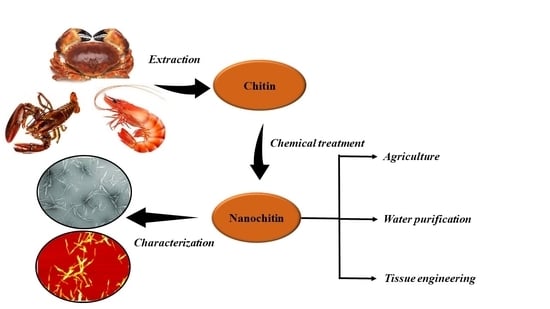
Extraction of Nanochitin from Marine Resources and Fabrication of Polymer Nanocomposites: Recent Advances
Abstract
1. Introduction
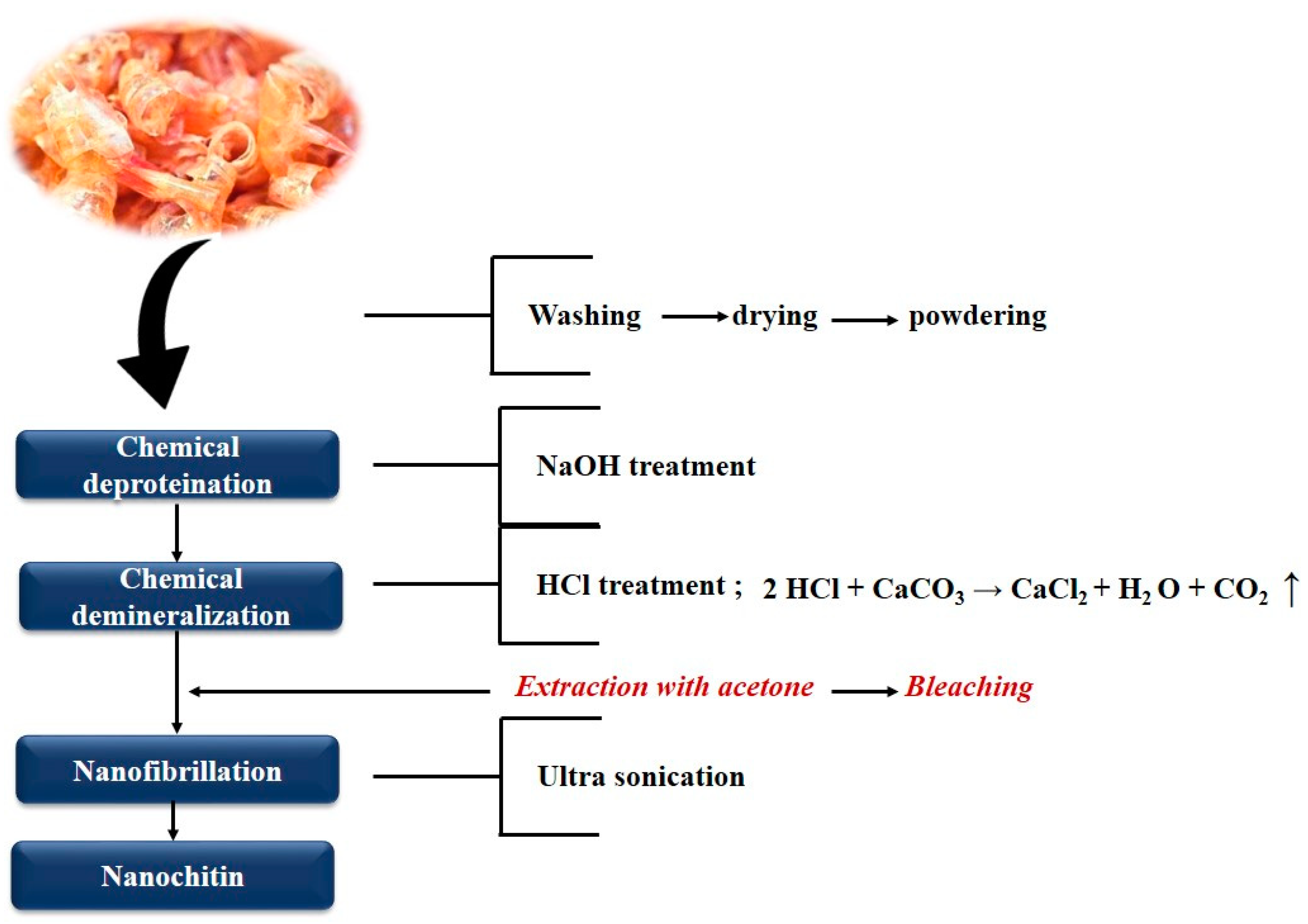
2. Extraction of Nanochitin
2.1. Chitin Nanowhiskers (CNW)
2.2. Chitin Nanofibrils (CNF) or Chitin Nanofibers
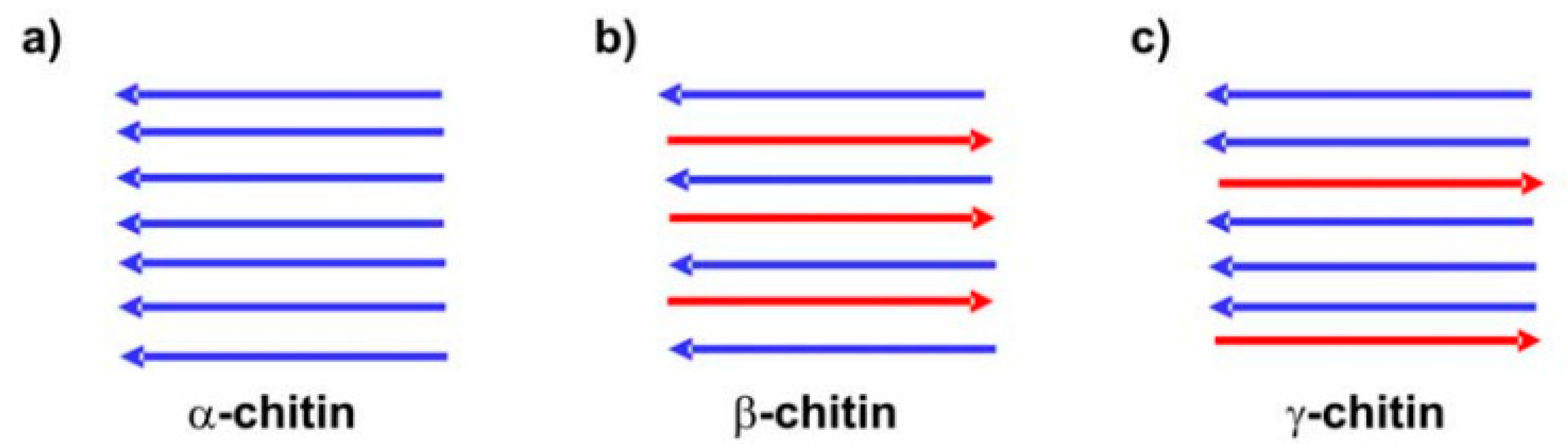
3. Properties of Nanochitin
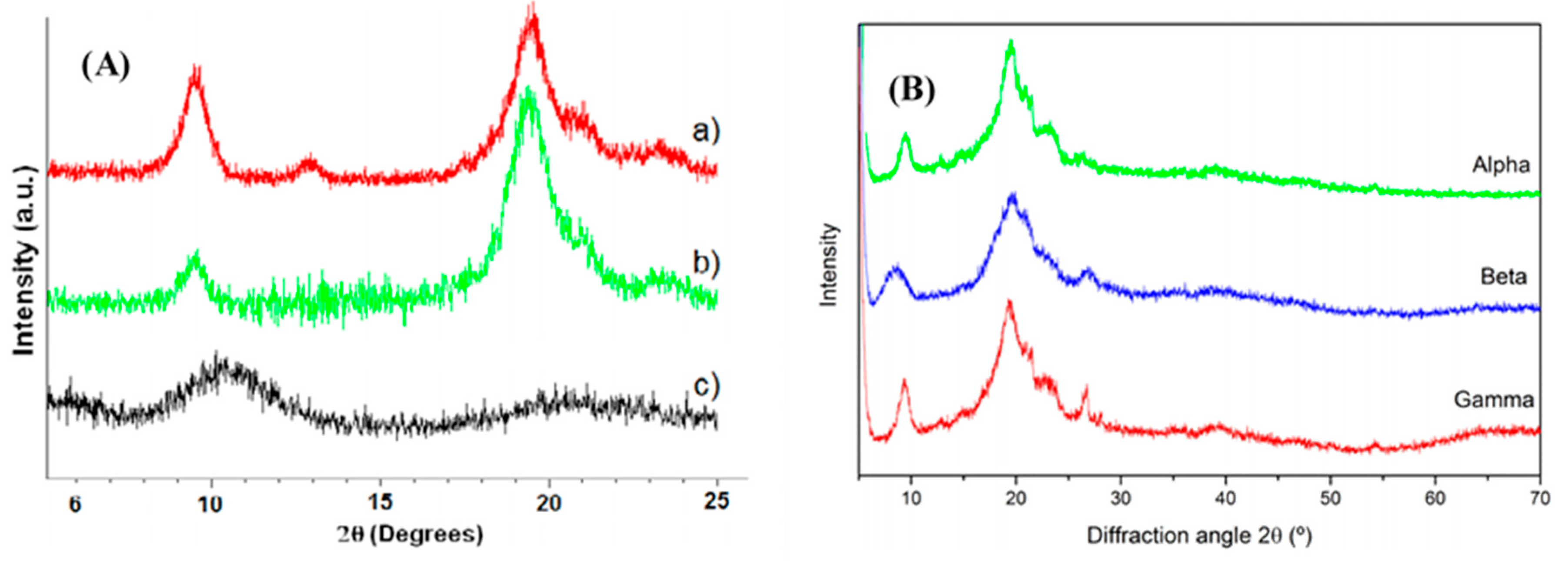
3.1. Physico-Chemical Properties
3.2. Morphology of Nanochitin
4. Nanochitin-Based Polymer Nanocomposites
4.1. Freeze-Drying
4.2. Casting/Solvent Evaporation
4.3. Extrusion
4.4. Electrospinning
5. Characterization of Chitin Nanowhiskers Containing Polymer Composites
5.1. Transmission Electron Microscopy
5.2. Scanning Electron Microscopy (SEM)
5.3. Mechanical Properties of Nanochitin Composites
5.4. Thermal Properties of Nanochitin Composites
5.5. Rheological Properties of Nanochitin-Based Composites
5.6. Barrier Properties of Nanochitin-Based Composites
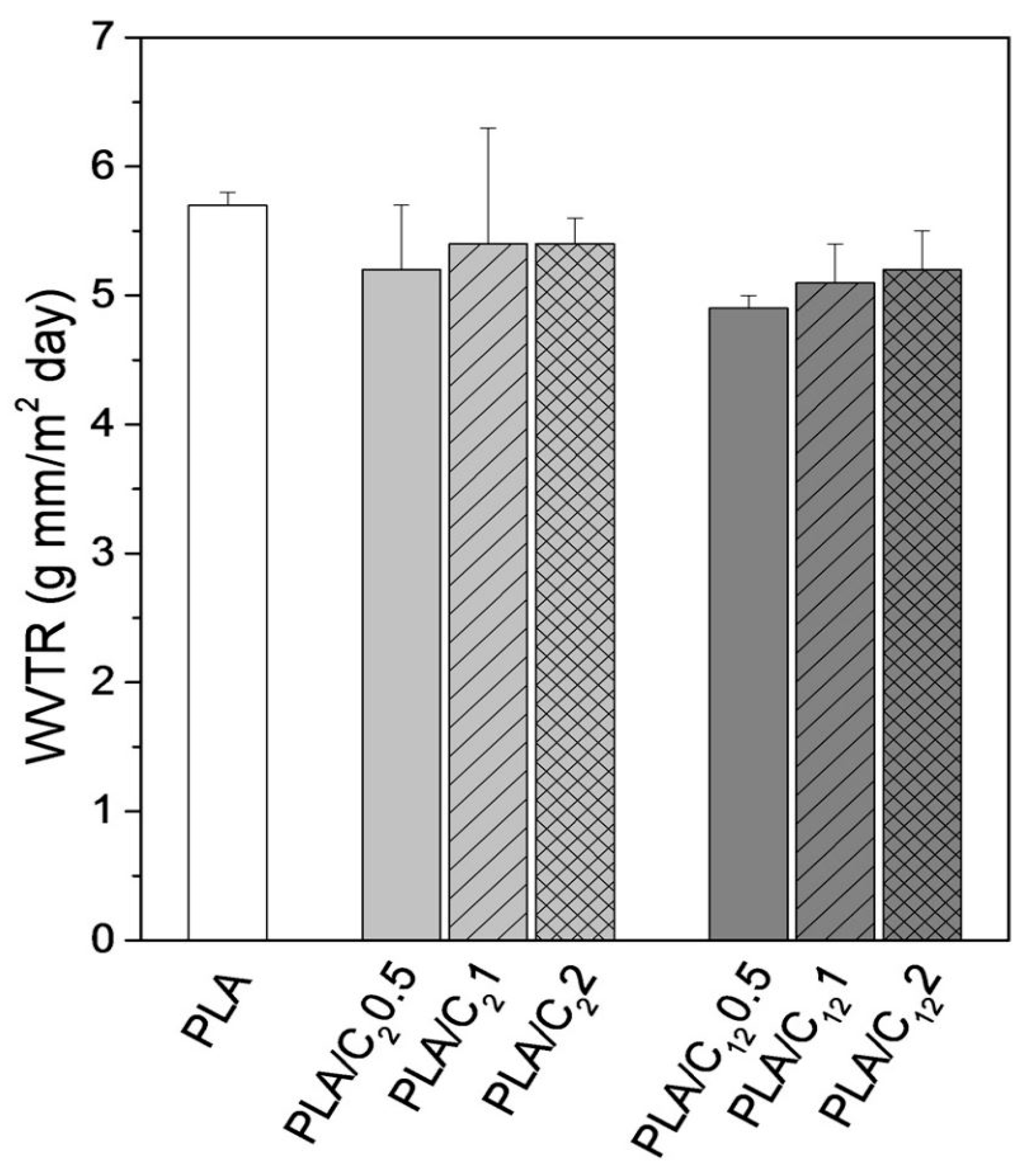
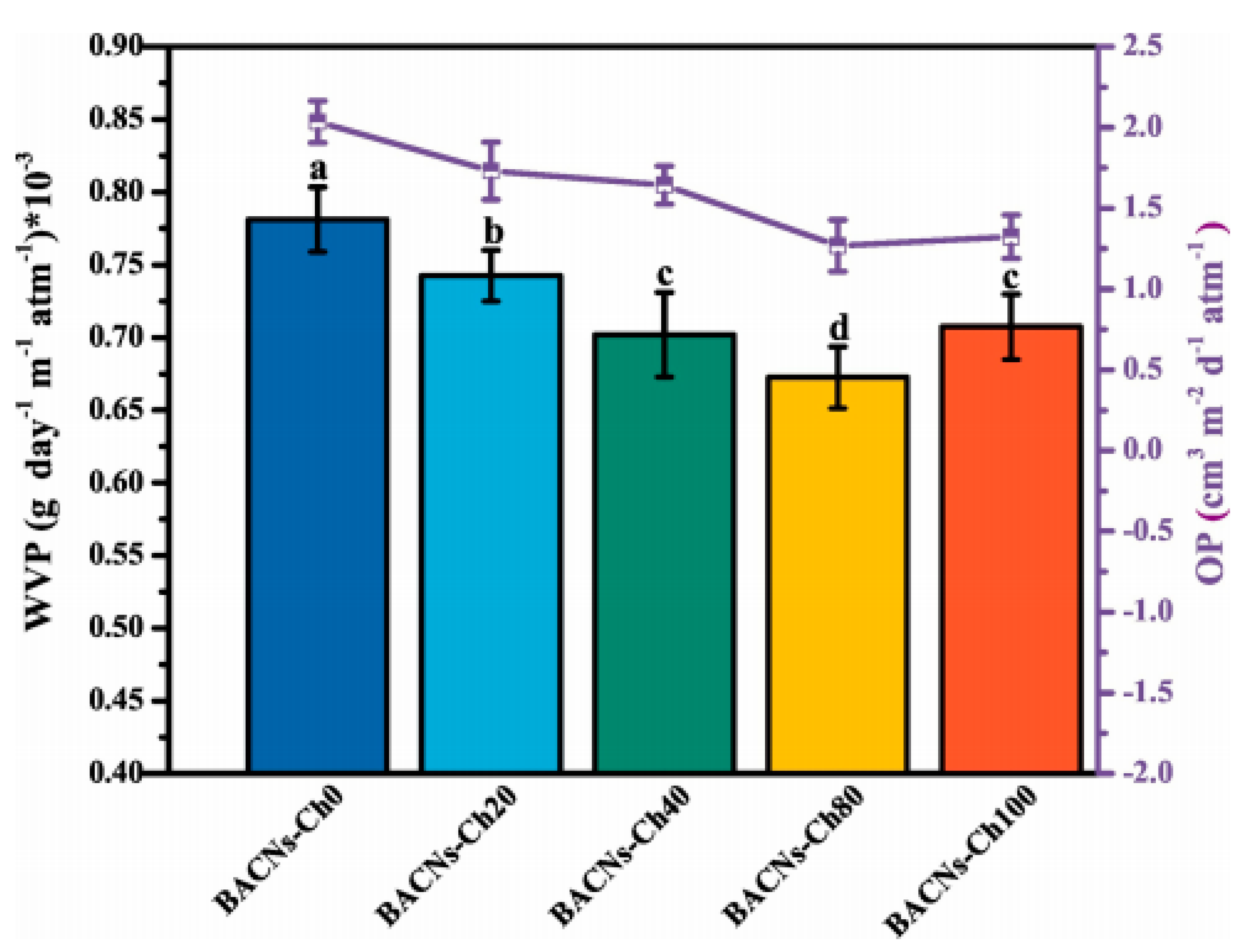
5.6.1. Water/Water Vapor Permeability (WVP)
5.6.2. Gas/Oxygen Permeability (OP)
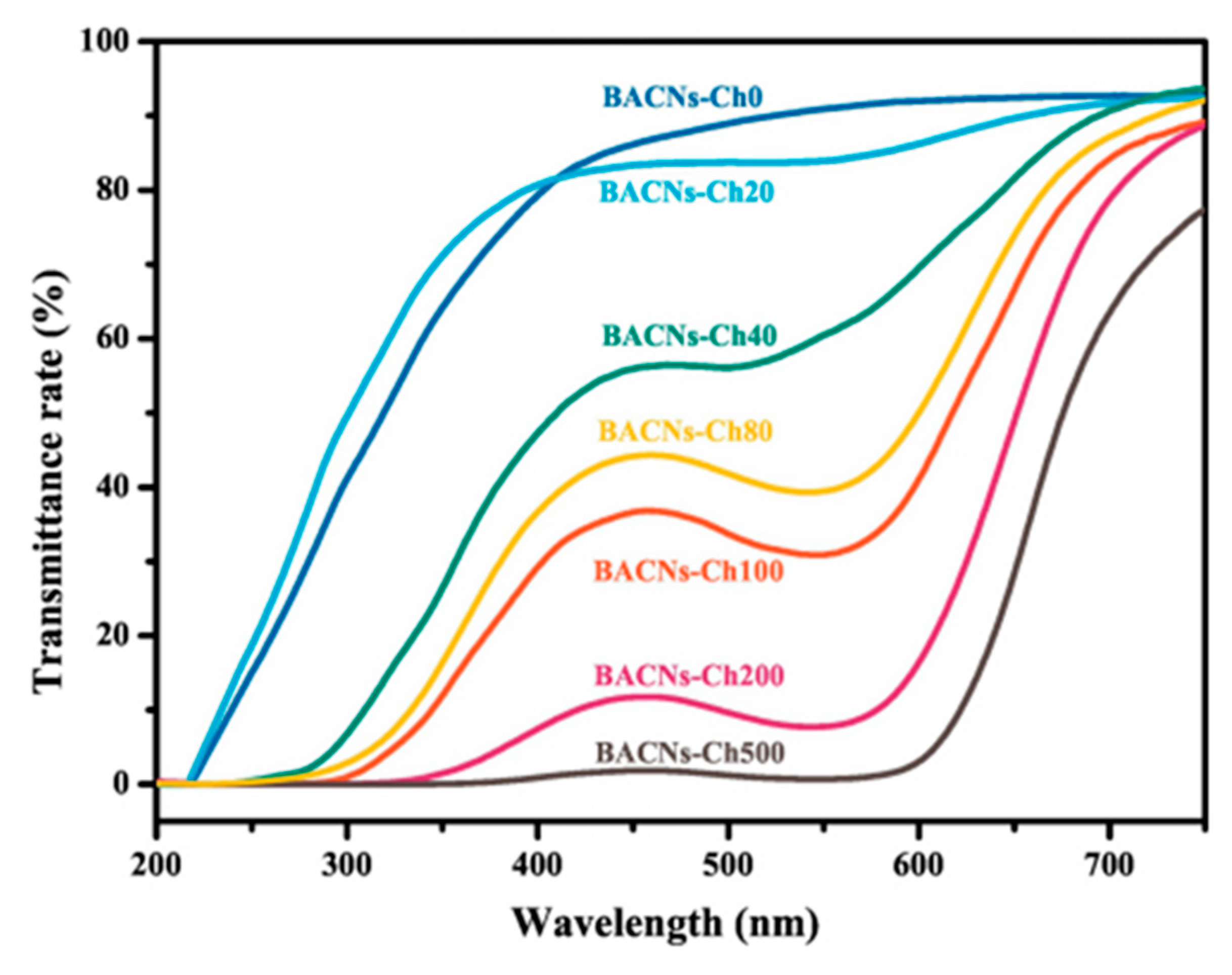
5.7. Optical Properties
6. Applications of Nanochitin
7. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Silva, T.H.; Alves, A.; Ferreira, B.M.; Oliveira, J.M.; Reys, L.L.; Ferreira, R.J.F.; Sousa, R.A.; Silva, S.S.; Mano, J.F.; Reis, R.L. Materials of marine origin: A review on polymers and ceramics of biomedical interest. Int. Mater. Rev. 2012, 57, 276–306. [Google Scholar] [CrossRef]
- Manivasagan, P.; Bharathiraja, S.; Moorthy, M.S.; Oh, Y.O.; Seo, H.; Oh, J. Marine Biopolymer-Based Nanomaterials as a Novel Platform for Theranostic Applications. Polym. Rev. 2017, 57, 631–667. [Google Scholar] [CrossRef]
- Austin, P.; Brine, C.; Castle, J.; Zikakis, J. Chitin: New facets of research. Science 1981, 212, 749–753. [Google Scholar] [CrossRef]
- Das, S.; Roy, D.; Sen, R. Utilization of Chitinaceous Wastes for the Production of Chitinase. In Advances in Food and Nutrition Research; Academic Press: Cambridge, MA, USA, 2016; pp. 27–46. [Google Scholar]
- Kadokawa, J. Preparation and Applications of Chitin Nanofibers/Nanowhiskers. In Biopolymer Nanocomposites; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013; pp. 131–151. [Google Scholar]
- Kumirska, J.; Czerwicka, M.; Kaczyński, Z.; Bychowska, A.; Brzozowski, K.; Thöming, J.; Stepnowski, P. Application of spectroscopic methods for structural analysis of chitin and chitosan. Mar. Drugs 2010, 8, 1567–1636. [Google Scholar] [CrossRef]
- Rudall, K.M. The Chitin/Protein Complexes of Insect Cuticles. In Advances in Insect Physiology; Academic Press: Cambridge, MA, USA, 1963; pp. 257–313. [Google Scholar]
- Kumirska, J.; Weinhold, M.X.; Thöming, J.; Stepnowski, P. Biomedical Activity of Chitin/Chitosan Based Materials—Influence of Physicochemical Properties Apart from Molecular Weight and Degree of N-Acetylation. Polymers 2011, 3, 1875–1901. [Google Scholar] [CrossRef]
- Younes, I.; Rinaudo, M. Chitin and chitosan preparation from marine sources. Structure, properties and applications. Mar. Drugs 2015, 13, 1133–1174. [Google Scholar] [CrossRef]
- Casadidio, C.; Peregrina, D.V.; Gigliobianco, M.R.; Deng, S.; Censi, R.; Di Martino, P. Chitin and Chitosans: Characteristics, Eco-Friendly Processes, and Applications in Cosmetic Science. Mar. Drugs 2019, 17, 369. [Google Scholar] [CrossRef]
- Chatelet, C.; Damour, O.; Domard, A. Influence of the degree of acetylation on some biological properties of chitosan films. Biomaterials 2001, 22, 261–268. [Google Scholar] [CrossRef]
- Nakajima, M.; Atsumi, K.; Kifune, K.; Miura, K.; Kanamaru, H. Chitin is an effective material for sutures. Jpn. J. Surg. 1986, 16, 418–424. [Google Scholar] [CrossRef]
