Effect of Metal-Ligand Coordination Complexes on Molecular Dynamics and Structure of Cross-Linked Poly(dimethylosiloxane)
Abstract
1. Introduction
2. Materials and Methods
2.1. Density Functional Theory Calculations
2.2. Differential Scanning Calorimetry (DSC)
2.3. Broadband Dielectric Spectroscopy (BDS)
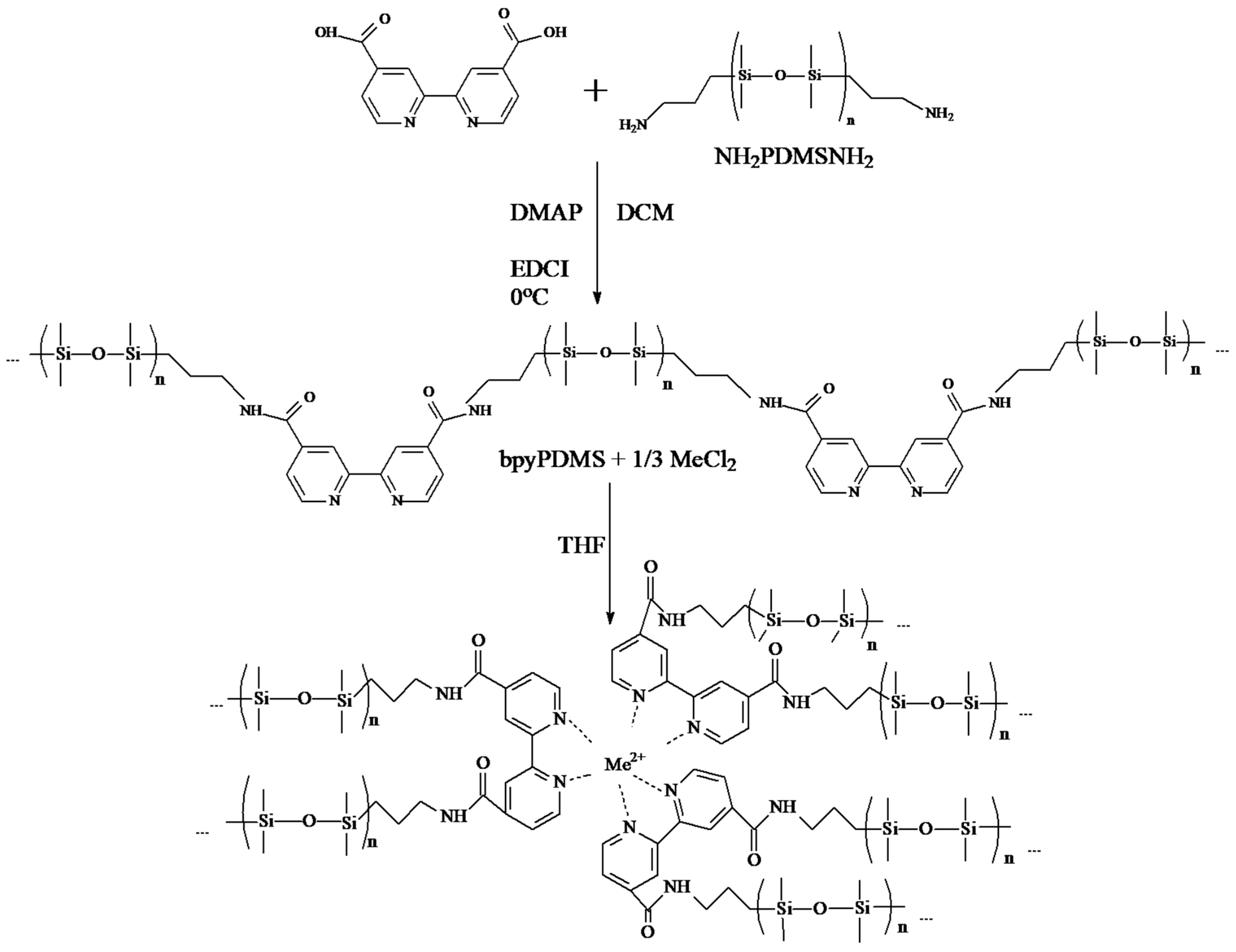
3. Results and Discussion
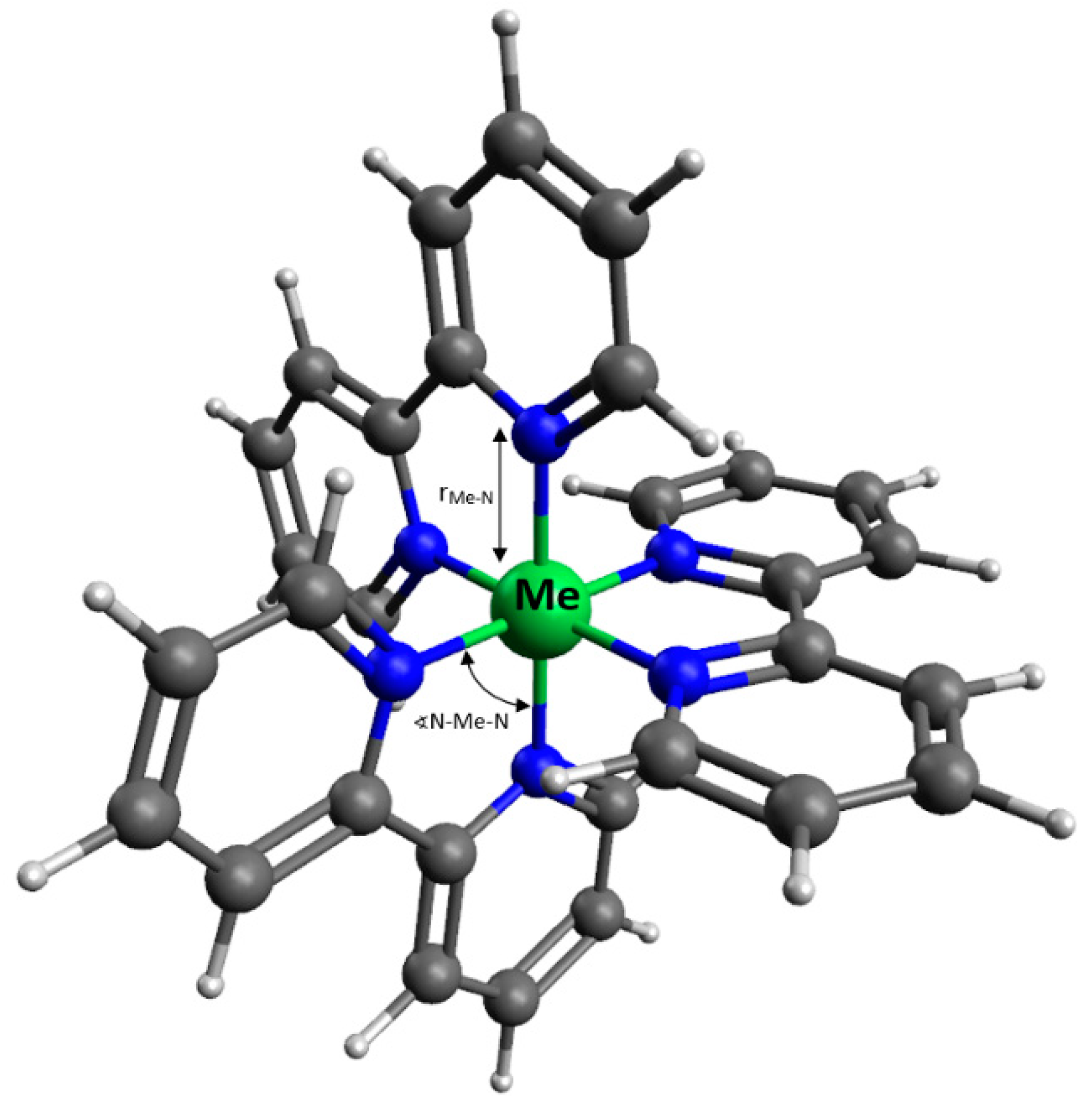
3.1. Density Functional Theory Calculations
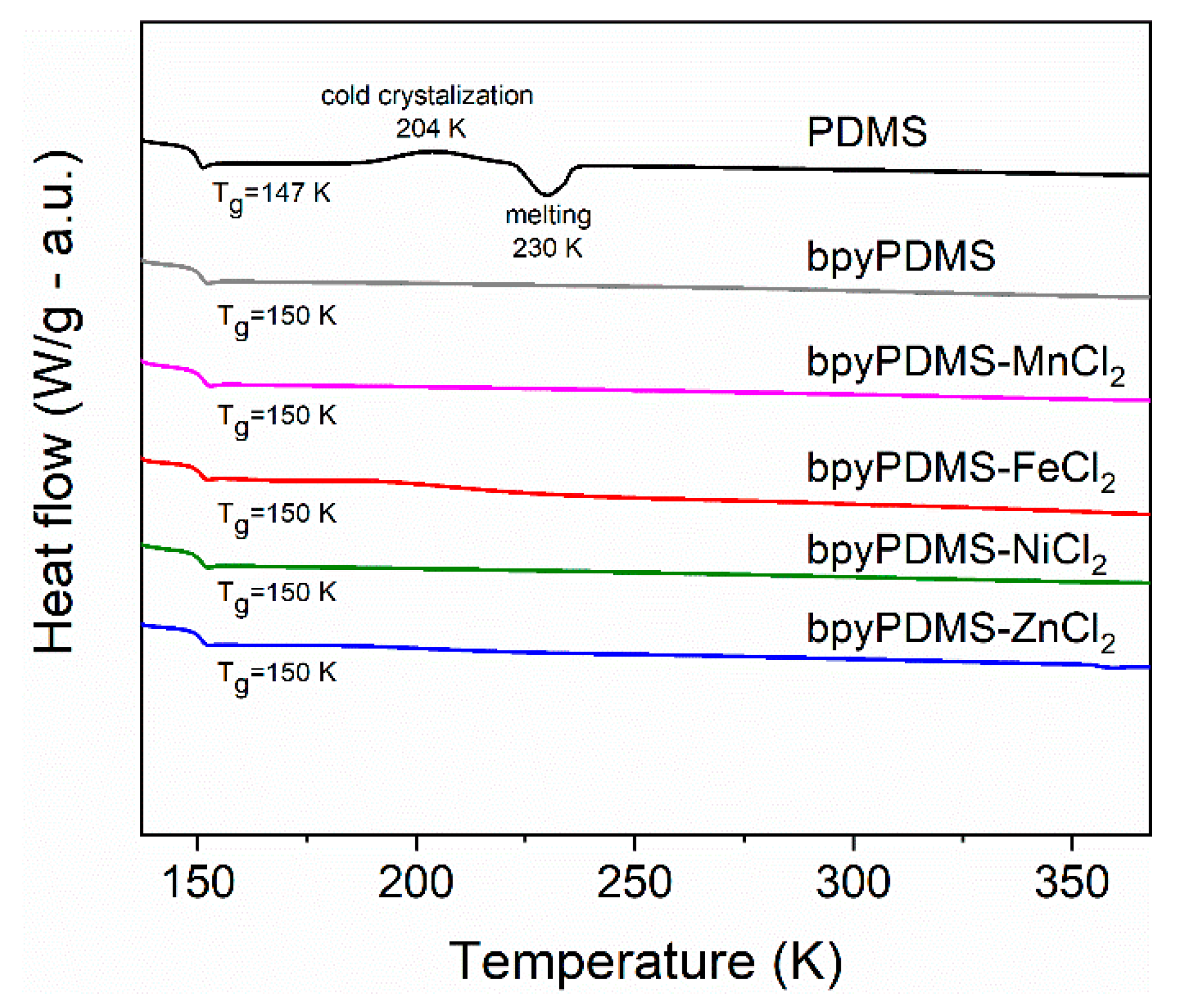
3.2. Differential Scanning Calorimetry (DSC)
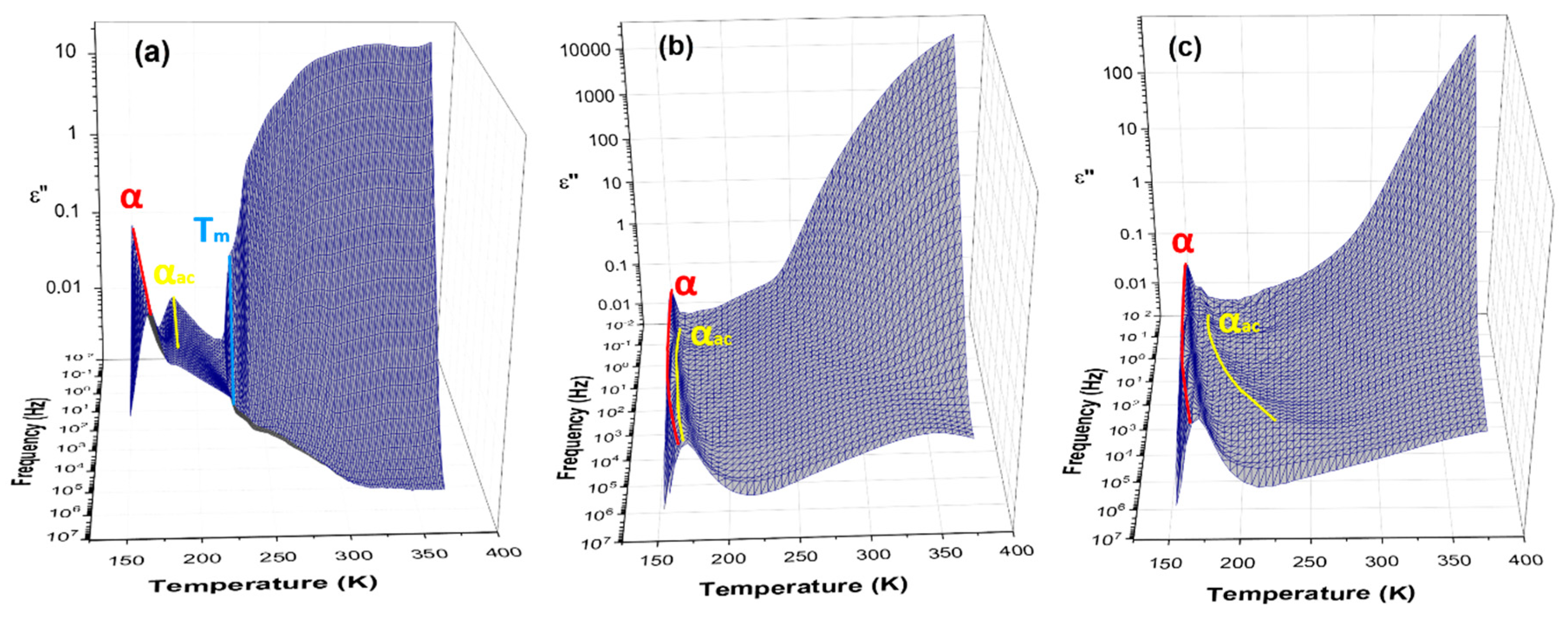
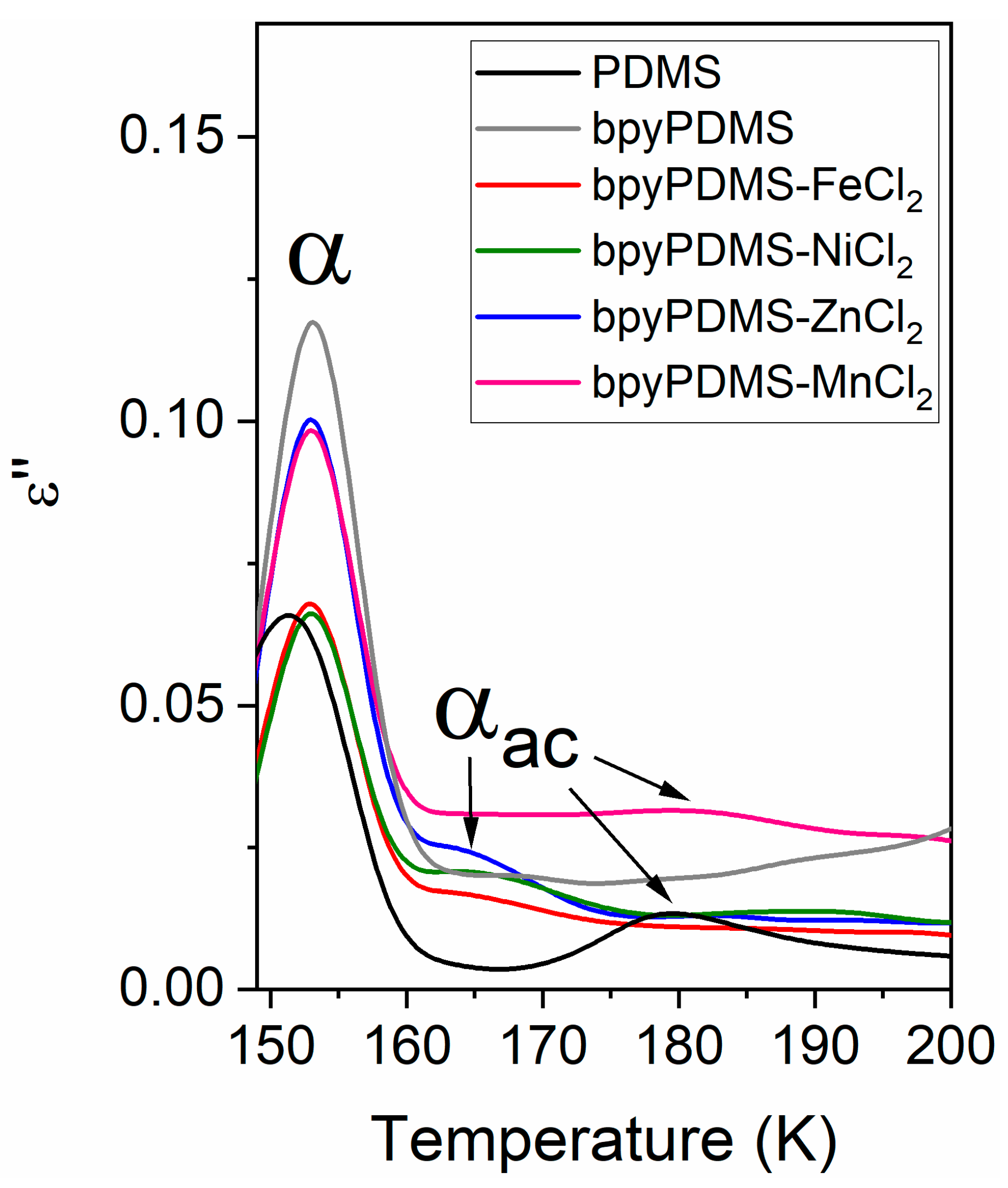
3.3. Broadband Dielectric Spectroscopy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fu, M.C.; Uno, T.; Ueda, M.; Higashihara, T. Investigation of polycyanurate/benzoxazine curing system. Microsyst. Technol. 2018, 24, 597–604. [Google Scholar] [CrossRef]
- Yu, H.; Wang, L.; Wang, Z.; Han, X.; Zhao, M. Alkyl-substituted carboxyl-containing polyaryletherketones and the crosslinking modifications with various bisphenols: Preparation and optical properties. Polymer 2010, 51, 3269–3276. [Google Scholar] [CrossRef]
- Han, S.-T.; Peng, H.; Sun, Q.; Venkatesh, S.; Chung, K.-S.; Lau, S.C.; Zhou, Y.; Roy, V.A.L. An Overview of the Development of Flexible Sensors. Adv. Mater. 2017, 29, 1700375. [Google Scholar] [CrossRef] [PubMed]
- Lai, S.; Zucca, A.; Cosseddu, P.; Greco, F.; Mattoli, V.; Bonfiglio, A. Ultra-conformable Organic Field-Effect Transistors and circuits for epidermal electronic applications. Org. Electron. Phys. Mater. Appl. 2017, 46, 60–67. [Google Scholar] [CrossRef]
