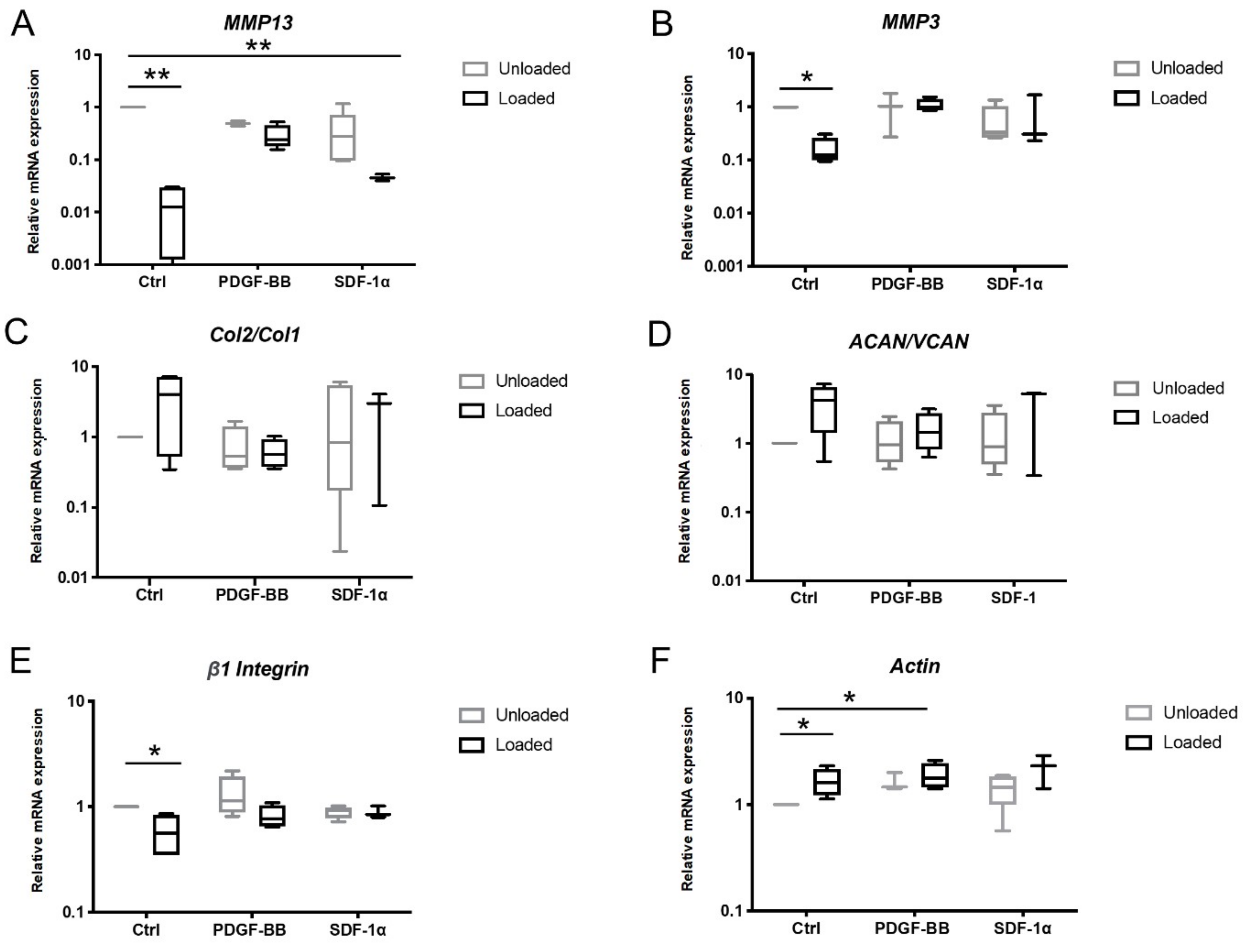
3.2. Biophysical and Biochemical Cues Influence Gene Expression within the Osteochondral Defect at Early Time Point
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Smith, G.D.; Knutsen, G.; Richardson, J.B. A clinical review of cartilage repair techniques. J. Bone Jt. Surg. Br. Vol. 2005, 87, 445–449. [Google Scholar] [CrossRef]
- Kreuz, P.C.; Steinwachs, M.; Erggelet, C.; Krause, S.; Konrad, G.; Uhl, M.; Südkamp, N. Results after microfracture of full-thickness chondral defects in different compartments in the knee. Osteoarthr. Cartil. 2006, 14, 1119–1125. [Google Scholar] [CrossRef]
- Gracitelli, G.C.; Meric, G.; Pulido, P.A.; McCauley, J.C.; Bugbee, W.D. Osteochondral Allograft Transplantation for Knee Lesions after Failure of Cartilage Repair Surgery. Cartilage 2015, 6, 98–105. [Google Scholar] [CrossRef]
- Brittberg, M.; Lindahl, A.; Nilsson, A.; Ohlsson, C.; Isaksson, O.; Peterson, L. Treatment of Deep Cartilage Defects in the Knee with Autologous Chondrocyte Transplantation. N. Engl. J. Med. 1994, 331, 889–895. [Google Scholar] [CrossRef]
- Gille, J.; Behrens, P.; Schulz, A.P.; Oheim, R.; Kienast, B. Matrix-Associated Autologous Chondrocyte Implantation. Cartilage 2016, 7, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Jo, C.H.; Gil Lee, Y.; Shin, W.H.; Kim, H.; Chai, J.W.; Jeong, E.; Kim, J.E.; Shim, H.; Shin, J.S.; Shin, I.S.; et al. Intra-Articular Injection of Mesenchymal Stem Cells for the Treatment of Osteoarthritis of the Knee: A Proof-of-Concept Clinical Trial. STEM CELLS 2014, 32, 1254–1266. [Google Scholar] [CrossRef]
- Gobbi, A.; Chaurasia, S.; Karnatzikos, G.; Nakamura, N. Matrix-Induced Autologous Chondrocyte Implantation versus Multipotent Stem Cells for the Treatment of Large Patellofemoral Chondral Lesions: A Nonrandomized Prospective Trial. Cartilage 2015, 6, 82–97. [Google Scholar] [CrossRef] [PubMed]
- Nejadnik, H.; Hui, J.H.P.; Choong, E.P.F.; Tai, B.-C.; Lee, E.H. Autologous Bone Marrow-Derived Mesenchymal Stem Cells Versus Autologous Chondrocyte Implantation. Am. J. Sports Med. 2010, 38, 1110–1116. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Luo, J. Thoughts on Chemistry, Manufacturing and Control of Cell Therapy Products for Clinical Application. Hum. Gene Ther. 2018. [Google Scholar] [CrossRef]
- Benthien, J.P.; Behrens, P. Autologous Matrix-Induced Chondrogenesis (AMIC). Cartilage 2010, 1, 65–68. [Google Scholar] [CrossRef]