- Wu, H.; Williams, G.R.; Wu, J.; Wu, J.; Niu, S.; Li, H.; Wang, H.; Zhu, L. Regenerated chitin fibers reinforced with bacterial cellulose nanocrystals as suture biomaterials. Carbohydr. Polym. 2018, 180, 304–313. [Google Scholar] [CrossRef]
- Di Rosa, M.; Distefano, G.; Zorena, K.; Malaguarnera, L. Chitinases and immunity: Ancestral molecules with new functions. Immunobiology 2016, 221, 399–411. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.G.; Da Silva, C.A.; Lee, J.Y.; Hartl, D.; Elias, J.A. Chitin regulation of immune responses: An old molecule with new roles. Curr. Opin. Immunol. 2008, 20, 684–689. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.G.; Da Silva, C.A.; Dela Cruz, C.S.; Ahangari, F.; Ma, B.; Kang, M.-J.; He, C.-H.; Takyar, S.; Elias, J.A. Role of Chitin and Chitinase/Chitinase-Like Proteins in Inflammation, Tissue Remodeling, and Injury. Annu. Rev. Physiol. 2011, 73, 479–501. [Google Scholar] [CrossRef]
- Karagozlu, M.Z.; Kim, S.-K. Anti-Cancer Effects of Chitin and Chitosan Derivatives. In Handbook of Anticancer Drugs from Marine Origin; Springer International Publishing: Cham, Switzerland, 2015; pp. 413–421. [Google Scholar]
- Bouhenna, M.; Salah, R.; Bakour, R.; Drouiche, N.; Abdi, N.; Grib, H.; Lounici, H.; Mameri, N. Effects of chitin and its derivatives on human cancer cells lines. Environ. Sci. Pollut. Res. 2015, 22, 15579–15586. [Google Scholar] [CrossRef] [PubMed]
- Rementería, A.; Abaitua, F.; García-Tobalina, R.; Hernando, F.; Pontón, J.; Sevilla, M.J. Resistance to candidiasis and macrophage activity in chitin-treated mice. FEMS Immunol. Med. Microbiol. 1997, 19, 223–230. [Google Scholar] [CrossRef]
- Ito, I.; Osaki, T.; Ifuku, S.; Saimoto, H.; Takamori, Y.; Kurozumi, S.; Imagawa, T.; Azuma, K.; Tsuka, T.; Okamoto, Y.; et al. Evaluation of the effects of chitin nanofibrils on skin function using skin models. Carbohydr. Polym. 2014, 101, 464–470. [Google Scholar] [CrossRef]
- Ito, I.; Yoneda, T.; Omura, Y.; Osaki, T.; Ifuku, S.; Saimoto, H.; Azuma, K.; Imagawa, T.; Tsuka, T.; Murahata, Y.; et al. Protective effect of chitin urocanate nanofibers against ultraviolet radiation. Mar. Drugs 2015, 101, 464–470. [Google Scholar] [CrossRef]
- Global Industry Analysts, I. (GIA) Chitin and Chitosan Derivatives: A Global Strategic Business Report; Global Industry Analysts, Inc.: San Jose, CA, USA, 2020. [Google Scholar]
- Mincea, M.; Negrulescu, A.; Ostafe, V. Preparation, modification, and applications of chitin nanowhiskers: A review. Rev. Adv. Mater. Sci. 2012, 30, 225–242. [Google Scholar]
- Gopi, S.; Balakrishnan, P.; Pius, A.; Thomas, S. Chitin nanowhisker (ChNW)-functionalized electrospun PVDF membrane for enhanced removal of Indigo carmine. Carbohydr. Polym. 2017, 165, 115–122. [Google Scholar] [CrossRef]
- Markovic, G.; Visakh, P.M. Applications of chitin based rubber nanocomposites. In Advanced Structured Materials; Springer: Berlin/Heidelberg, Germany, 2017; Volume 56, pp. 51–69. [Google Scholar]
- Zhang, M.; Haga, A.; Sekiguchi, H.; Hirano, S. Structure of insect chitin isolated from beetle larva cuticle and silkworm (Bombyx mori) pupa exuvia. Int. J. Biol. Macromol. 2000, 27, 99–105. [Google Scholar] [CrossRef]
- Zeng, J.B.; He, Y.S.; Li, S.L.; Wang, Y.Z. Chitin whiskers: An overview. Biomacromolecules 2012, 13, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Saito, T.I. Chitin nanocrystals prepared by TEMPO-mediated oxidation of α-chitin. Biomacromolecules 2008, 9, 192–198. [Google Scholar] [CrossRef]
- Gopalan Nair, K.; Dufresne, A. Crab Shell Chitin Whisker Reinforced Natural Rubber Nanocomposites. 1. Processing and Swelling Behavior. Biomacromolecules 2003, 4, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Nair, K.G.; Dufresne, A.; Gandini, A.; Belgacem, M.N. Crab shell chitin whiskers reinforced natural rubber nanocomposites. 3. Effect of Chemical Modification of chitin whiskers. Biomacromolecules 2003, 4, 1835–1842. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Sun, Q.; She, X.; Xia, Y.; Liu, Y.; Li, J.; Yang, D. Fabrication and characterisation of α-chitin nanofibers and highly transparent chitin films by pulsed ultrasonication. Carbohydr. Polym. 2013, 98, 1497–1504. [Google Scholar] [CrossRef]
