3.1. Co-Pyrolysis of the Main Polymeric Components Based on TG Experiments
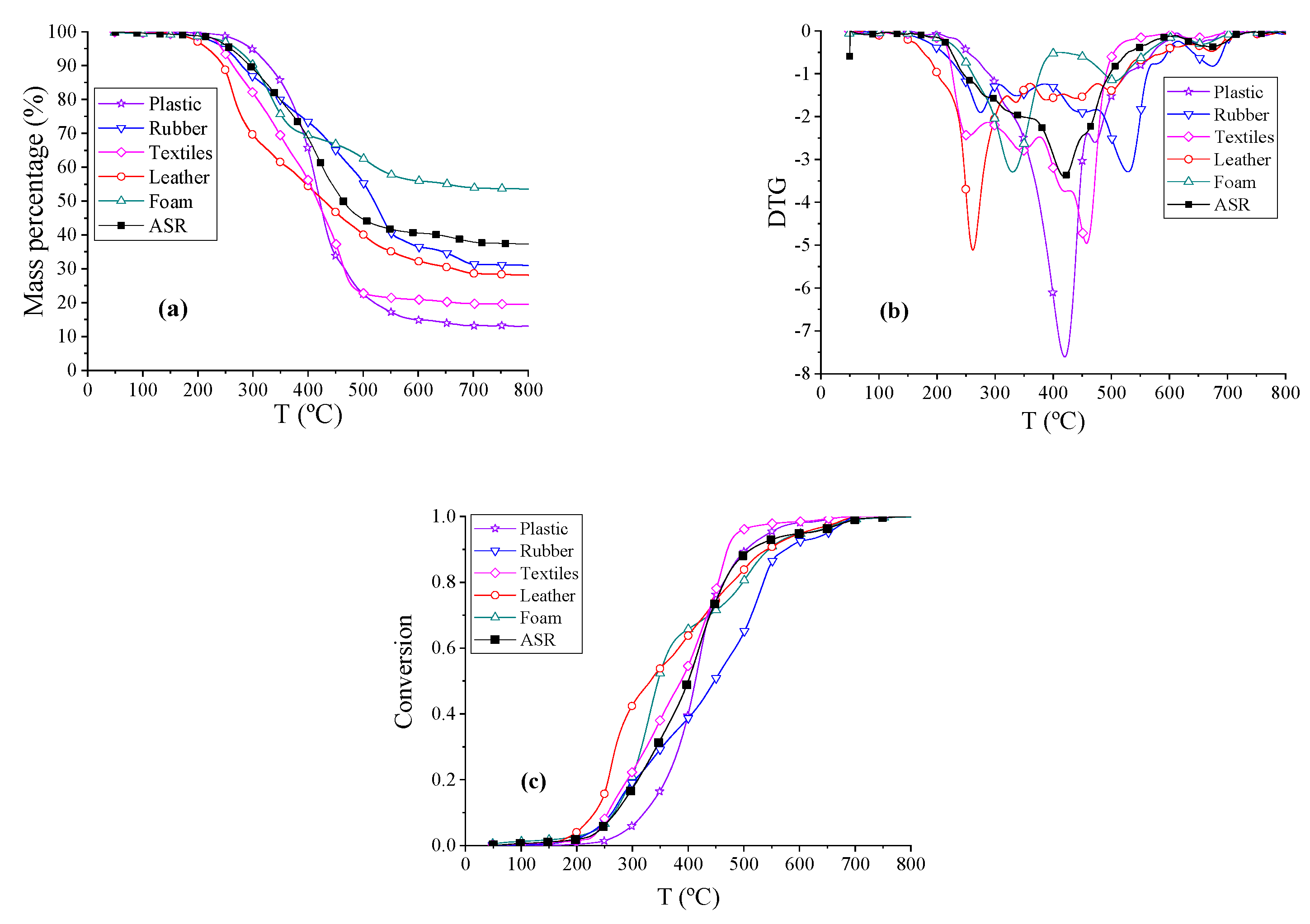
The thermogravimetry (TG) analysis curves, derivative thermogravimetry analysis (DTG) curves of ASR, and the pyrolysis of the five main components at a constant heating rate of 10 °C/min under a nitrogen atmosphere are shown in
Figure 2a,b. Conversion (α) vs. temperature (T) “reaction profiles” for ASR and the main components of ASR were calculated with the TG data and the results are shown in
Figure 2c. From these curves, we found that DTG curves of textiles, leather, rubber, and foam have two obvious peaks, whereas the DTG curves of plastic and ASR feature just one peak. There are more volatile products in the pyrolysis of plastics and textiles, but more solid products in the pyrolysis of foam and rubber. Let
Td,
Tf, and
rꝏ represent the starting temperature, the finishing temperature and the total weight loss rates, respectively. We defined the pyrolysis as the beginning when conversion α = 0.1 and the finish when α = 0.95, thus, non-isothermal pyrolysis yielding an 85% weight loss starts at temperature around
Td and finishes at temperature around
Tf. The total weight loss rates
rꝏ, and the DTG peak temperatures,
Td and
Tf, for the thermal decomposition processes are presented in
Table 3. Combining
Figure 2a–c, the weight percentages of samples fell quickly in the temperature range from 255 to 535 °C. The ranges of the pyrolysis temperature for actual ASR, MixASR, P50R50, P50L50, P50T50, and P50F50 were similar from 50 to 600 °C. Violent pyrolysis reactions of leather and textiles occurred at lower temperatures. The pyrolysis of ASR was not a simple superposition of the components, so it was necessary to analyze the interactions between the components in the pyrolysis process.