- Zeng, W.; Shu, L.; Li, Q.; Chen, S.; Wang, F.; Tao, X.M. Fiber-based wearable electronics: A review of materials, fabrication, devices, and applications. Adv. Mater. 2014, 26, 5310–5336. [Google Scholar] [CrossRef]
- Lai, S.; Viola, F.A.; Cosseddu, P.; Bonfiglio, A. Floating gate, organic field-effect transistor-based sensors towards biomedical applications fabricated with large-area processes over flexible substrates. Sensors 2018, 18, 688. [Google Scholar] [CrossRef]
- Rivnay, J.; Inal, S.; Salleo, A.; Owens, R.M.; Berggren, M.; Malliaras, G.G. Organic electrochemical transistors. Nat. Rev. Mater. 2018, 3. [Google Scholar] [CrossRef]
- Zhang, Z.; Du, J.; Zhang, D.; Sun, H.; Yin, L.; Ma, L.; Chen, J.; Ma, D.; Cheng, H.M.; Ren, W. Rosin-enabled ultraclean and damage-free transfer of graphene for large-area flexible organic light-emitting diodes. Nat. Commun. 2017, 8, 1–9. [Google Scholar] [CrossRef]
- Liu, Y.; He, K.; Chen, G.; Leow, W.R.; Chen, X. Nature-Inspired Structural Materials for Flexible Electronic Devices. Chem. Rev. 2017, 117, 12893–12941. [Google Scholar] [CrossRef]
- Yan, J.; Pietrasik, J.; Wypych-Puszkarz, A.; Ciekanska, M.; Matyjaszewski, K. Synthesis of High k Nanoparticles by Controlled Radical Polymerization. In Solution-Processable Components for Organic Electronic Devices; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2019; pp. 181–226. ISBN 9783527813872. [Google Scholar]
- Dünki, S.J.; Tress, M.; Kremer, F.; Ko, S.Y.; Nüesch, F.A.; Varganici, C.D.; Racles, C.; Opris, D.M. Fine-tuning of the dielectric properties of polysiloxanes by chemical modification. RSC Adv. 2015, 5, 50054–50062. [Google Scholar] [CrossRef]