- Salaberria, A.M.; Labidi, J.; Fernandes, S.C.M. Different routes to turn chitin into stunning nano-objects. Eur. Polym. J. 2015, 68, 503–515. [Google Scholar] [CrossRef]
- Zubillaga, V.; Alonso-Varona, A.; Fernandes, S.C.M.; Salaberria, A.M.; Palomares, T. Adipose-Derived Mesenchymal Stem Cell Chondrospheroids Cultured in Hypoxia and a 3D Porous Chitosan/Chitin Nanocrystal Scaffold as a Platform for Cartilage Tissue Engineering. Int. J. Mol. Sci. 2020, 21, 1004. [Google Scholar] [CrossRef]
- Robles, E.; Salaberria, A.M.; Herrera, R.; Fernandes, S.C.M.; Labidi, J. Self-bonded composite films based on cellulose nanofibers and chitin nanocrystals as antifungal materials. Carbohydr. Polym. 2016, 144, 41–49. [Google Scholar] [CrossRef]
- Labidi, A.; Salaberria, A.M.; Fernandes, S.C.M.; Labidi, J.; Abderrabba, M. Adsorption of copper on chitin-based materials: Kinetic and thermodynamic studies. J. Taiwan Inst. Chem. Eng. 2016, 65, 140–148. [Google Scholar] [CrossRef]
- Lu, Y.; Weng, L.; Zhang, L. Morphology and properties of soy protein isolate thermoplastics reinforced with chitin whiskers. Biomacromolecules 2004, 5, 1046–1051. [Google Scholar] [CrossRef]
- Morin, A.; Dufresne, A. Nanocomposites of chitin whiskers from Riftia tubes and poly(caprolactone). Macromolecules 2002, 35, 2190–2199. [Google Scholar] [CrossRef]
- Sriupayo, J.; Supaphol, P.; Blackwell, J.; Rujiravanit, R. Preparation and characterization of α-chitin whisker-reinforced chitosan nanocomposite films with or without heat treatment. Carbohydr. Polym. 2005, 62, 130–136. [Google Scholar] [CrossRef]
- Revol, J.F.; Marchessault, R.H. In vitro chiral nematic ordering of chitin crystallites. Int. J. Biol. Macromol. 1993, 15, 329–335. [Google Scholar] [CrossRef]
- Li, J.; Revol, J.F.; Marchessault, R.H. Rheological properties of aqueous suspensions of chitin crystallites. J. Colloid Interface Sci. 1996, 183, 365–373. [Google Scholar] [CrossRef]
- Salaberria, A.M.; Diaz, R.H.; Labidi, J.; Fernandes, S.C.M. Preparing valuable renewable nanocomposite films based exclusively on oceanic biomass-Chitin nanofillers and chitosan. React. Funct. Polym. 2015, 89, 31–39. [Google Scholar] [CrossRef]
- Ifuku, S.; Nogi, M.; Abe, K.; Yoshioka, M.; Morimoto, M.; Saimoto, H.; Yano, H. Preparation of chitin nanofibers with a uniform width as α-chitin from crab shells. Biomacromolecules 2009, 10, 1584–1588. [Google Scholar] [CrossRef]
- Ifuku, S.; Nogi, M.; Yoshioka, M.; Morimoto, M.; Yano, H.; Saimoto, H. Fibrillation of dried chitin into 10-20 nm nanofibers by a simple grinding method under acidic conditions. Carbohydr. Polym. 2010, 81, 134–139. [Google Scholar] [CrossRef]
- Salaberria, A.M.; Fernandes, S.C.M.; Diaz, R.H.; Labidi, J. Processing of α-chitin nanofibers by dynamic high pressure homogenization: Characterization and antifungal activity against A. niger. Carbohydr. Polym. 2015, 116, 286–291. [Google Scholar] [CrossRef]
- Mushi, N.E.; Butchosa, N.; Salajkova, M.; Zhou, Q.; Berglund, L.A. Nanostructured membranes based on native chitin nanofibers prepared by mild process. Carbohydr. Polym. 2014, 112, 255–263. [Google Scholar] [CrossRef]
- Ifuku, S.; Nomura, R.; Morimoto, M.; Saimoto, H. Preparation of Chitin Nanofibers from Mushrooms. Materials 2011, 4, 1417–1425. [Google Scholar] [CrossRef]
- Cai, J.; Xiong, Z.; Zhou, M.; Tan, J.; Zeng, F.; Lin, S.; Xiong, H. Thermal properties and crystallization behavior of thermoplastic starch/poly(É”-caprolactone) composites. Carbohydr. Polym. 2014, 102, 746–754. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, Y.; Nge, T.T.; Takemura, A.; Hori, N.; Ono, H. Characterization of uniaxially aligned chitin film by 2D FT-IR spectroscopy. Biomacromolecules 2005, 6, 1941–1947. [Google Scholar] [CrossRef] [PubMed]