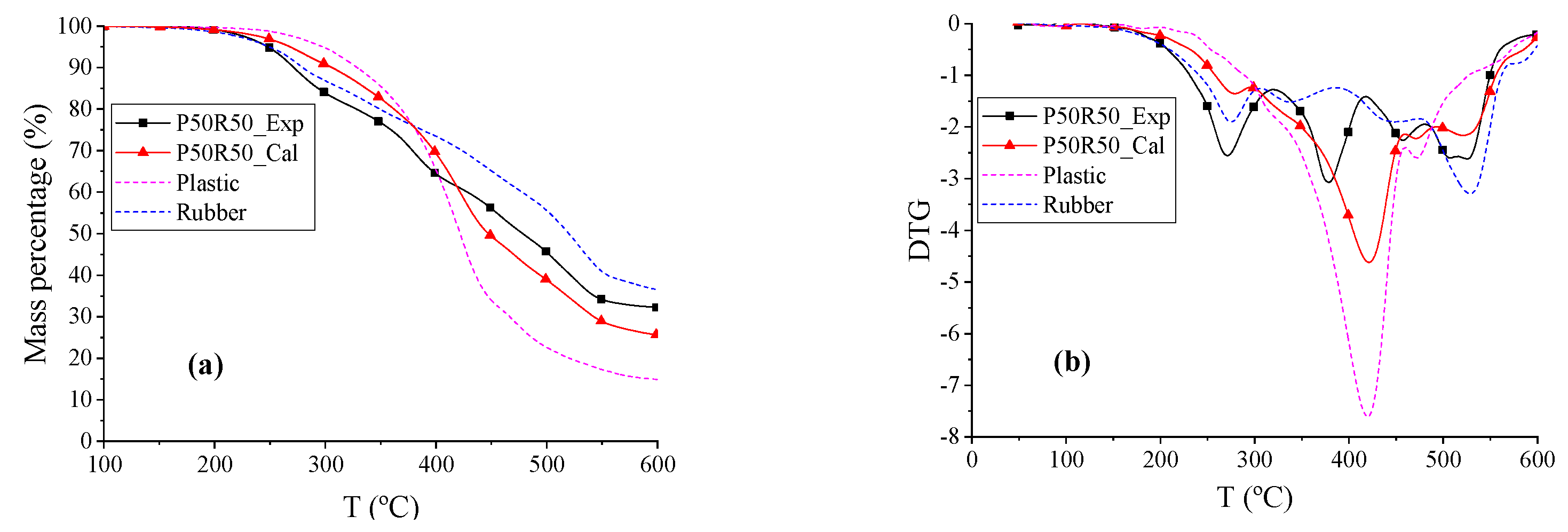
The TG and DTG curves of plastic (P), rubber (R), and P50R50 are shown in
Figure 3. The beginning reaction temperature
Td of the blend P50R50 was 5.26 °C lower than R and 63.48 °C lower than P. The rubber radicals are hypothesized to catalyze the formation of radicals in plastic samples; this causes a positive synergistic effect in the system until the entire rubber sample is expected to pyrolyze completely [
48]. The DTG curve of P50R50_Exp had three peaks, where the first peak and the third peak were mainly due to rubber pyrolysis, and the second peak was led by plastic pyrolysis. Within temperature ranges from 258.16 to 418.86 °C, the weight loss rate of P50R50_Exp was higher than P50R50_Cal, whereas the exact opposite was observed in temperature ranges from 418.86 to 600 °C. As can be seen from the temperature changes, rubber and plastics reduce each other’s pyrolysis temperature in co-pyrolysis process. However, the total weight loss rates
rꝏ of P50R50_Exp were 6.55% higher than P50R50_Cal; thus, more solid phases were formed. The main reason for large amount of char is that rubber contains more inorganic impurities and black carbon, where a high ash content leads to more solid products [
35]. This char of rubber pyrolysis hinders the pyrolysis of the plastic, and the volatiles formed are suspected to deposit in the char, which causes a negative synergy in the system [
48,
