Dyeing and Antibacterial Properties of Chemically Recycled PET Thermal-Bonded Nonwovens Dyed with Terminalia chebula Dye
Abstract
1. Introduction
2. Experimental
2.1. Materials
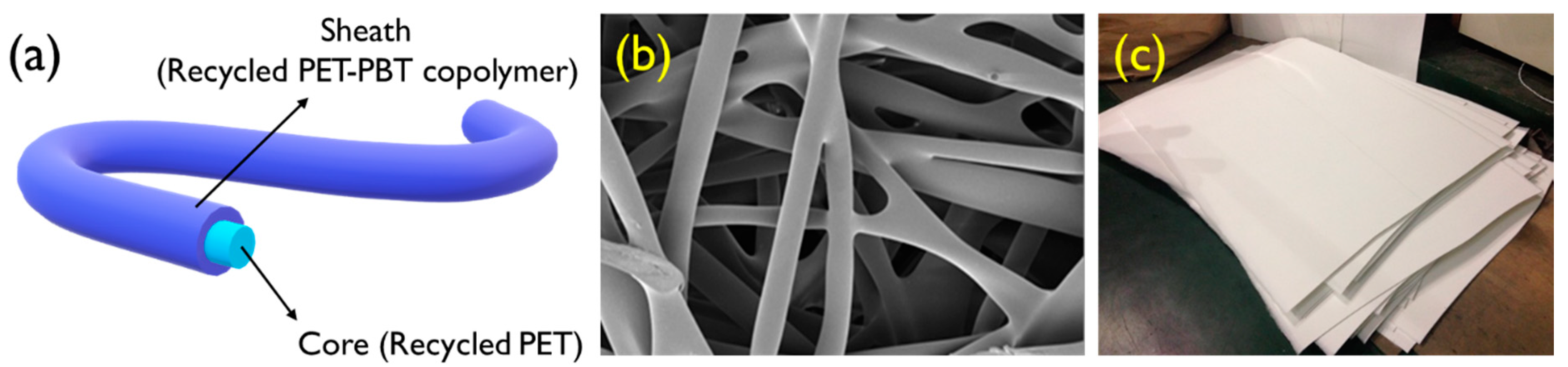
2.2. Preparation of Themal-Bonded Nonwoven
2.3. Dyeing Procedure
2.4. Characterization
3. Result and Discussion
3.1. Analysis of the Components of the T. Chebula Dye
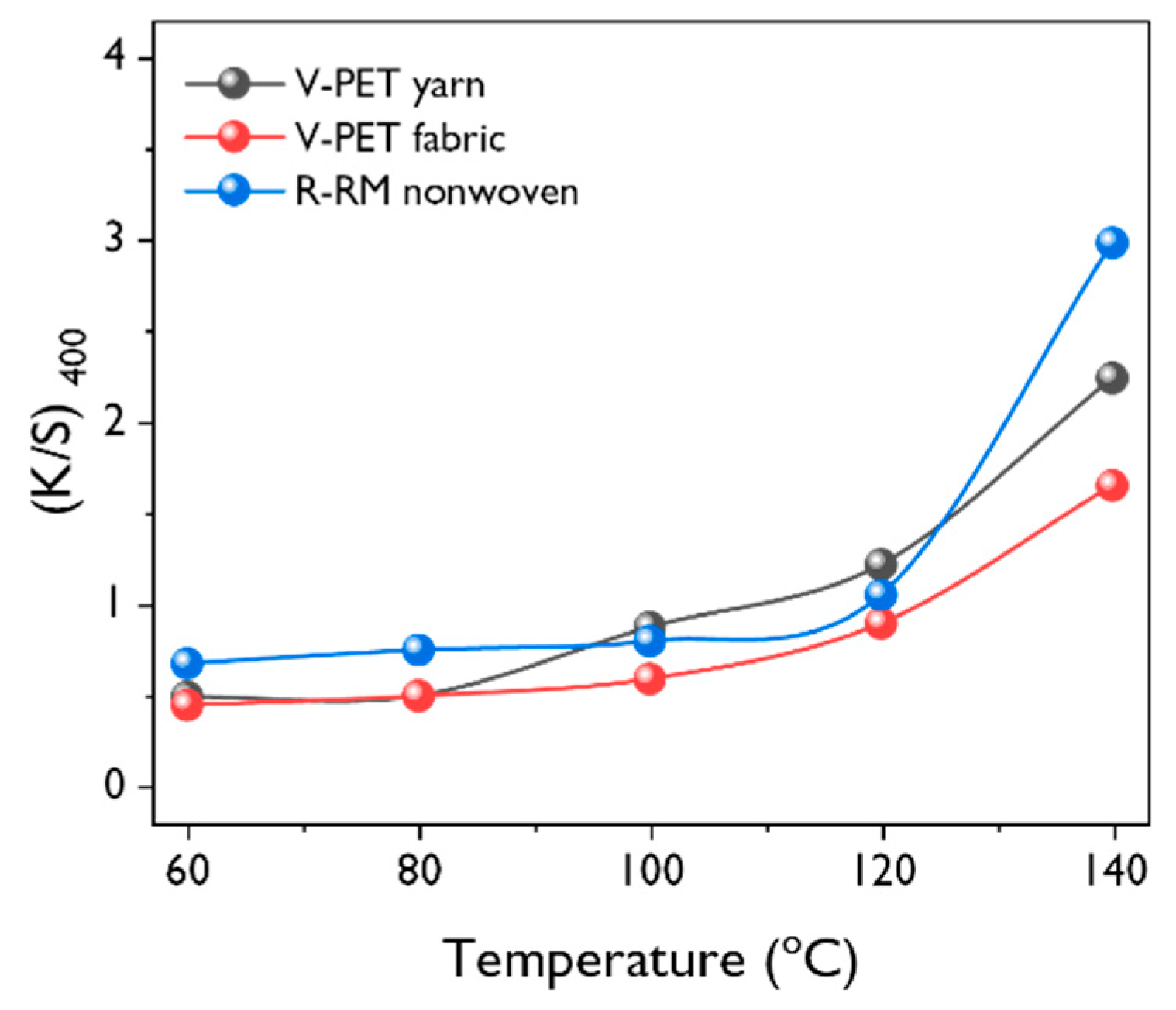
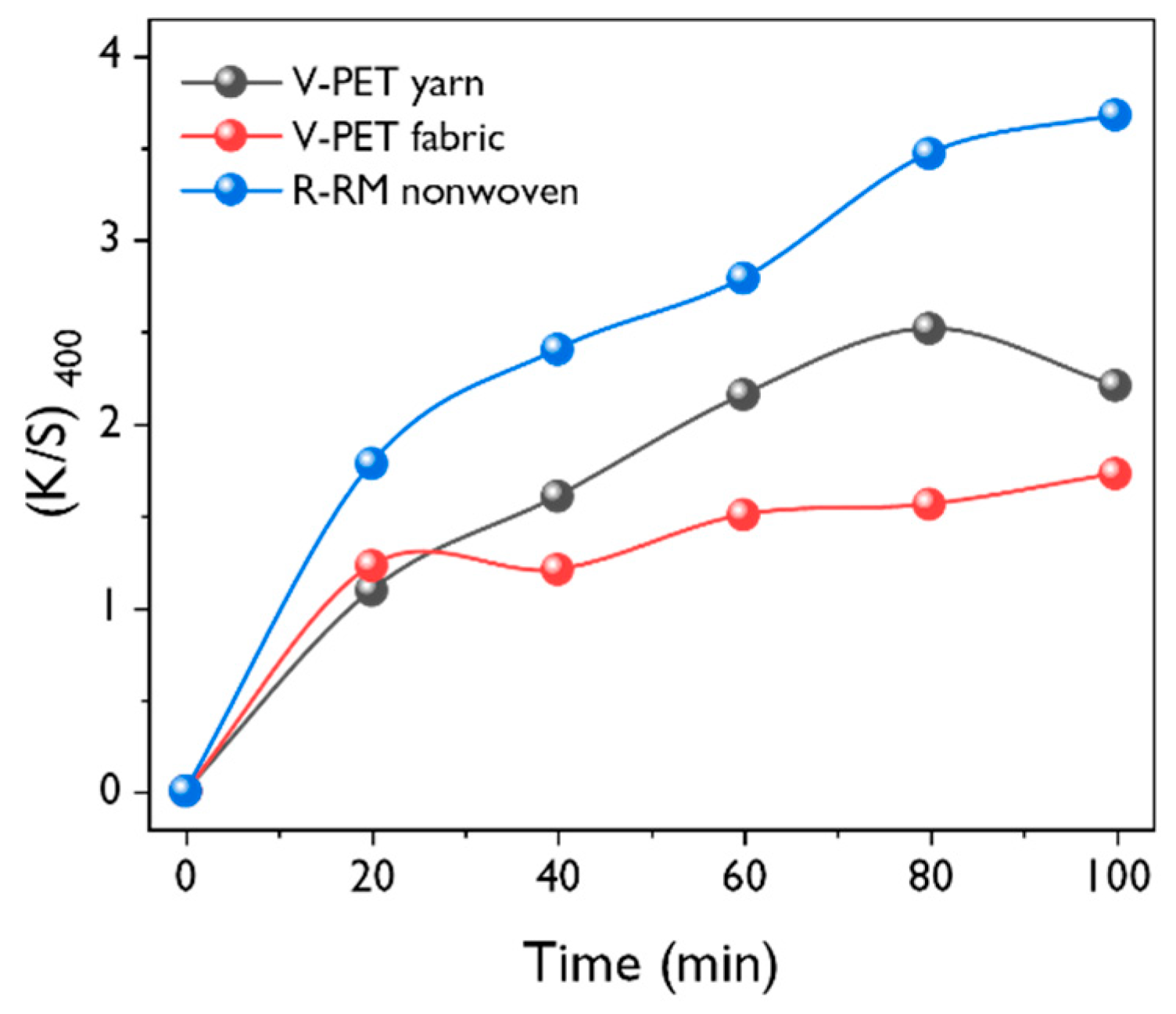
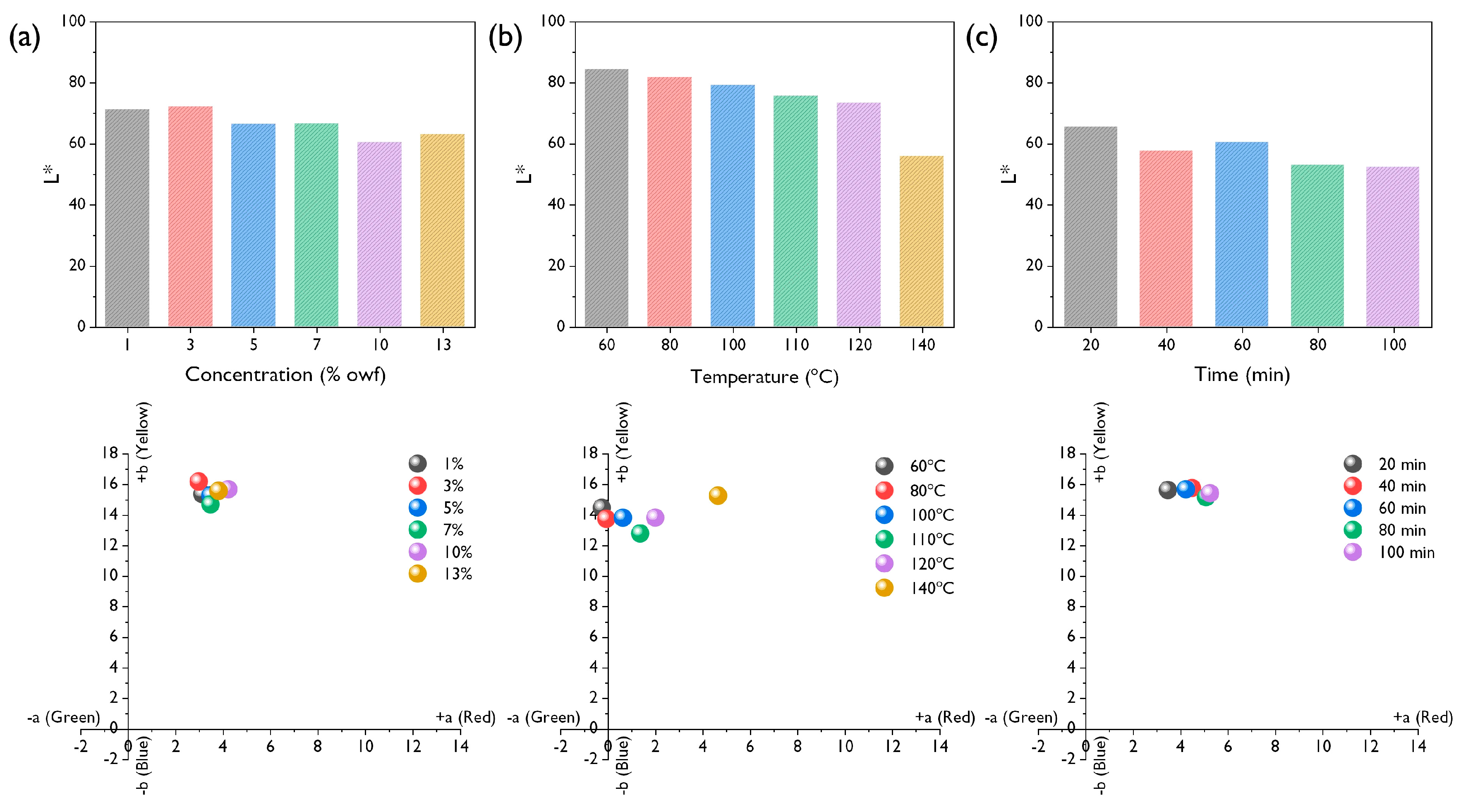
3.2. Effect of Dyeing Conditions on Dyeability
3.3. Color Fastness
3.4. Morphology
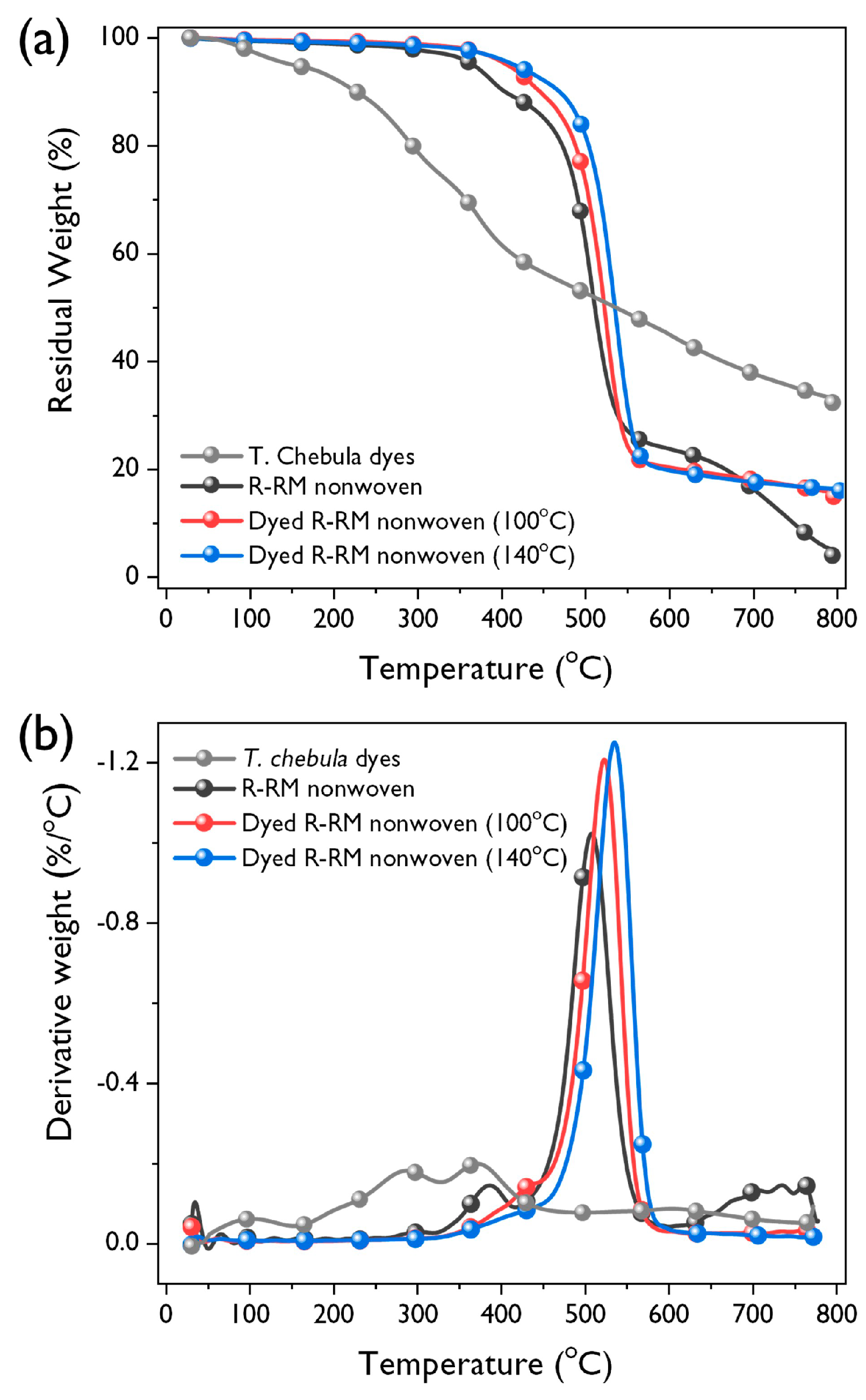
3.5. TGA Analysis
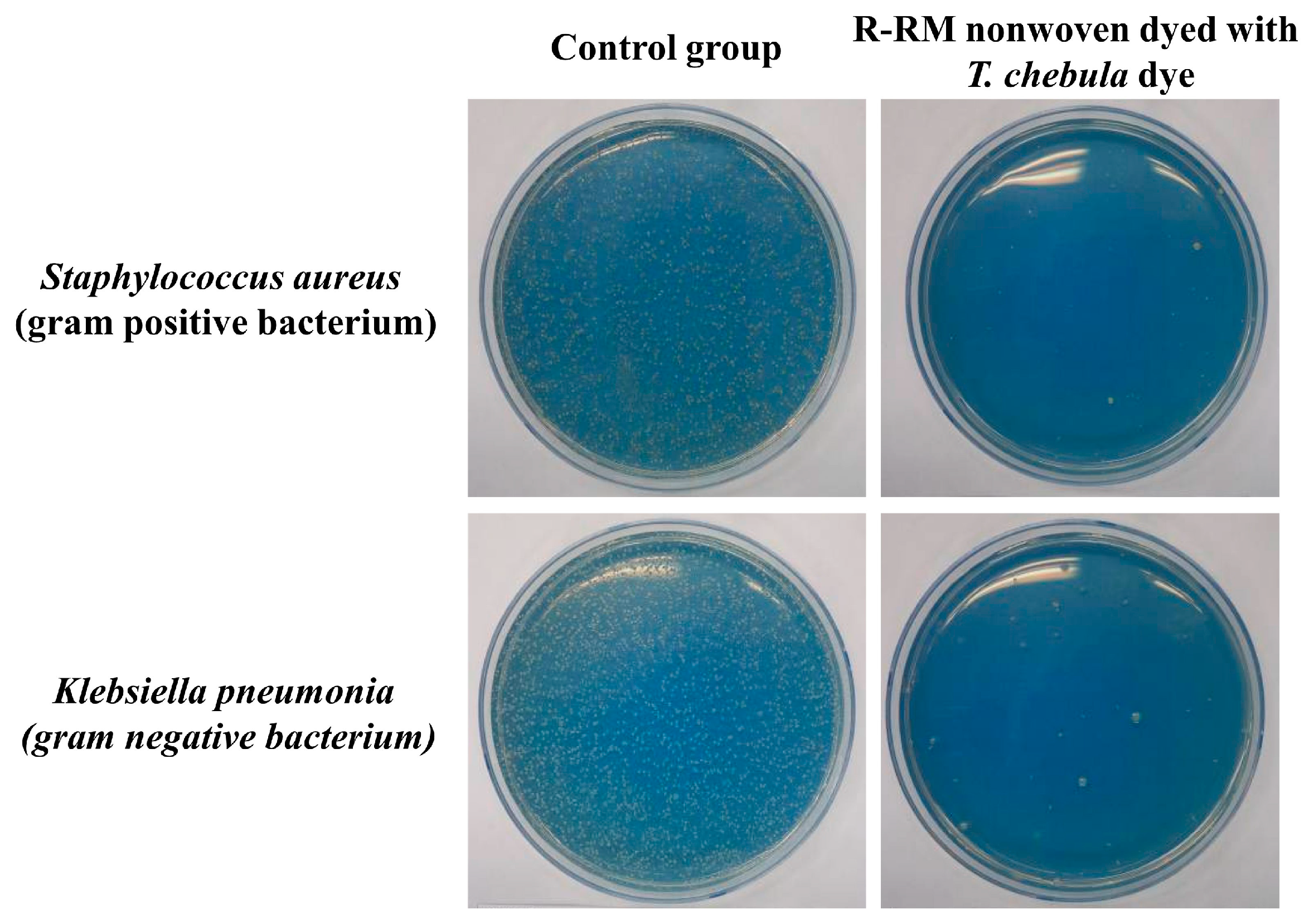
3.6. Antibacterial Activity
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Park, S.H.; Kim, S.H. Poly (ethylene terephthalate) recycling for high value added textiles. Fash. Text. 2014, 1, 1–17. [Google Scholar] [CrossRef]
- Kim, H.N.; Park, C.K.; Kim, I.S.; Kim, S.H. Compatibilization of immiscible blends of polypropylene and isosorbide containing copolyester with silica nanoparticles. Polym. Eng. Sci. 2020, 60, 1365–1376. [Google Scholar] [CrossRef]