- Callister, W.D.; Rethwisch, D.G. Materials Science and Engineering: An Introduction; John Wiley and Sons: Hoboken, NJ, USA, 2014; ISBN 9781118324578 1118324579 9781118477700 1118477707. [Google Scholar]
- Bosq, N.; Guigo, N.; Persello, J.; Sbirrazzuoli, N. Melt and glass crystallization of PDMS and PDMS silica nanocomposites. Phys. Chem. Chem. Phys. 2014, 16, 7830–7840. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, G.; Wang, K.; Chen, X.Y. TiO2 nanoparticles modified polydimethylsiloxane with fast response time and increased dielectric constant. J. Micromech. Microeng. 2012, 22. [Google Scholar] [CrossRef]
- Bele, A.; Cazacu, M.; Stiubianu, G.; Vlad, S.; Ignat, M. Polydimethylsiloxane-barium titanate composites: Preparation and evaluation of the morphology, moisture, thermal, mechanical and dielectric behavior. Compos. Part B Eng. 2015, 68, 237–245. [Google Scholar] [CrossRef]
- Li, C.H.; Wang, C.; Keplinger, C.; Zuo, J.L.; Jin, L.; Sun, Y.; Zheng, P.; Cao, Y.; Lissel, F.; Linder, C.; et al. A highly stretchable autonomous self-healing elastomer. Nat. Chem. 2016, 8, 618–624. [Google Scholar] [CrossRef] [PubMed]
- Rao, Y.L.; Chortos, A.; Pfattner, R.; Lissel, F.; Chiu, Y.C.; Feig, V.; Xu, J.; Kurosawa, T.; Gu, X.; Wang, C.; et al. Stretchable self-healing polymeric dielectrics cross-linked through metal-ligand coordination. J. Am. Chem. Soc. 2016, 138, 6020–6027. [Google Scholar] [CrossRef] [PubMed]
- Oh, I.; Jeon, S.I.; Chung, I.J.; Ahn, C.H. Self-Healable Dielectric Polydimethylsiloxane Composite Based on Zinc-Imidazole Coordination Bond. Macromol. Res. 2019, 27, 435–443. [Google Scholar] [CrossRef]