- Wijesena, R.N.; Tissera, N.; Kannangara, Y.Y.; Lin, Y.; Amaratunga, G.A.J.; de Silva, K.M.N. A method for top down preparation of chitosan nanoparticles and nanofibers. Carbohydr. Polym. 2015, 117, 731–738. [Google Scholar] [CrossRef]
- Kaya, M.; Mujtaba, M.; Ehrlich, H.; Salaberria, A.M.; Baran, T.; Amemiya, C.T.; Galli, R.; Akyuz, L.; Sargin, I.; Labidi, J. On chemistry of γ-chitin. Carbohydr. Polym. 2017, 176, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Daraghmeh, N.H.; Chowdhry, B.Z.; Leharne, S.A.; Al Omari, M.M.; Badwan, A.A. Chitin. In Profiles of Drug Substances, Excipients and Related Methodology; Brittain, H., Ed.; Elsevier: Cambridge, MA, USA, 2011; pp. 35–102. [Google Scholar]
- Favier, V.; Chanzy, H.; Cavaillé, J.Y. Polymer Nanocomposites Reinforced by Cellulose Whiskers. Macromolecules 1995, 28, 6365–6367. [Google Scholar] [CrossRef]
- Habibi, Y.; Lucia, L.A.; Rojas, O.J. Cellulose nanocrystals: Chemistry, self-assembly, and applications. Chem. Rev. 2010, 110, 3479–3500. [Google Scholar] [CrossRef]
- Fan, Y.; Saito, T.; Isogai, A. Individual chitin nano-whiskers prepared from partially deacetylated α-chitin by fibril surface cationization. Carbohydr. Polym. 2010, 79, 1046–1051. [Google Scholar] [CrossRef]
- Saralegi, A.; Fernandes, S.C.M.; Alonso-Varona, A.; Palomares, T.; Foster, E.J.; Weder, C.; Eceiza, A.; Corcuera, M.A. Shape-memory bionanocomposites based on chitin nanocrystals and thermoplastic polyurethane with a highly crystalline soft segment. Biomacromolecules 2013, 14, 4475–4482. [Google Scholar] [CrossRef]
- Zubillaga, V.; Salaberria, A.M.; Palomares, T.; Alonso-Varona, A.; Kootala, S.; Labidi, J.; Fernandes, S.C.M. Chitin Nanoforms Provide Mechanical and Topological Cues to Support Growth of Human Adipose Stem Cells in Chitosan Matrices. Biomacromolecules 2018, 19, 3000–3012. [Google Scholar] [CrossRef]
- Salaberria, A.M.; Diaz, R.H.; Andrés, M.A.; Fernandes, S.C.M.; Labidi, J. The antifungal activity of functionalized chitin nanocrystals in poly (Lactid Acid) films. Materials 2017, 10, 546. [Google Scholar] [CrossRef]
- Herrera, N.; Roch, H.; Salaberria, A.M.; Pino-Orellana, M.A.; Labidi, J.; Fernandes, S.C.M.; Radic, D.; Leiva, A.; Oksman, K. Functionalized blown films of plasticized polylactic acid/chitin nanocomposite: Preparation and characterization. Mater. Des. 2016, 92, 846–852. [Google Scholar] [CrossRef]
- Salaberria, A.M.; Labidi, J.; Fernandes, S.C.M. Chitin nanocrystals and nanofibers as nano-sized fillers into thermoplastic starch-based biocomposites processed by melt-mixing. Chem. Eng. J. 2014, 256, 356–364. [Google Scholar] [CrossRef]
- Zhang, X.; Elsayed, I.; Navarathna, C.; Schueneman, G.T.; Hassan, E.B. Biohybrid Hydrogel and Aerogel from Self-Assembled Nanocellulose and Nanochitin as a High-Efficiency Adsorbent for Water Purification. ACS Appl. Mater. Interfaces 2019, 11, 46714–46725. [Google Scholar] [CrossRef] [PubMed]
- Garcia, I.; Azcune, I.; Casuso, P.; Carrasco, P.M.; Grande, H.-J.; Cabañero, G.; Katsigiannopoulos, D.; Grana, E.; Dimos, K.; Karakassides, M.A.; et al. Carbon nanotubes/chitin nanowhiskers aerogel achieved by quaternization-induced gelation. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Salaberria, A.M.; Diaz, R.H.; Labidi, J.; Fernandes, S.C.M. Role of chitin nanocrystals and nanofibers on physical, mechanical and functional properties in thermoplastic starch films. Food Hydrocoll. 2015, 46, 93–102. [Google Scholar] [CrossRef]
- Liu, M.; Peng, Q.; Luo, B.; Zhou, C. The improvement of mechanical performance and water-response of carboxylated SBR by chitin nanocrystals. Eur. Polym. J. 2015, 68, 190–206. [Google Scholar] [CrossRef]
- Dufresne, A. Processing of polymer nanocomposites reinforced with polysaccharide nanocrystals. Molecules 2010, 15, 4111–4128. [Google Scholar] [CrossRef]
- Chi-Yan Li, S.; Sun, Y.C.; Guan, Q.; Naguib, H. Effects of chitin nanowhiskers on the thermal, barrier, mechanical, and rheological properties of polypropylene nanocomposites. RSC Adv. 2016, 6, 72086–72095. [Google Scholar] [CrossRef]