- Lee, J.H.; Lim, K.S.; Hahm, W.G.; Kim, S.H. Properties of recycled and virgin poly (ethylene terephthalate) blend fibers. J. Appl. Polym. Sci. 2013, 128, 1250–1256. [Google Scholar] [CrossRef]
- Lee, S.H.; Han, T.H.; Kim, S.H. Thermal shrinkage of chemically recycled and virgin poly (ethylene terephthalate) blends. Macromol. Res. 2014, 22, 782–787. [Google Scholar] [CrossRef]
- Choi, Y.J.; Kim, S.H. Characterization of recycled polyethylene terephthalates and polyethylene terephthalate–nylon6 blend knitted fabrics. Text. Res. J. 2015, 85, 337–345. [Google Scholar] [CrossRef]
- Choi, Y.J.; Kim, I.; Kim, S.H. Effect of heat-setting on the physical properties of chemically recycled polyester nonwoven fabrics. Text. Res. J. 2019, 89, 498–509. [Google Scholar] [CrossRef]
- Kim, E.S.; Oh, H.J.; Kim, H.-J.; Kim, C.G.; Park, S.Y.; Jeong, Y.G.; Hahm, W.-G. Effect of Polycondensation Catalyst on Fiber Structure Development in High-Speed Melt Spinning of Poly (Ethylene Terephthalate). Polymers 2019, 11, 1931. [Google Scholar] [CrossRef]