- Saad, G.R.; Morsi, R.E.; Mohammady, S.Z.; Elsabee, M.Z. Dielectric relaxation of monoesters based poly (styrene-co-maleic anhydride) copolymer. J. Polym. Res. 2008, 15, 115–123. [Google Scholar] [CrossRef]
- Kirst, K.U.; Kremer, F.; Pakula, T.; Hollingshurst, J. Molecular dynamics of cyclic and linear poly (dimethylsiloxanes). Colloid Polym. Sci. 1994, 272, 1420–1429. [Google Scholar] [CrossRef]
- Kirst, K.U.; Kremer, F.; Litvinov, V.M. Broad-Band Dielectric Spectroscopy on the Molecular Dynamics of Bulk and Adsorbed Poly (dimethylsiloxane). Macromolecules 1993, 26, 975–980. [Google Scholar] [CrossRef]
- Jancelewicz, M.; Nowaczyk, G.; Makrocka-Rydzyk, M.; Wypych, A.; Fojud, Z.; Jurga, S.; Maciejewski, H. Molecular dynamics in grafted polydimethylsiloxanes. J. Non-Cryst. Solids 2010, 356, 669–675. [Google Scholar] [CrossRef]
- You, Y.; Huang, W.; Zhang, A.; Lin, Y. A facile and controllable synthesis of dual-crosslinked elastomers based on linear bifunctional polydimethylsiloxane oligomers. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 3760–3768. [Google Scholar] [CrossRef]
- Wang, D.P.; Lai, J.C.; Lai, H.Y.; Mo, S.R.; Zeng, K.Y.; Li, C.H.; Zuo, J.L. Distinct Mechanical and Self-Healing Properties in Two Polydimethylsiloxane Coordination Polymers with Fine-Tuned Bond Strength. Inorg. Chem. 2018, 57, 3232–3242. [Google Scholar] [CrossRef] [PubMed]