49]. The experimental curves do not fit well with the calculated curves; thus, the co-pyrolysis of plastic and rubber cannot be simply analyzed via a parallel superposition model.
The TG and DTG curves of plastics (P), textiles (T), and P50T50 are shown in
Figure 4. The beginning reaction temperature Td of P50T50 was 78.74 °C higher than T, and 13.15 °C higher than P. The DTG peak temperatures and Tf of P, P50T50_Exp, and P50T50_Cal were similar, and the shape and overall trend of the P50T50_Exp and P50T50_Cal curves were also similar to that of P. Within temperature ranges from 248.95 to 385.37 °C, the weight loss rate of P50T50_Exp was lower than P50T50_Cal, whereas the exact opposite was observed in temperature ranges from 385.37 to 600 °C. When the temperature was below 385.37 °C, both textiles and plastics featured slow pyrolysis reactions. According to shape of the TG/DTG curves, the co-pyrolysis of plastic and textiles is dominated by plastic and produces more volatile products.
The TG and DTG curves of plastic (P), leather (L), and P50L50 are shown in
Figure 5. The beginning reaction temperature
Td of the blend P50T50 was 27.47 °C higher than L and 61.88 °C lower than P. The experimental curves fit well with the calculated curves via the parallel reaction model. The DTG curve of blend P50L50 has two peaks. The temperature of the first peak is similar to the peak temperature of L, whereas the temperature of the second peak is similar to the peak temperature of P. The reaction rate of P50L50 was faster than plastic and lower than leather, and the total weight loss rates
rꝏ of P50L50_Exp and P50L50_Cal were the same. Therefore, the co-pyrolysis of plastic and leather could be analyzed using the parallel superposition model.