- Bag, A.; Bhattacharyya, S.K.; Chattopadhyay, R.R. The development of Terminalia chebula Retz. (Combretaceae) in clinical research. Asian Pac. J. Trop. Biomed. 2013, 3, 244–252. [Google Scholar] [CrossRef]
- Chowdhury, S.A.; Vijayaraghavan, R.; MacFarlane, D. Distillable ionic liquid extraction of tannins from plant materials. Green Chem. 2010, 12, 1023–1028. [Google Scholar] [CrossRef]
- Muhammad, S.; Khan, B.A.; Akhtar, N.; Mahmood, T.; Rasul, A.; Hussain, I.; Khan, H.; Badshah, A. The morphology, extractions, chemical constituents and uses of Terminalia chebula: A review. J. Med. Plants Res. 2012, 6, 4772–4775. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, J.; Tang, R.-C.; Zhang, J. Simultaneous dyeing and functionalization of silk with three natural yellow dyes. Ind. Crops Prod. 2015, 64, 224–232. [Google Scholar] [CrossRef]
- Phan, K.; Van Den Broeck, E.; Van Speybroeck, V.; De Clerck, K.; Raes, K.; De Meester, S. The potential of anthocyanins from blueberries as a natural dye for cotton: A combined experimental and theoretical study. Dyes Pigments 2020, 176, 108180. [Google Scholar] [CrossRef]