- Rizvi, R.; Cochrane, B.; Naguib, H.; Lee, P.C. Fabrication and characterization of melt-blended polylactide-chitin composites and their foams. J. Cell. Plast. 2011, 47, 283–300. [Google Scholar] [CrossRef]
- Joseph, B.; Augustine, R.; Kalarikkal, N.; Thomas, S.; Seantier, B.; Grohens, Y. Recent advances in electrospun polycaprolactone based scaffolds for wound healing and skin bioengineering applications. Mater. Today Commun. 2019, 19, 319–335. [Google Scholar] [CrossRef]
- Junkasem, J.; Rujiravanit, R.; Supaphol, P. Fabrication of α-chitin whisker-reinforced poly(vinyl alcohol) nanocomposite nanofibres by electrospinning. Nanotechnology 2006, 17, 4519. [Google Scholar] [CrossRef]
- Gopi, S.; Kargl, R.; Kleinschek, K.S.; Pius, A.; Thomas, S. Chitin nanowhisker—Inspired electrospun PVDF membrane for enhanced oil-water separation. J. Environ. Manag. 2018, 228, 249–259. [Google Scholar] [CrossRef]
- Anwer, M.A.S.; Wang, J.; Guan, A.; Naguib, H.E. Chitin nano-whiskers (CNWs) as a bio-based bio-degradable reinforcement for epoxy: Evaluation of the impact of CNWs on the morphological, fracture, mechanical, dynamic mechanical, and thermal characteristics of DGEBA epoxy resin. RSC Adv. 2019, 9, 11063–11076. [Google Scholar] [CrossRef]
- Alonso, B.; Belamie, E. Chitin-Silica Nanocomposites by Self-Assembly. Angew. Chem. 2010, 122, 8377–8380. [Google Scholar] [CrossRef]
- Qin, A.; Li, X.; Zhao, X.; Liu, D.; He, C. Preparation and characterization of nano-chitin whisker reinforced PVDF membrane with excellent antifouling property. J. Membr. Sci. 2015, 480, 1–10. [Google Scholar] [CrossRef]
- Mathew, A.P.; Laborie, M.-P.G.; Oksman, K. Cross-Linked Chitosan/Chitin Crystal Nanocomposites with Improved Permeation Selectivity and pH Stability. Biomacromolecules 2009, 10, 1627–1632. [Google Scholar] [CrossRef]
- Zhou, Y.; Fu, S.; Pu, Y.; Pan, S.; Ragauskas, A.J. Preparation of aligned porous chitin nanowhisker foams by directional freeze-casting technique. Carbohydr. Polym. 2014, 112, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Liang, K.; Wang, X.; Ji, Y. Fabrication of Nanocomposite Bioelastomer Porous Scaffold Based on Chitin Nanocrystal Supported Emulsion-Freeze-Casting. ACS Sustain. Chem. Eng. 2017, 5, 3305–3313. [Google Scholar] [CrossRef]
- Mushi, N.E.; Utsel, S.; Berglund, L.A. Nanostructured biocomposite films of high toughness based on native chitin nanofibers and chitosan. Front. Chem. 2014, 2, 99. [Google Scholar] [CrossRef] [PubMed]
- Nie, J.; Mou, W.; Ding, J.; Chen, Y. Bio-based epoxidized natural rubber/chitin nanocrystals composites: Self-healing and enhanced mechanical properties. Compos. Part B Eng. 2019, 172, 152–160. [Google Scholar] [CrossRef]
- Bie, D.; Jiang, L.; Zhu, M.; Miao, W.; Wang, Z. Effect of Chitin Nanocrystals on the Formation of Shish-Kebab Crystals in Bimodal Polyethylene Injection Bar. Polym. Sci.-Ser. A 2019, 61, 627–634. [Google Scholar] [CrossRef]
- Peng, C.; Xu, J.; Chen, G.; Tian, J.; He, M. The preparation of α-chitin nanowhiskers-poly (vinyl alcohol) hydrogels for drug release. Int. J. Biol. Macromol. 2019, 131, 336–342. [Google Scholar] [CrossRef]
- Meng, D.; Xie, J.; Waterhouse, G.I.N.; Zhang, K.; Zhao, Q.; Wang, S.; Qiu, S.; Chen, K.; Li, J.; Ma, C.; et al. Biodegradable Poly(butylene adipate-co-terephthalate) composites reinforced with bio-based nanochitin: Preparation, enhanced mechanical and thermal properties. J. Appl. Polym. Sci. 2019, 137, 48485. [Google Scholar] [CrossRef]
- Coltelli, M.; Cinelli, P.; Gigante, V.; Aliotta, L.; Morganti, P.; Panariello, L.; Lazzeri, A. Chitin Nanofibrils in Poly (Lactic Acid) (PLA) Nanocomposites: Dispersion and Thermo-Mechanical Properties. Int. J. Mol. Sci. 2019, 20, 504. [Google Scholar] [CrossRef] [PubMed]