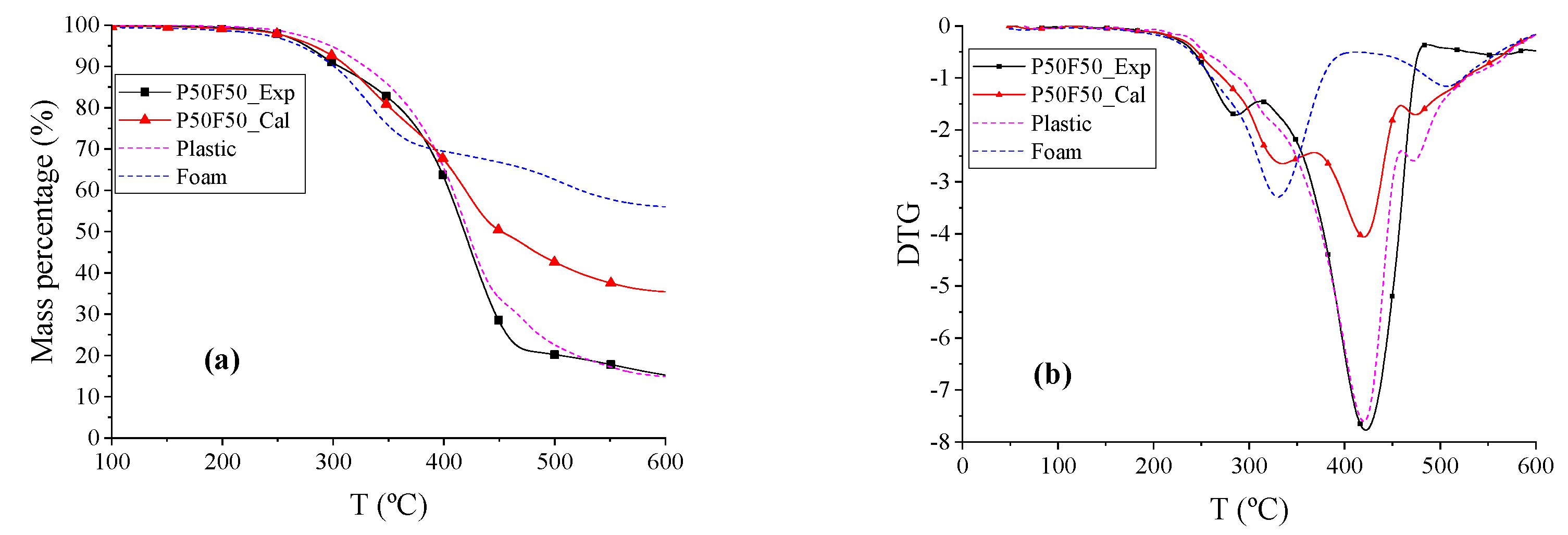
The TG and DTG curves of plastic (P), foam (F), and P50F50 are shown in
Figure 6. The beginning reaction temperature
Td of the blend P50F50 was 28.84 °C higher than T and 25.63 °C lower than P. The DTG peak temperatures of P, P50F50_Exp, and P50F50_Cal are similar, and the shape and overall trend of the P50F50_Exp and P50F50_Cal curves are also similar to that of P, and the total weight loss rates of P50F50 and P were the same at 534.01 °C. Therefore, the co-pyrolysis of plastic and foam is dominated by plastic, and the decomposition of foam was increased and changed by plastic here.