- Ghaheh, F.S.; Mortazavi, S.M.; Alihosseini, F.; Fassihi, A.; Nateri, A.S.; Abedi, D. Assessment of antibacterial activity of wool fabrics dyed with natural dyes. J. Clean. Prod. 2014, 72, 139–145. [Google Scholar] [CrossRef]
- Shabbir, M.; Islam, S.U.; Bukhari, M.N.; Rather, L.J.; Khan, M.A.; Mohammad, F. Application of Terminalia chebula natural dye on wool fiber—evaluation of color and fastness properties. Text. Cloth. Sustain. 2017, 2, 1. [Google Scholar] [CrossRef]
- Pantoja-Castro, M.A.; González-Rodríguez, H. Study by infrared spectroscopy and thermogravimetric analysis of tannins and tannic acid. Rev. Latinoam. Química 2011, 39, 107–112. [Google Scholar]
- Jung, J.S.; Kim, S.H. Application of smectite for textile dyeing and fastness improvement. RSC Adv. 2019, 9, 36631–36639. [Google Scholar] [CrossRef]
- Pisitsak, P.; Hutakamol, J.; Thongcharoen, R.; Phokaew, P.; Kanjanawan, K.; Saksaeng, N. Improving the dyeability of cotton with tannin-rich natural dye through pretreatment with whey protein isolate. Ind. Crops Prod. 2016, 79, 47–56. [Google Scholar] [CrossRef]