- Williams, Z.H.; Burwell, E.D.; Chiomento, A.E.; Demsko, K.J.; Pawlik, J.T.; Harris, S.O.; Yarolimek, M.R.; Whitney, M.B.; Hambourger, M.; Schwab, A.D. Rubber-elasticity and electrochemical activity of iron (II) tris (bipyridine) crosslinked poly (dimethylsiloxane) networks. Soft Matter 2017, 13, 6542–6554. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09, Revision A.02; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Hehre, W.J.; Ditchfield, K.; Pople, J.A. Self-consistent molecular orbital methods. XII. Further extensions of gaussian-type basis sets for use in molecular orbital studies of organic molecules. J. Chem. Phys. 1972, 56, 2257–2261. [Google Scholar] [CrossRef]
- Rassolov, V.A.; Ratner, M.A.; Pople, J.A.; Redfern, P.C.; Curtiss, L.A. 6-31G* Basis Set for Third-Row Atoms. J. Comput. 2001, 22, 976–984. [Google Scholar] [CrossRef]
- Hay, P.J.; Wadt, W.R. Ab initio effective core potentials for molecular calculations. Potentials for K to Au including the outermost core orbitale. J. Chem. Phys. 1985, 82, 299–310. [Google Scholar] [CrossRef]
- Reed, A.E.; Curtiss, L.A.; Weinhold, F. Intermolecular Interactions from a Natural Bond Orbital, Donor-Acceptor Viewpoint. Chem. Rev. 1988, 88, 899–926. [Google Scholar] [CrossRef]
- Glendening, E.D.; Reed, A.E.; Carpenter, J.E.; Weinhold, F. NBO Version 3.1.; ScienceOpen: Berlin, Germany, 2001. [Google Scholar]
- Gerasimova, T.P.; Katsyuba, S.A. Bipyridine and phenanthroline IR-spectral bands as indicators of metal spin state in hexacoordinated complexes of Fe (ii), Ni (ii) and Co (ii). Dalt. Trans. 2013, 42, 1787–1797. [Google Scholar] [CrossRef]