- Fossum, V.; Hansen, A.K.; Wilsgaard, T.; Knutsen, G. Collagen-Covered Autologous Chondrocyte Implantation Versus Autologous Matrix-Induced Chondrogenesis: A Randomized Trial Comparing 2 Methods for Repair of Cartilage Defects of the Knee. Orthop. J. Sports Med. 2019, 7. [Google Scholar] [CrossRef] [PubMed]
- Qu, F.; Guilak, F.; Mauck, R.L. Cell migration: Implications for repair and regeneration in joint disease. Nat. Rev. Rheumatol. 2019, 15, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Sundararaghavan, H.G.; Burdick, J.A. Gradients with Depth in Electrospun Fibrous Scaffolds for Directed Cell Behavior. Biomacromolecules 2011, 12, 2344–2350. [Google Scholar] [CrossRef] [PubMed]
- Loebel, C.; Szczesny, S.E.; Cosgrove, B.D.; Alini, M.; Zenobi-Wong, M.; Mauck, R.L.; Eglin, D. Cross-Linking Chemistry of Tyramine-Modified Hyaluronan Hydrogels Alters Mesenchymal Stem Cell Early Attachment and Behavior. Biomacromolecules 2017, 18, 855–864. [Google Scholar] [CrossRef]
- Yu, Y.; Brouillette, M.J.; Seol, D.; Zheng, H.; Buckwalter, J.A.; Martin, J.A. Use of Recombinant Human Stromal Cell-Derived Factor 1? Loaded Fibrin/Hyaluronic Acid Hydrogel Networks to Achieve Functional Repair of Full-Thickness Bovine Articular Cartilage Via Homing of Chondrogenic Progenitor Cells. Arthritis Rheumatol. 2015, 67, 1274–1285. [Google Scholar] [CrossRef]
- Vainieri, M.; Lolli, A.; Kops, N.; D’Atri, D.; Eglin, D.; Yayon, A.; Alini, M.; Grad, S.; Sivasubramaniyan, K.; Van Osch, G.J. Evaluation of biomimetic hyaluronic-based hydrogels with enhanced endogenous cell recruitment and cartilage matrix formation. Acta Biomater. 2019, 101, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Lolli, A.; Sivasubramaniyan, K.; Vainieri, M.L.; Oieni, J.; Kops, N.; Yayon, A.; Van Osch, G.J. Hydrogel-based delivery of antimiR-221 enhances cartilage regeneration by endogenous cells. J. Control. Release 2019, 309, 220–230. [Google Scholar] [CrossRef]
- Salinas, C.N.; Anseth, K.S. The enhancement of chondrogenic differentiation of human mesenchymal stem cells by enzymatically regulated RGD functionalities. Biomaterials 2008, 29, 2370–2377. [Google Scholar] [CrossRef]
- Janssen, L.M.; Der Maur, C.D.I.; Bos, P.K.; Hardillo, J.A.; Van Osch, G.J.V.M. Short-Duration Enzymatic Treatment Promotes Integration of a Cartilage Graft in a Defect. Ann. Otol. Rhinol. Laryngol. 2006, 115, 461–468. [Google Scholar] [CrossRef]
- Grad, S.; Loparic, M.; Peter, R.; Stolz, M.; Aebi, U.; Alini, M. Sliding motion modulates stiffness and friction coefficient at the surface of tissue engineered cartilage. Osteoarthr. Cartil. 2012, 20, 288–295. [Google Scholar] [CrossRef]