- Ghoreishian, S.M.; Maleknia, L.; Mirzapour, H.; Norouzi, M. Antibacterial properties and color fastness of silk fabric dyed with turmeric extract. Fibers Polym. 2013, 14, 201–207. [Google Scholar] [CrossRef]
- Bae, J.; Hong, K.H. Optimized Dyeing Process for Enhancing the Functionalities of Spent Coffee Dyed Wool Fabrics Using a Facile Extraction Process. Polymers 2019, 11, 574. [Google Scholar] [CrossRef] [PubMed]
- Sasivatchutikool, P.; Nakpathom, M. Application of Natural Dye Extracted from Cassia Fistula Ripe Pods for Dyeing of Silk Fabric. Fibers Polym. 2019, 20, 1841–1849. [Google Scholar] [CrossRef]
- Avinc, O.; Celik, A.; Gedik, G.; Yavas, A. Natural dye extraction from waste barks of Turkish red pine (Pinus brutia Ten.) timber and eco-friendly natural dyeing of various textile fibers. Fibers Polym. 2013, 14, 866–873. [Google Scholar] [CrossRef]
- Sutlović, A.; Brlek, I.; Ljubić, V.; Glogar, M.I. Optimization of Dyeing Process of Cotton Fabric with Cochineal Dye. Fibers Polym. 2020, 21, 555–563. [Google Scholar] [CrossRef]
- Kiratitanavit, W.; Xia, Z.; Singh, A.; Mosurkal, R.; Nagarajan, R. Tannic Acid: A Bio-based Intumescent Char-forming Additive for Nylon 6. Combustion 2016, 9, 10. [Google Scholar]