- Sahraee, S.; Milani, J.M.; Ghanbarzadeh, B.; Hamishekar, H. Effect of corn oil on physical, thermal, and antifungal properties of gelatin-based nanocomposite films containing nano chitin. LWT-Food Sci. Technol. 2017, 76, 33–39. [Google Scholar] [CrossRef]
- Li, D.; Gao, H.; Li, M.; Chen, G.; Guan, L.; He, M.; Tian, J.; Cao, R. Nanochitin/metal ion dual reinforcement in synthetic polyacrylamide network-based nanocomposite hydrogels. Carbohydr. Polym. 2020, 236, 116061. [Google Scholar] [CrossRef] [PubMed]
- Oun, A.A.; Rhim, J. Preparation of multifunctional carboxymethyl cellulose-based films incorporated with chitin nanocrystal and grapefruit seed extract. Int. J. Biol. Macromol. 2019. [Google Scholar] [CrossRef]
- Xu, J.; Zhou, Z.; Cai, J.; Tian, J. Conductive biomass-based composite wires with cross-linked anionic nanocellulose and cationic nanochitin as scaffolds. Int. J. Biol. Macromol. 2019, 156, 1183–1190. [Google Scholar] [CrossRef]
- Sun, J.; Du, Y.; Ma, J.; Li, Y.; Wang, L.; Lu, Y.; Zou, J.; Pang, J.; Wu, C. Transparent bionanocomposite films based on konjac glucomannan, chitosan, and TEMPO-oxidized chitin nanocrystals with enhanced mechanical and barrier properties. Int. J. Biol. Macromol. 2019, 138, 866–873. [Google Scholar] [CrossRef]
- Teacă, C.A.; Bodîrlău, R. Rheology and Processing of Nanocellulose, Nanochitin, and Nanostarch/Polymer Bionanocomposites. In Rheology and Processing of Polymer Nanocomposites; Wiley: Hoboken, NJ, USA, 2016; pp. 453–490. ISBN 9781118969809. [Google Scholar]
- Rošic, R.; Pelipenko, J.; Kocbek, P.; Baumgartner, S.; Bešter-Rogač, M.; Kristl, J. The role of rheology of polymer solutions in predicting nanofiber formation by electrospinning. Eur. Polym. J. 2012, 48, 1374–1384. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.A.; El Mehtedi, M.; Mattioli-belmonte, M. Emerging Biomedical Applications of Nano-Chitins and Nano-Chitosans Obtained via Advanced Eco-Friendly Technologies from Marine Resources. Mar. Drugs 2014, 12, 5468–5502. [Google Scholar] [CrossRef] [PubMed]
- González, J.A.; Mazzobre, M.F.; Villanueva, M.E.; Díaz, L.E.; Copello, G.J. Chitin hybrid materials reinforced with graphene oxide nanosheets: Chemical and mechanical characterisation. RSC Adv. 2014, 4, 16480–16488. [Google Scholar] [CrossRef]
- Li, M.; Ge, S.; Liu, Q.; Li, M.; Liu, J.; Lu, H.; Li, F.; Zhang, S. Enhanced mechanical properties and gelling ability of gelatin hydrogels reinforced with chitin whiskers. Food Hydrocoll. 2017, 75, 1–12. [Google Scholar] [CrossRef]
- Zhou, H.; Lv, S.; Liu, J.; Tan, Y.; Muriel Mundo, J.L.; Bai, L.; Rojas, O.J.; McClements, D.J. Modulation of Physicochemical Characteristics of Pickering Emulsions: Utilization of Nanocellulose- and Nanochitin-Coated Lipid Droplet Blends. J. Agric. Food Chem. 2020, 68, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Zhong, T.; Wolcott, M.P.; Liu, H.; Wang, J. Developing chitin nanocrystals for flexible packaging coatings. Carbohydr. Polym. 2019, 226, 115276. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, R.; Xu, P.; Yu, J.; Liu, L.; Fan, Y. Physical nanochitin/microemulsion composite hydrogels for hydrophobic Nile Red release under in vitro physiological conditions. Cellulose 2019, 26, 1221–1230. [Google Scholar] [CrossRef]
- Liu, L.; Bai, L.; Tripathi, A.; Yu, J.; Wang, Z.; Borghei, M.; Fan, Y.; Rojas, O.J. High Axial Ratio Nanochitins for Ultrastrong and Shape-Recoverable Hydrogels and Cryogels via Ice Templating. ACS Nano 2019, 13, 2927–2935. [Google Scholar] [CrossRef]
- Thomas, M.S.; Koshy, R.R.; Mary, S.K.; Thomas, S.A.; Pothan, L. Properties of Composites. In Starch, Chitin and Chitosan Based Composites and Nanocomposites; Springer International Publishing: Cham, Switzerland, 2019; pp. 19–42. ISBN 9783030031589. [Google Scholar]
- Ge, Y.; Li, Y.; Bai, Y.; Yuan, C.; Wu, C.; Hu, Y. Intelligent gelatin/oxidized chitin nanocrystals nanocomposite films containing black rice bran anthocyanins for fish freshness monitorings. Int. J. Biol. Macromol. 2019. [Google Scholar] [CrossRef]