- Thorpe, S.D.; Nagel, T.; Carroll, S.F.; Kelly, D.J. Modulating Gradients in Regulatory Signals within Mesenchymal Stem Cell Seeded Hydrogels: A Novel Strategy to Engineer Zonal Articular Cartilage. PLoS ONE 2013, 8, e60764. [Google Scholar] [CrossRef] [PubMed]
- Schätti, O.; Grad, S.; Goldhahn, J.; Salzmann, G.; Li, Z.; Alini, M.; Stoddart, M.J. A combination of shear and dynamic compression leads to mechanically induced chondrogenesis of human mesenchymal stem cells. Eur. Cells Mater. 2011, 22, 214–225. [Google Scholar] [CrossRef] [PubMed]
- Fahy, N.; Alini, M.; Stoddart, M.J. Mechanical stimulation of mesenchymal stem cells: Implications for cartilage tissue engineering. J. Orthop. Res. 2017, 36. [Google Scholar] [CrossRef] [PubMed]
- O’Conor, C.J.; Case, N.; Guilak, F. Mechanical regulation of chondrogenesis. Stem Cell Res. Ther. 2013, 4, 61. [Google Scholar] [CrossRef] [PubMed]
- Glatt, V.; Evans, C.H.; Stoddart, M.J. Regenerative rehabilitation: The role of mechanotransduction in orthopaedic regenerative medicine. J. Orthop. Res. 2019, 37, 1263–1269. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Guo, S.S.; Fässler, R. Integrin-mediated mechanotransduction. J. Cell Boil. 2016, 215, 445–456. [Google Scholar] [CrossRef]
- Cui, Y.; Hameed, F.M.; Yang, B.; Lee, K.; Pan, C.Q.; Park, S.; Sheetz, M. Cyclic stretching of soft substrates induces spreading and growth. Nat. Commun. 2015, 6, 1–8. [Google Scholar] [CrossRef]
- Aragona, M.; Panciera, T.; Manfrin, A.; Giulitti, S.; Michielin, F.; Elvassore, N.; Dupont, S.; Piccolo, S. A Mechanical Checkpoint Controls Multicellular Growth through YAP/TAZ Regulation by Actin-Processing Factors. Cell 2013, 154, 1047–1059. [Google Scholar] [CrossRef]
- Huelsz-Prince, G.; Belkin, A.M.; Van Bavel, E.; Bakker, E.N. Activation of Extracellular Transglutaminase 2 by Mechanical Force in the Arterial Wall. J. Vasc. Res. 2013, 50, 383–395. [Google Scholar] [CrossRef]
- Chiquet-Ehrismann, R.; Tannheimer, M.; Koch, M.; Brunner, A.; Spring, J.; Martin, D.; Baumgärtner, S.; Chiquet, M. Tenascin-C expression by fibroblasts is elevated in stressed collagen gels. J. Cell Boil. 1994, 127, 2093–2101. [Google Scholar] [CrossRef]
- Chiquet-Ehrismann, R.; Chiquet, M. Tenascins: Regulation and putative functions during pathological stress. J. Pathol. 2003, 200, 488–499. [Google Scholar] [CrossRef] [PubMed]