- Auriemma, M.; Piscitelli, A.; Pasquino, R.; Cerruti, P.; Malinconico, M.; Grizzuti, N. Blending poly (3-hydroxybutyrate) with tannic acid: Influence of a polyphenolic natural additive on the rheological and thermal behavior. Eur. Polym. J. 2015, 63, 123–131. [Google Scholar] [CrossRef]
- Laoutid, F.; Karaseva, V.; Costes, L.; Brohez, S.; Mincheva, R.; Dubois, P. Novel bio-based flame retardant systems derived from tannic acid. J. Renew. Mater. 2018, 6, 559–572. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, S.H.; Kim, S.H. Fabrication of Bio-Based Polyurethane Nanofibers Incorporated with a Triclosan/Cyclodextrin Complex for Antibacterial Applications. RSC Adv. 2020, 10, 3450–3458. [Google Scholar] [CrossRef]
- Guo, L.; Yang, Z.-Y.; Tang, R.-C.; Yuan, H.-B. Preliminary Studies on the Application of Grape Seed Extract in the Dyeing and Functional. Modif. Cotton Fabric Biomol. 2020, 10, 220. [Google Scholar]





| Characteristics | R-RM Fiber |
|---|---|
| Linear density (denier) | 4.7 |
| Tenacity (cN/tex) | 32.5 |
| Elongation (%) | 52.3 |