- Hubbe, M.A.; Tyagi, P.; Pal, L. Nanopolysaccharides in Barrier Composites. In Advanced Functional Materials from Nanopolysaccharides; Springer: Singapore, 2019; pp. 321–366. [Google Scholar]
- Tran, T.H.; Nguyen, H.-L.; Hwang, D.S.; Lee, J.Y.; Cha, H.G.; Koo, J.M.; Hwang, S.Y.; Park, J.; Oh, D.X. Five Different Chitin Nanomaterials from Identical Source with Different Advantageous Functions and Performances. Carbohydr. Polym. 2018, 205, 392–400. [Google Scholar] [CrossRef]
- Morganti, P. Bionanotechnology to Save the Environment; MDPI: Basel, Switzerland, 2018; ISBN 9783038426929. [Google Scholar]
- Sahraee, S.; Milani, J.M.; Ghanbarzadeh, B.; Hamishehkar, H. Development of emulsion films based on bovine gelatin-nano chitin-nano ZnO for cake packaging. Food Sci. Nutr. 2020, 8, 1303–1312. [Google Scholar] [CrossRef]
- Sahraee, S.; Ghanbarzadeh, B.; Milani, J.M.; Hamishehkar, H. Development of Gelatin Bionanocomposite Films Containing Chitin and ZnO. Food Bioprocess Technol. 2017, 10, 1441–1453. [Google Scholar] [CrossRef]
- Larbi, F.; García, A.; Luis, J.; Hamou, A.; Puiggalí, J. Comparison of nanocrystals and nanofibers produced from shrimp shell α-chitin: From energy production to material cytotoxicity and Pickering emulsion properties. Carbohydr. Polym. 2018, 196, 385–397. [Google Scholar] [CrossRef] [PubMed]
- Shankar, S.; Reddy, J.P.; Rhim, J.-W.; Kim, H.-Y. Preparation, characterization, and antimicrobial activity of chitin nanofibrils reinforced carrageenan nanocomposite films. Carbohydr. Polym. 2015, 117, 468–475. [Google Scholar] [CrossRef]
- Yang, Y.N.; Lu, K.Y.; Wang, P.; Ho, Y.C.; Tsai, M.L.; Mi, F.L. Development of bacterial cellulose/chitin multi-nanofibers based smart films containing natural active microspheres and nanoparticles formed in situ. Carbohydr. Polym. 2020, 228, 115370. [Google Scholar] [CrossRef]
- Satam, C.C.; Irvin, C.W.; Coffey, C.J.; Geran, R.K.; Ibarra-Rivera, R.; Shofner, M.L.; Meredith, J.C. Controlling Barrier and Mechanical Properties of Cellulose Nanocrystals by Blending with Chitin Nanofibers. Biomacromolecules 2019, 21, 545–555. [Google Scholar] [CrossRef] [PubMed]
- Satam, C.C.; Irvin, C.W.; Lang, A.W.; Jallorina, J.C.R.; Shofner, M.L.; Reynolds, J.R.; Meredith, J.C. Spray-Coated Multilayer Cellulose Nanocrystal-Chitin Nanofiber Films for Barrier Applications. ACS Sustain. Chem. Eng. 2018, 6, 10637–10644. [Google Scholar] [CrossRef]
- Manjula, K.; Podile, A.R. Chitin-supplemented formulations improve biocontrol and plant growth promoting efficiency of Bacillus subtilis AF 1. Can. J. Microbiol. 2001, 47, 618–625. [Google Scholar] [CrossRef]
- Sid Ahmed, A.; Ezziyyani, M.; Pérez Sánchez, C.; Candela, M.E. Effect of chitin on biological control activity of Bacillus spp. and Trichoderma harzianum against root rot disease in pepper (Capsicum annuum) plants. Eur. J. Plant Pathol. 2003, 109, 633–637. [Google Scholar] [CrossRef]
- Sharp, R. A Review of the Applications of Chitin and Its Derivatives in Agriculture to Modify Plant-Microbial Interactions and Improve Crop Yields. Agronomy 2013, 3, 757–793. [Google Scholar] [CrossRef]
- Xue, W.; Han, Y.; Tan, J.; Wang, Y.; Wang, G.; Wang, H. Effects of Nanochitin on the Enhancement of the Grain Yield and Quality of Winter Wheat. J. Agric. Food Chem. 2018, 66, 6637–6645. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, Y.; Han, Y.; Li, D.; Zhang, Z.; Zhu, X.; Tan, J.; Wang, H. The stimulatory effects of nanochitin whisker on carbon and nitrogen metabolism and on the enhancement of grain yield and crude protein of winter wheat. Molecules 2019, 24, 1752. [Google Scholar] [CrossRef]