- Chang, N.-J.; Lin, C.-C.; Shie, M.-Y.; Yeh, M.-L.; Li, C.-F.; Liang, P.-I.; Lee, K.-W.; Shen, P.-H.; Chu, C.-J. Positive effects of cell-free porous PLGA implants and early loading exercise on hyaline cartilage regeneration in rabbits. Acta Biomater. 2015, 28, 128–137. [Google Scholar] [CrossRef]
- Im, G.-I. Endogenous Cartilage Repair by Recruitment of Stem Cells. Tissue Eng. Part B Rev. 2016, 22, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Geçkil, H.; Xu, F.; Zhang, X.; Moon, S.; Demirci, U. Engineering hydrogels as extracellular matrix mimics. Nanomedicine 2010, 5, 469–484. [Google Scholar] [CrossRef]
- Antunes, B.P.; Vainieri, M.L.; Alini, M.; Monsonego-Ornan, E.; Grad, S.; Yayon, A. Enhanced chondrogenic phenotype of primary bovine articular chondrocytes in Fibrin-Hyaluronan hydrogel by multi-axial mechanical loading and FGF18. Acta Biomater. 2020, 105, 170–179. [Google Scholar] [CrossRef]
- Vainieri, M.; Wahl, D.; Alini, M.; Van Osch, G.; Grad, S. Mechanically stimulated osteochondral organ culture for evaluation of biomaterials in cartilage repair studies. Acta Biomater. 2018, 81, 256–266. [Google Scholar] [CrossRef] [PubMed]
- Melle, M.L.D.V.-V.; Mandl, E.W.; Kops, N.; Koevoet, W.J.; Verhaar, J.A.N.; Van Osch, G.J. An Osteochondral Culture Model to Study Mechanisms Involved in Articular Cartilage Repair. Tissue Eng. Part C: Methods 2012, 18, 45–53. [Google Scholar] [CrossRef]
- Li, Z.; Kaplan, K.M.; Wertzel, A.; Peroglio, M.; Amit, B.; Alini, M.; Grad, S.; Yayon, A. Biomimetic fibrin–hyaluronan hydrogels for nucleus pulposus regeneration. Regen. Med. 2014, 9, 309–326. [Google Scholar] [CrossRef]
- Pereira, C.L.; Teixeira, G.Q.; Ferreira, J.R.; D’Este, M.; Eglin, D.; Alini, M.; Grad, S.; Barbosa, M.; Gonçalves, R.; Alini, M. Stromal Cell Derived Factor-1-Mediated Migration of Mesenchymal Stem Cells Enhances Collagen Type II Expression in Intervertebral Disc. Tissue Eng. Part A 2018, 24, 1818–1830. [Google Scholar] [CrossRef]
- Wimmer, M.A.; Grad, S.; Kaup, T.; Hanni, M.; Schneider, E.; Gogolewski, S.; Alini, M. Tribology approach to the engineering and study of articular cartilage. Tissue Eng. 2004, 10, 1436–1445. [Google Scholar] [CrossRef]
- Lee, C.R.; Grad, S.; MacLean, J.J.; Iatridis, J.C.; Alini, M. Effect of mechanical loading on mRNA levels of common endogenous controls in articular chondrocytes and intervertebral disk. Anal. Biochem. 2005, 341, 372–375. [Google Scholar] [CrossRef] [PubMed]