| Modulus at 1% (cN/tex) | 172.2 |
| Crimp number (ea/2.5 cm) | 12.0 |
| Crimp stability (%) | 57.3 |
| Untreated | R-RM Nonwoven | |||||
 | ||||||
| Dye conc. (%owf) | 1 | 3 | 5 | 7 | 10 | 13 |
 |  |  |  |  |  | |
| Temperature (°C) | 60 | 80 | 100 | 120 | 140 | |
 |  |  |  |  | ||
| Time (min) | 20 | 40 | 60 | 80 | ||
 |  |  |  |
| Colorfastness | R-RM Nonwoven | |||
|---|---|---|---|---|
| Light | Color change | 2–3 | ||
| Rubbing | Dry | 4–5 | ||
| Wet | 4 | |||
| Perspiration | Acidic | Color change | 4–5 | |
| Stain | Cotton | 4–5 | ||
| PET | 4–5 | |||
| Alkalic | Color change | 4–5 | ||
| Stain | Cotton | 4–5 | ||
| PET | 4–5 | |||
| TGA in N2 Atmosphere | |||
|---|---|---|---|
| T5% (°C) a | TD (°C) b | Char Yield (%) | |
| T. chebula dye | 150 | 370 | 32 |
| R-RM nonwoven | 367 | 508 | 4 |
| R-RM nonwoven dyed at 100 °C | 407 | 524 | 14 |
| R-RM nonwoven dyed at 140 °C | 415 | 536 | 16 |
| Sample | R-RM Nonwoven | |
|---|---|---|
| Bacteria Type | S. aureus (ATCC 6538) | K. pneumonia (ATCC 4352) |
| Initial average number of bacteria (CFU 1) | 1.8 × 104 | 1.8 × 104 |