- Wang, M.; England, J.; Weyhermüller, T.; Wieghardt, K. Molecular and electronic structures of the members of the electron transfer series [Mn (bpy) 3] n (n = 2+, 1+, 0, 1-) and [Mn (tpy) 2] m (m = 4+, 3+, 2+, 1+, 0). An experimental and density functional theory study. Inorg. Chem. 2014, 53, 2276–2287. [Google Scholar] [CrossRef]
- Lund, R.; Alegría, A.; Goitandía, L.; Colmenero, J.; González, M.A.; Lindner, P. Dynamical and structural aspects of the cold crystallization of poly (dimethylsiloxane) (PDMS). Macromolecules 2008, 41, 1364–1376. [Google Scholar] [CrossRef]
- Callaghan, P.T.; Samulski, E.T. Molecular weight dependence of nuclear spin correlations in PDMS networks. Macromolecules 2000, 33, 3795–3802. [Google Scholar] [CrossRef]
- Fulcher, G.S. Analysis of Recent Measurements of the Viscosity of Glasses.—II. J. Am. Ceram. Soc. 1925, 8, 789–794. [Google Scholar] [CrossRef]
- Tammann, G.; Hesse, W. Die Abhängigkeit der Viscosität von der Temperatur bie unterkühlten Flüssigkeiten. Z. Anorg. Allg. Chem. 1926, 156, 245–257. [Google Scholar] [CrossRef]
- Korus, J.; Hempel, E.; Beiner, M.; Kahle, S.; Donth, E. Temperature dependence of a glass transition cooperativity. Acta Polym. 1997, 48, 369–378. [Google Scholar] [CrossRef]
- Lorthioir, C.; Alegría, A.; Colmenero, J.; Deloche, B. Heterogeneity of the segmental dynamics of poly (dimethylsiloxane) in a diblock lamellar mesophase: Dielectric relaxation investigations. Macromolecules 2004, 37, 7808–7817. [Google Scholar] [CrossRef]
- Santangelo, P.G.; Roland, C.M. Molecular weight dependence of fragility in polystyrene. Macromolecules 1998, 31, 4581–4585. [Google Scholar] [CrossRef]
- Sokolov, A.P.; Novikov, V.N.; Ding, Y. Why many polymers are so fragile. J. Phys. Condens. Matter 2007, 19. [Google Scholar] [CrossRef]