- Iseki, T.; Rothrauff, B.B.; Kihara, S.; Sasaki, H.; Yoshiya, S.; Fu, F.H.; Tuan, R.S.; Gottardi, R. Dynamic Compressive Loading Improves Cartilage Repair in an In Vitro Model of Microfracture: Comparison of 2 Mechanical Loading Regimens on Simulated Microfracture Based on Fibrin Gel Scaffolds Encapsulating Connective Tissue Progenitor Cells. Am. J. Sports Med. 2019, 47, 2188–2199. [Google Scholar] [CrossRef] [PubMed]
- Vukasovic, A.; Asnaghi, M.A.; Kostesic, P.; Quasnichka, H.; Cozzolino, C.; Pusic, M.; Hails, L.; Trainor, N.; Krause, C.; Figallo, E.; et al. Bioreactor-manufactured cartilage grafts repair acute and chronic osteochondral defects in large animal studies. Cell Prolif. 2019, 52, e12653. [Google Scholar] [CrossRef] [PubMed]
- Mueller-Rath, R.; Gavenis, K.; Gravius, S.; Andereya, S.; Mumme, T.; Schneider, U. In Vivo cultivation of human articular chondrocytes in a nude mouse-based contained defect organ culture model. Bio-Med. Mater. Eng. 2007, 17, 357–366. [Google Scholar]
- Schuller, G.C.; Tichy, B.; Majdisova, Z.; Jagersberger, T.; van Griensven, M.; Marlovits, S.; Redl, H. An In Vivo mouse model for human cartilage regeneration. J. Tissue Eng. Regen. Med. 2008, 2, 202–209. [Google Scholar] [CrossRef]
- Friedl, P.; Wolf, K. Plasticity of cell migration: A multiscale tuning model. J. Exp. Med. 2010, 207. [Google Scholar] [CrossRef]
- Takao, S.; Taya, M.; Chiew, C. Mechanical stress-induced cell death in breast cancer cells. Boil. Open 2019, 8, bio043133. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, X.; Lv, J.; Zeng, L.; Wei, X.; Wei, L. Stromal Cell-Derived Factor-1 Accelerates Cartilage Defect Repairing by Recruiting Bone Marrow Mesenchymal Stem Cells and Promoting Chondrogenic Differentiation*. Tissue Eng. Part A 2017, 23, 1160–1168. [Google Scholar] [CrossRef]
- Lee, C.H.; Cook, J.L.; Mendelson, A.; Moioli, E.K.; Yao, H.; Mao, J.J. Regeneration of the articular surface of the rabbit synovial joint by cell homing: A proof of concept study. Lancet 2010, 376, 440–448. [Google Scholar] [CrossRef]
- Zhang, W.; Lian, Q.; Li, D.; Wang, K.; Hao, D.; Bian, W.; He, J.; Jin, Z. Cartilage Repair and Subchondral Bone Migration Using 3D Printing Osteochondral Composites: A One-Year-Period Study in Rabbit Trochlea. BioMed Res. Int. 2014, 2014, 1–16. [Google Scholar] [CrossRef]
- Madry, H.; Cucchiarini, M. Signalling pathways in osteochondral defect regeneration. In A Tissue Regeneration Approach to Bone and Cartilage Repai; Zreiqat, H., Dunstan, C.R., Rosen, V., Eds.; Springer: Cham, Switzerland, 2014; pp. 219–228. [Google Scholar]