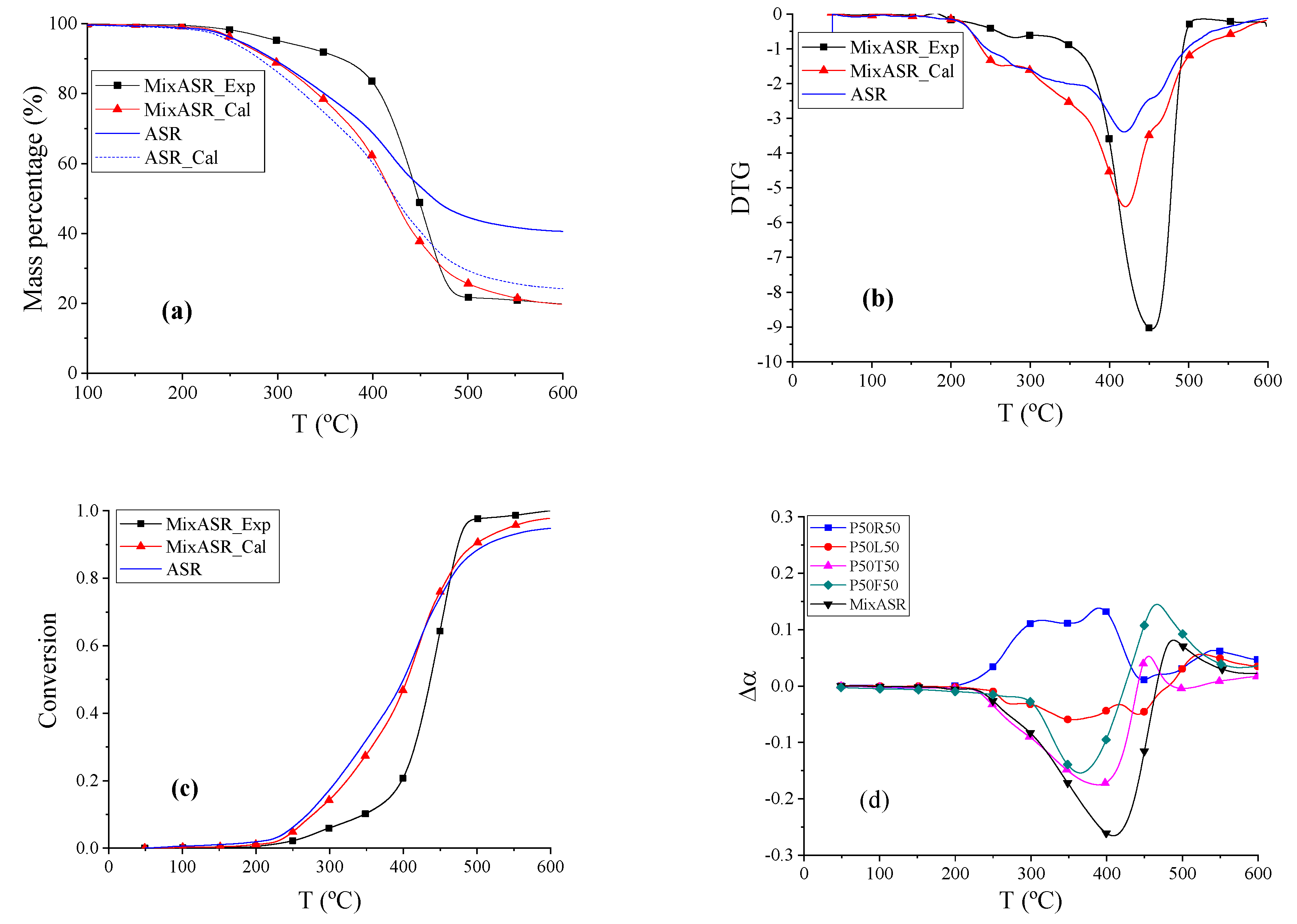
The TG, DTG, and conversion curves of actual ASR, MixASR_Exp, and MixASR_Cal are shown in
Figure 7a–c. The beginning reaction temperatures
Td of ASR, MixASR_Exp, and MixASR_Cal were 270 °C, 347.07 °C, and 277.69 °C, respectively. According to the data shown in
Figure 7b, the DTG peak temperatures of ASR, MixASR_Exp, and MixASR_Cal were 418.76 °C, 453.97 °C and 420.22 °C, respectively. Within temperature ranges from 211.88 to 410.33 °C, the weight loss rate of MixASR_Exp was higher than MixASR_Cal, whereas the exact opposite was observed in temperature ranges from 410.33 to 600 °C, as shown in
Figure 7a. The shape and overall trend of the conversion curves of Mix_ASR and ASR are similar. By fitting the TG data of ASR based on the material composition of ASR (shown as ASR_cal curve in
Figure 7c), we found that the total weight loss rate
rꝏ of the fitted TG curve was 75.7763% and the total weight loss rate
rꝏ of MixASR was 80.1983%. The pyrolysis mechanism of the blend includes these five components and was inconsistent with parallel superposition model. However, the pyrolytic trend and conversion curve shape of MixASR_Cal and ASR were similar, so that the kinetic analysis of the studied ASR could be predicted with a parallel superposition model based on the TG experiments and analysis of each of the main components.