- Roland, C.M.; Nagi, K.L. Segmental relaxation in poly (dimethylsiloxane). Macromolecules 1996, 29, 5747–5750. [Google Scholar] [CrossRef]
- Roland, C.M.; Ngai, K.L. Normalization of the Temperature Dependence of Segmental Relaxation Times. Macromolecules 1992, 25, 5765–5768. [Google Scholar] [CrossRef]
- Dudowicz, J.; Freed, K.F.; Douglas, J.F. The glass transition temperature of polymer melts. J. Phys. Chem. B 2005, 109, 21285–21292. [Google Scholar] [CrossRef] [PubMed]




| Complex | Cationic Metal Electronic Structure | Ground State (S) |
|---|---|---|
| [Fe(bpy)3]2+ | [Ar]4s03d6 | 0 |
| [Ni(bpy)3]2+ | [Ar]4s03d8 | 2/3 |
| [Zn(bpy)3]2+ | [Ar]4s03d10 | 0 |
| [Mn(bpy)3]2+ | [Ar]4s03d5 | 5/2 |
| [Fe(bpy)3]2+ | [Zn(bpy)3]2+ | [Ni(bpy)3]2+ | [Mn(bpy)3]2+ | |
|---|---|---|---|---|
| rMe-N [Å] | 1.9544 | 2.2594 | 2.1407 | 2.3115 |
| [Å] | 0.0004 | 0.0009 | 0.0006 | 0.0011 |
| N–Me–N [°] | 89.26 | 87.78 | 88.51 | 87.55 |
| [°] | 5.33 | 10.22 | 8.08 | 11.36 |
| µb [D] | 3.09 | 21.05 | 12.86 | 19.20 |
| Sample | τ (s) | τmax (s) | Δε | αHN | βHN |
|---|---|---|---|---|---|
| PDMS | 1.1 × 10−5 | 4.8 × 10−6 | 0.66 | 0.79 | 0.49 |
| bpyPDMS | 1.3 × 10−5 | 1.0 × 10−5 | 0.67 | 0.59 | 0.85 |
| bpyPDMS-FeCl2 | 9.8 × 10−6 | 7.6 × 10−6 | 0.43 | 0.60 | 0.85 |
| bpyPDMS-NiCl2 | 1.1 × 10−5 | 8.9 × 10−6 | 0.40 | 0.59 | 0.88 |
| bpyPDMS-ZnCl2 | 1.2 × 10−5 | 9.2 × 10−6 | 0.60 | 0.59 | 0.86 |
| bpyPDMS-MnCl2 | 1.1 × 10−5 | 1.0 × 10−5 | 0.54 | 0.55 | 0.96 |
| Sample | Ln|τ0 (s)| | B | T0 (K) | TgDRS (K) | F |
|---|---|---|---|---|---|
| PDMS | −30.0 ± 0.7 | 588 ± 36 | 129.9 ± 0.8 | 150.8 | 88 |