- Kimpton, L.; Schwab, A.; Ehlicke, F.; Waters, S.; Please, C.; Whiteley, J.; Byrne, H. A mathematical model for cell infiltration and proliferation in a chondral defect. Math. Biosci. 2017, 292, 46–56. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, A.; Kelly, D.J. Unravelling the Role of Mechanical Stimuli in Regulating Cell Fate During Osteochondral Defect Repair. Ann. Biomed. Eng. 2016, 44, 3446–3459. [Google Scholar] [CrossRef] [PubMed]
- Morales, T.I. Chondrocyte moves: Clever strategies? Osteoarthritis and cartilage/OARS. Osteoarthr. Res. Soc. 2007, 15, 861–871. [Google Scholar] [CrossRef]
- Kirilak, Y.; Pavlos, N.J.; Willers, C.; Han, R.; Feng, H.; Xu, J.; Asokananthan, N.; Stewart, G.; Henry, P.; Wood, D.; et al. Fibrin sealant promotes migration and proliferation of human articular chondrocytes: Possible involvement of thrombin and protease-activated receptors. Int. J. Mol. Med. 2006, 17. [Google Scholar] [CrossRef]
- Chubinskaya, S.; Di Matteo, B.; Lovato, L.; Iacono, F.; Robinson, D.; Kon, E. Agili-C implant promotes the regenerative capacity of articular cartilage defects in an ex vivo model. Knee Surg. Sports Traumatol. Arthrosc. 2018, 27, 1953–1964. [Google Scholar] [CrossRef]
- Orth, P.; Cucchiarini, M.; Kaul, G.; Ong, M.; Gräber, S.; Kohn, D.; Madry, H. Temporal and spatial migration pattern of the subchondral bone plate in a rabbit osteochondral defect model. Osteoarthr. Cartil. 2012, 20, 1161–1169. [Google Scholar] [CrossRef]
- Grad, S.; Gogolewski, S.; Alini, M.; Wimmer, M.A. Effects of simple and complex motion patterns on gene expression of chondrocytes seeded in 3D scaffolds. Tissue Eng. 2006, 12, 3171–3179. [Google Scholar] [CrossRef]
- Martin, I.; Jakob, M.; Schäfer, D.; Dick, W.; Spagnoli, G.; Heberer, M. Quantitative analysis of gene expression in human articular cartilage from normal and osteoarthritic joints. Osteoarthr. Cartil. 2001, 9, 112–118. [Google Scholar] [CrossRef]
- Kechagia, J.Z.; Ivaska, J.; Roca-Cusachs, P. Integrins as biomechanical sensors of the microenvironment. Nat. Rev. Mol. Cell Boil. 2019, 20, 457–473. [Google Scholar] [CrossRef]
- Paluch, E.; Nelson, C.M.; Biais, N.; Fabry, B.; Moeller, J.; Pruitt, B.L.; Wollnik, C.; Kudryasheva, G.; Rehfeldt, F.; Federle, W. Mechanotransduction: Use the force(s). BMC Boil. 2015, 13, 47. [Google Scholar] [CrossRef]
- Taraballi, F.; Bauza, G.; McCulloch, P.; Harris, J.; Tasciotti, E. Concise Review: Biomimetic Functionalization of Biomaterials to Stimulate the Endogenous Healing Process of Cartilage and Bone Tissue. STEM CELLS Transl. Med. 2017, 6, 2186–2196. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Grad, S.; Stoddart, M.J.; Niemeyer, P.; Südkamp, N.P.; Pestka, J.; Alini, M.; Chen, J.; Salzmann, G.M. Bioreactor-Induced Chondrocyte Maturation Is Dependent on Cell Passage and Onset of Loading. Cartilage 2013, 4, 165–176. [Google Scholar] [CrossRef] [PubMed]