To compare the co-pyrolysis characteristics of various mixtures, including P50R50, P50T50, P50L50, P50F50, and MixASR, the data were further processed by subtracting the calculated value from the experimental value, and the results are shown in
Figure 7d. The reaction trend of mixture P50R50 was different than MixASR, whereas the reaction trends of mixtures P50T50, P50L50, and P50F50 are similar to that of MixASR. Considering the compositions of the studied ASR (
Table 1), rubber only accounts for 2.2%, and according to the above analysis, the co-pyrolysis characteristics of P50T50 and P50F50 were determined by plastic, where the co-pyrolysis of plastic and leather could be analyzed by the parallel superposition model, thus, linear superposition equations such as Equations (3)–(7) could be applied to predict the pyrolysis characteristics of ASR with the results of each of the components. According to the above analysis, the selection of the ASR pyrolysis temperature in the actual process can be determined based on the pyrolysis mechanism of plastic and textiles. This is mainly because they account for the largest proportion in the ASR and can influence the pyrolysis temperature of other polymeric components.
3.2. Pyrolytic Product Yields and Characterization of Gaseous Products
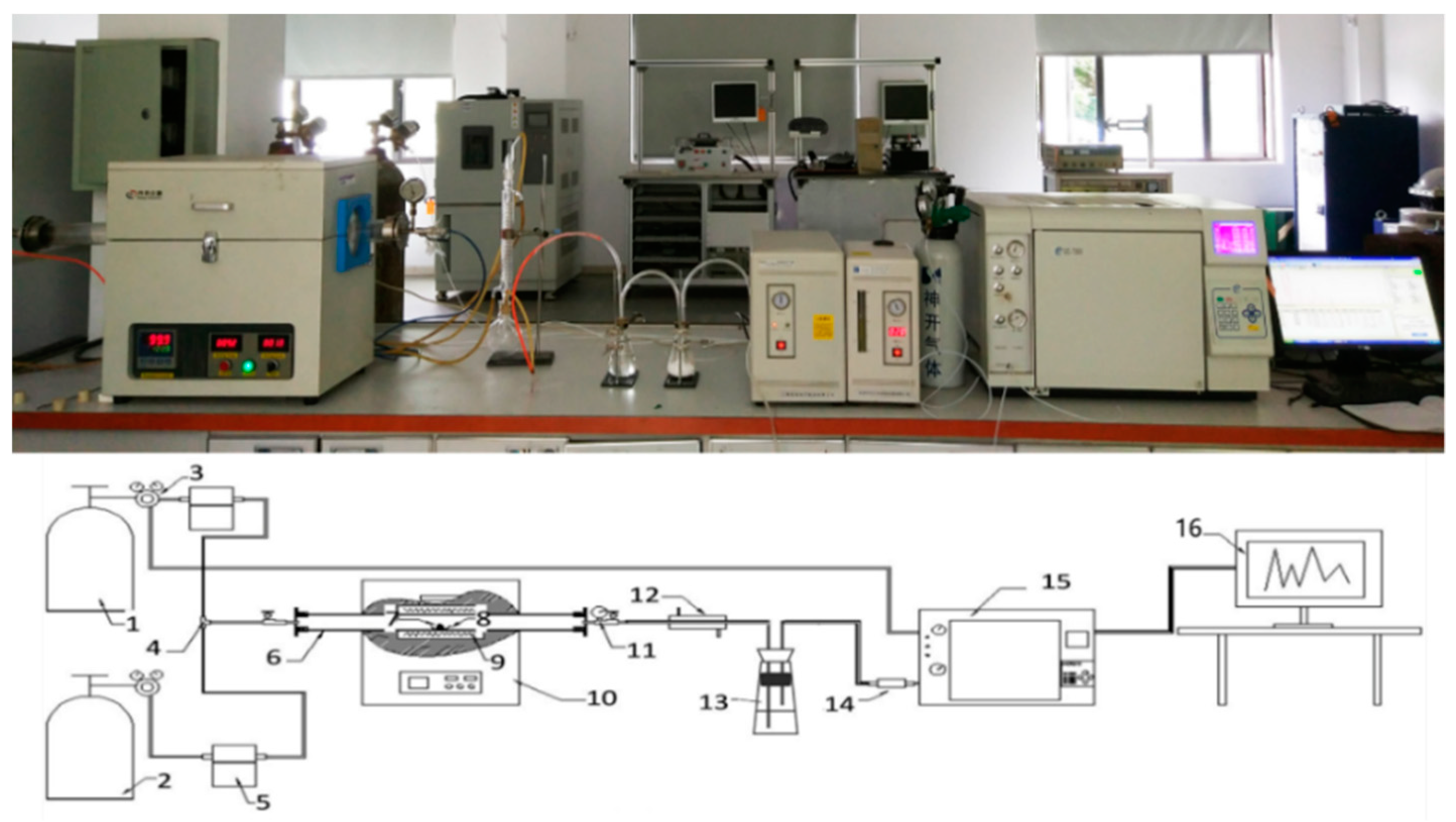
The pyrolytic product yields of plastic, textiles, rubber, leather, foam, P50R50, P50L50, P50T50, P50F50, ASR, and MixASR were obtained by the laboratory-scale pyrolysis experimental platform (
Figure 1), and the results are shown in
Table 4. With increasing finished pyrolysis temperature, the masses of solid and liquid products in all samples decreased, while gas products increased. Comparing the experimental results with the calculated results by the parallel superposition model of P50R50, P50L50, P50T50, P50F50, and MixASR, shown in