| bpyPDMS | −34.9 ± 0.6 | 860 ± 38 | 126.7 ± 0.7 | 152.5 | 86 |
| bpyPDMS-FeCl2 | −34.0 ± 0.9 | 792 ± 54 | 127.8 ± 1.0 | 152.5 | 86 |
| bpyPDMS-NiCl2 | −34.8 ± 1.3 | 854 ±80 | 126.6 ± 1.5 | 152.5 | 85 |
| bpyPDMS-ZnCl2 | −33.2 ± 1.1 | 760 ± 65 | 128.3 ± 1.2 | 152.5 | 86 |
| bpyPDMS-MnCl2 | −34.5 ± 1.0 | 838 ± 61 | 126.9 ± 1.1 | 152.5 | 85 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wrzesińska, A.; Bobowska, I.; Maczugowska, P.; Małolepsza, J.; Błażewska, K.M.; Wypych-Puszkarz, A. Effect of Metal-Ligand Coordination Complexes on Molecular Dynamics and Structure of Cross-Linked Poly(dimethylosiloxane). Polymers 2020, 12, 1680. https://doi.org/10.3390/polym12081680
Wrzesińska A, Bobowska I, Maczugowska P, Małolepsza J, Błażewska KM, Wypych-Puszkarz A. Effect of Metal-Ligand Coordination Complexes on Molecular Dynamics and Structure of Cross-Linked Poly(dimethylosiloxane). Polymers. 2020; 12(8):1680. https://doi.org/10.3390/polym12081680
Chicago/Turabian StyleWrzesińska, Angelika, Izabela Bobowska, Paulina Maczugowska, Joanna Małolepsza, Katarzyna M. Błażewska, and Aleksandra Wypych-Puszkarz. 2020. "Effect of Metal-Ligand Coordination Complexes on Molecular Dynamics and Structure of Cross-Linked Poly(dimethylosiloxane)" Polymers 12, no. 8: 1680. https://doi.org/10.3390/polym12081680
APA StyleWrzesińska, A., Bobowska, I., Maczugowska, P., Małolepsza, J., Błażewska, K. M., & Wypych-Puszkarz, A. (2020). Effect of Metal-Ligand Coordination Complexes on Molecular Dynamics and Structure of Cross-Linked Poly(dimethylosiloxane). Polymers, 12(8), 1680. https://doi.org/10.3390/polym12081680