| Gene | Sequence or Cat. nr. | |
|---|---|---|
| COL1A2 | Primer forward (5′-3′) | TGC AGT AAC TTC GTG CCT AGC A |
| Primer reverse (5′-3′) | CGC GTG GTC CTC TAT CTC CA | |
| Probe (5′FAM- 3′TAMRA) | CAT GCC AAT CCT TAC AAG AGG CAA CTG C | |
| COL2A1 | Primer forward (5′-3′) | AAG AAA CAC ATC TGG TTT GGA GAA A |
| Primer reverse (5′- 3′) | TGG GAG CCA GGT TGT CAT C | |
| Probe (5′FAM- 3′TAMRA) | CAA CGG TGG CTT CCA CTT CAG CTA TGG | |
| ACAN | Primer forward (5′-3′) | CCA ACG AAA CCT ATG ACG TGT ACT |
| Primer reverse (5′- 3′) | GCA CTC GTT GGC TGC CTC | |
| Probe (5′FAM- 3′TAMRA) | ATG TTG CAT AGA AGA CCT CGC CCT CCA T | |
| MMP-3 | Primer forward (5′-3′) | GGC TGC AAG GGA CAA GGA A |
| Primer reverse (5′-3′) | CAA ACT GTT TCG TAT CCT TTG CAA | |
| Probe (5′FAM- 3′TAMRA) | CAC CAT GGA GCT TGT TCA GCA ATA TCT AGA AAA C | |
| MMP-13 | Primer forward (5′-3′) | CCA TCT ACA CCT ACA CTG GCA AAA G |
| Primer reverse (5′-3′) | GTC TGG CGT TTT GGG ATG TT | |
| Probe (5′FAM-3′TAMRA) | TCT CTC TAT GGT CCA GGA GAT GAA GAC CCC | |
| VCAN | Cat. nr. | Bt03217632_m1 |
| TFB1M | Cat. nr. | Bt03269747_m1 |
| ACTB | Cat. nr. | Bt03279174_g1 |
| RPLP0 | Cat. nr. | Bt03218086_m1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vainieri, M.L.; Alini, M.; Yayon, A.; van Osch, G.J.V.M.; Grad, S. Mechanical Stress Inhibits Early Stages of Endogenous Cell Migration: A Pilot Study in an Ex Vivo Osteochondral Model. Polymers 2020, 12, 1754. https://doi.org/10.3390/polym12081754
Vainieri ML, Alini M, Yayon A, van Osch GJVM, Grad S. Mechanical Stress Inhibits Early Stages of Endogenous Cell Migration: A Pilot Study in an Ex Vivo Osteochondral Model. Polymers. 2020; 12(8):1754. https://doi.org/10.3390/polym12081754
Chicago/Turabian StyleVainieri, Maria L., Mauro Alini, Avner Yayon, Gerjo J. V. M. van Osch, and Sibylle Grad. 2020. "Mechanical Stress Inhibits Early Stages of Endogenous Cell Migration: A Pilot Study in an Ex Vivo Osteochondral Model" Polymers 12, no. 8: 1754. https://doi.org/10.3390/polym12081754
APA StyleVainieri, M. L., Alini, M., Yayon, A., van Osch, G. J. V. M., & Grad, S. (2020). Mechanical Stress Inhibits Early Stages of Endogenous Cell Migration: A Pilot Study in an Ex Vivo Osteochondral Model. Polymers, 12(8), 1754. https://doi.org/10.3390/polym12081754