Table 5, numerous trends can be observed. For the production of liquid products of P50R50, the experimental results were much lower than those calculated by the parallel superposition model. This was the same for the production of solid products from P50F50. This proved once again that interactions occur among the components in the pyrolysis process, rather than simple linear superposition reaction relationships. Particularly, the analysis of P50R50 and P50F50 using linear superposition theory had large errors. For pyrolytic product yields of MixASR at 500 °C and 600 °C pyrolysis temperatures, the differences between the experimental results and calculated results by the parallel superposition model were relatively small. These results were the same as the result of the TG analysis in the previous section. The yield of pyrolytic gas products was determined by the thermal decomposition of organic substances in the ASR and the final temperature. A similar trend was also observed in [
35], where the polymeric fraction of ASR was pyrolyzed in a laboratory-scale screw type reactor under various conditions.
The characterizations of the gas products of P50R50, P50L50, P50T50, P50F50, ASR, and MixASR were analyzed by a gas chromatograph and the results are shown in
Table 6. In terms of energy recovery and use, syngas with a high calorific value was expected to be a product of ASR pyrolysis. The experimental data showed that production of H
2, CO, and CH
4 was greatest at 600 °C. The characterizations of gas products of ASR and MixASR pyrolysis were consistent. However, the production of CO
2 was always high, which would lead to a decrease in the calorific value of the syngas. Therefore, CO2 should be further eliminated and the calorific value of syngas should be improved. The total proportion of H2, CO, and CH4 in gas products of ASR pyrolysis exceeded 30% at finished temperatures of 600 °C and 700 °C. Therefore, pyrolysis synthesis of syngas is a promising direction for ASR recovery and treatment.