| A 2 | 1.8 × 104 | 1.8 × 104 |
| B 3 | 9.2 × 106 | 3.8 × 107 |
| Cytostatic activity | 2.7 | 3.3 |
| Cytostatic efficiency (%) | 99.9 | 99.9 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.H.; Jung, J.S.; Kim, S.H. Dyeing and Antibacterial Properties of Chemically Recycled PET Thermal-Bonded Nonwovens Dyed with Terminalia chebula Dye. Polymers 2020, 12, 1675. https://doi.org/10.3390/polym12081675
Lee JH, Jung JS, Kim SH. Dyeing and Antibacterial Properties of Chemically Recycled PET Thermal-Bonded Nonwovens Dyed with Terminalia chebula Dye. Polymers. 2020; 12(8):1675. https://doi.org/10.3390/polym12081675
Chicago/Turabian StyleLee, Joo Hyung, Jong Sun Jung, and Seong Hun Kim. 2020. "Dyeing and Antibacterial Properties of Chemically Recycled PET Thermal-Bonded Nonwovens Dyed with Terminalia chebula Dye" Polymers 12, no. 8: 1675. https://doi.org/10.3390/polym12081675
APA StyleLee, J. H., Jung, J. S., & Kim, S. H. (2020). Dyeing and Antibacterial Properties of Chemically Recycled PET Thermal-Bonded Nonwovens Dyed with Terminalia chebula Dye. Polymers, 12(8), 1675. https://doi.org/10.3390/polym12081675