- Liang, R.; Li, X.; Yuan, W.; Jin, S.; Hou, S.; Wang, M.; Wang, H. Antifungal Activity of Nanochitin Whisker against Crown Rot Diseases of Wheat. J. Agric. Food Chem. 2018, 66, 9907–9913. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Jiang, S.; Jiao, Y.; Wang, H. Synergistic effects of nanochitin on inhibition of tobacco root rot disease. Int. J. Biol. Macromol. 2017, 99, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Qin, Y.; Yang, J.; Li, M.; Xiong, L.; Sun, Q. Enhanced antibacterial activity of lysozyme immobilized on chitin nanowhiskers. Food Chem. 2017, 221, 1507–1513. [Google Scholar] [CrossRef] [PubMed]
- Torres-Rendon, J.G.; Femmer, T.; De Laporte, L.; Thomas Tigges, K.R.; Gremse, F.; Zafarnia, S.; Lederle, W.; Ifuku, S.; Wessling, M.; Hardy, J.G.; et al. Bioactive Gyroid Scaffolds Formed by Sacrificial Templating of Nanocellulose and Nanochitin Hydrogels as Instructive Platforms for Biomimetic Tissue Engineering. Adv. Mater. 2015, 27, 2989–2995. [Google Scholar] [CrossRef] [PubMed]
- Izumi, R.; Komada, S.; Ochi, K.; Karasawa, L.; Osaki, T.; Murahata, Y.; Tsuka, T.; Imagawa, T.; Itoh, N.; Okamoto, Y.; et al. Favorable effects of superficially deacetylated chitin nanofibrils on the wound healing process. Carbohydr. Polym. 2015, 123, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Wu, J.; Li, D.; Shi, C.; Chen, G.; He, M.; Tian, J. High-strength paper enhanced by chitin nanowhiskers and its potential bioassay applications. Int. J. Biol. Macromol. 2020, 150, 885–893. [Google Scholar] [CrossRef]
- Azuma, K.; Ifuku, S. Nanofibers based on chitin: A new functional food. Pure Appl. Chem. 2016, 88, 605–619. [Google Scholar] [CrossRef]
- Ye, W.; Liu, L.; Yu, J.; Liu, S.; Yong, Q.; Fan, Y. Hypolipidemic activities of partially deacetylated α-chitin nanofibers/nanowhiskers in mice. Food Nutr. Res. 2018, 62. [Google Scholar] [CrossRef]
- Wu, C.; Sun, J.; Zheng, P.; Kang, X.; Chen, M.; Li, Y.; Ge, Y.; Hu, Y.; Pang, J. Preparation of an intelligent film based on chitosan/oxidized chitin nanocrystals incorporating black rice bran anthocyanins for seafood spoilage monitoring. Carbohydr. Polym. 2019, 222, 115006. [Google Scholar] [CrossRef] [PubMed]
- Krivoshapkin, P.V.; Ivanets, A.I.; Torlopov, M.A.; Mikhaylov, V.I.; Srivastava, V.; Sillanpää, M.; Prozorovich, V.G.; Kouznetsova, T.F.; Koshevaya, E.D.; Krivoshapkina, E.F. Nanochitin/manganese oxide-biodegradable hybrid sorbent for heavy metal ions. Carbohydr. Polym. 2019, 210, 135–143. [Google Scholar] [CrossRef]
- Goto, M.; Ifuku, S.; Azuma, K.; Arima, H.; Kaneko, S.; Iohara, D.; Hirayama, F.; Anraku, M. Preparation and evaluation of freeze dried surface-deacetylated chitin nanofiber/sacran pellets for use as an extended-release excipient. Int. J. Biol. Macromol. 2019, 124, 888–894. [Google Scholar] [CrossRef]
- Khorasani, A.C.; Shojaosadati, S.A. Improvement of probiotic survival in fruit juice and under gastrointestinal conditions using pectin-nanochitin-nanolignocellulose as a novel prebiotic gastrointestinal-resistant matrix. Appl. Food Biotechnol. 2017, 4, 179–191. [Google Scholar] [CrossRef]
- Gopi, S.; Pius, A.; Thomas, S. Enhanced adsorption of crystal violet by synthesized and characterized chitin nano whiskers from shrimp shell. J. Water Process Eng. 2016, 14, 1–8. [Google Scholar] [CrossRef]
- Wu, J.; Cheng, X.; Li, Y.; Yang, G. Constructing biodegradable nanochitin-contained chitosan hydrogel beads for fast and efficient removal of Cu(II) from aqueous solution. Carbohydr. Polym. 2019, 211, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Cheng, X.; Yang, G. Preparation of nanochitin-contained magnetic chitosan microfibers via continuous injection gelation method for removal of Ni(II) ion from aqueous solution. Int. J. Biol. Macromol. 2019, 125, 404–413. [Google Scholar] [CrossRef]
- Poskela, A.; Miettunen, K.; Borghei, M.; Vapaavuori, J.; Greca, L.G.; Lehtonen, J.; Solin, K.; Ago, M.; Lund, P.D.; Rojas, O.J. Nanocellulose and Nanochitin Cryogels Improve the Efficiency of Dye Solar Cells. ACS Sustain. Chem. Eng. 2019, 7, 10257–10265. [Google Scholar] [CrossRef]


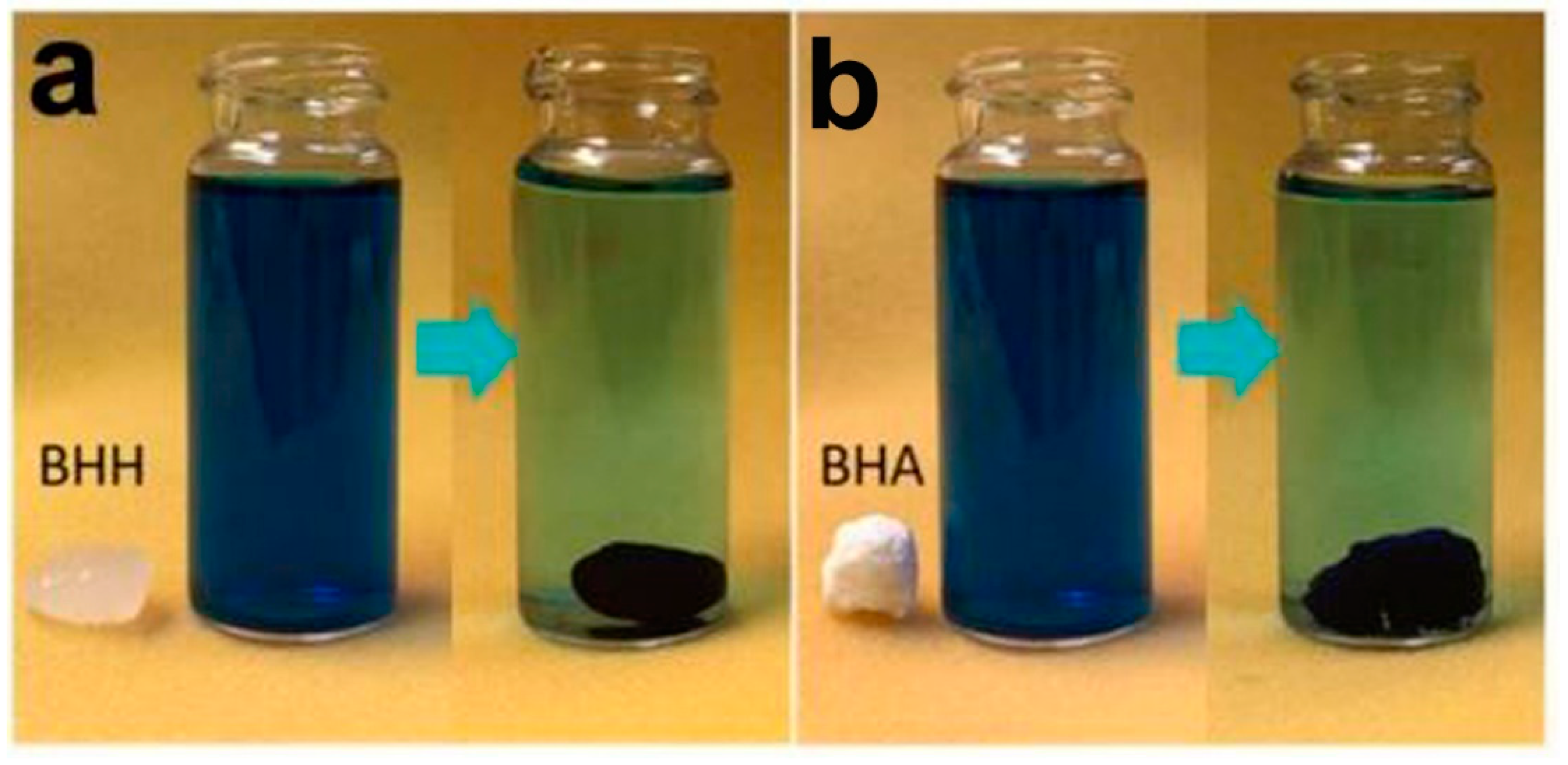
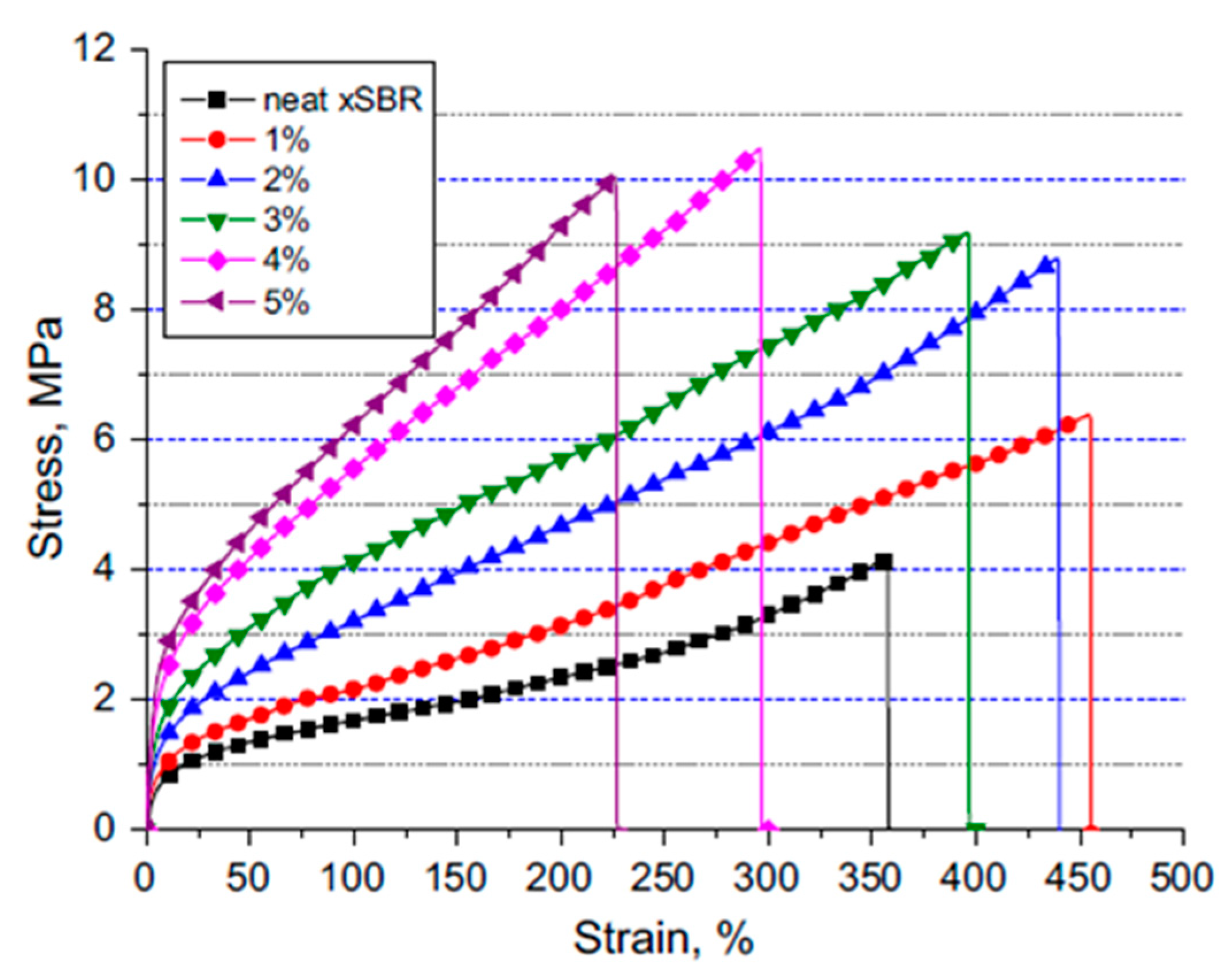
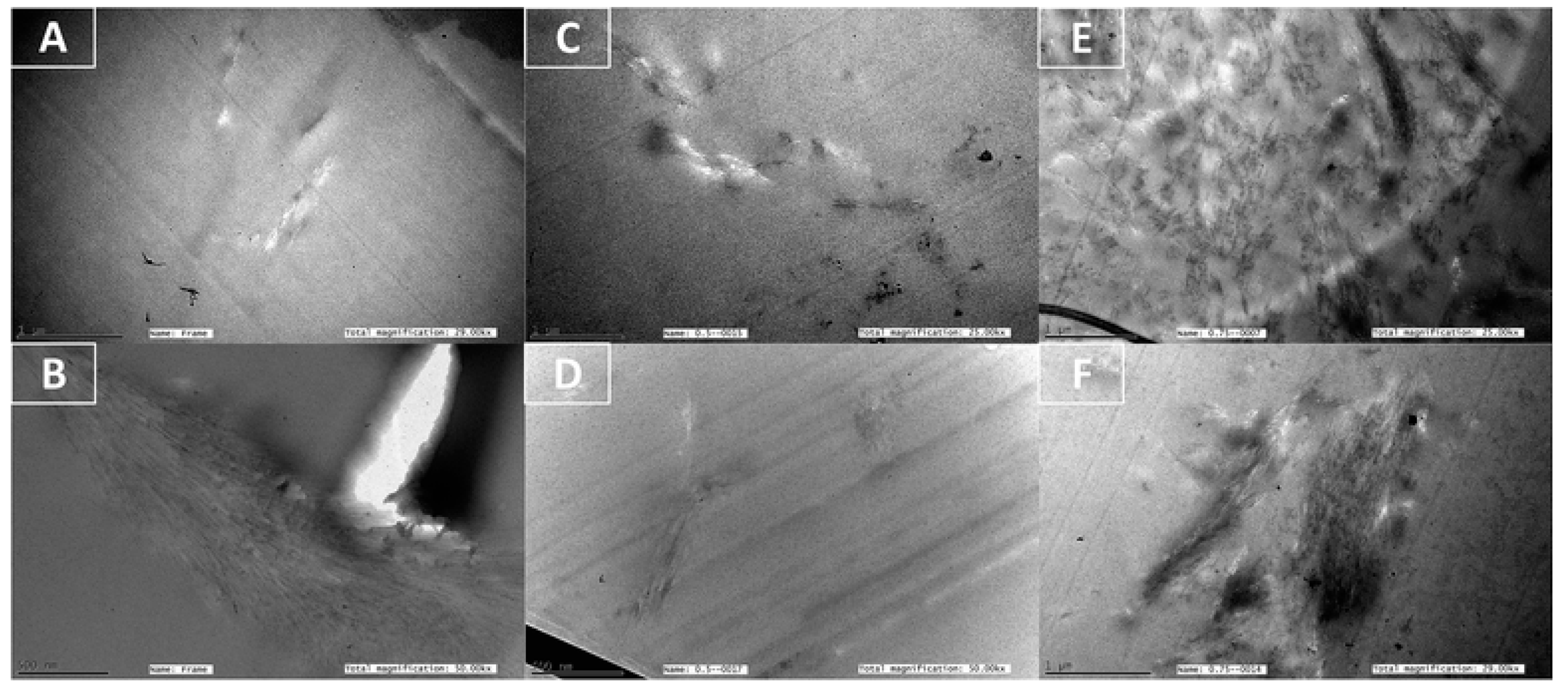


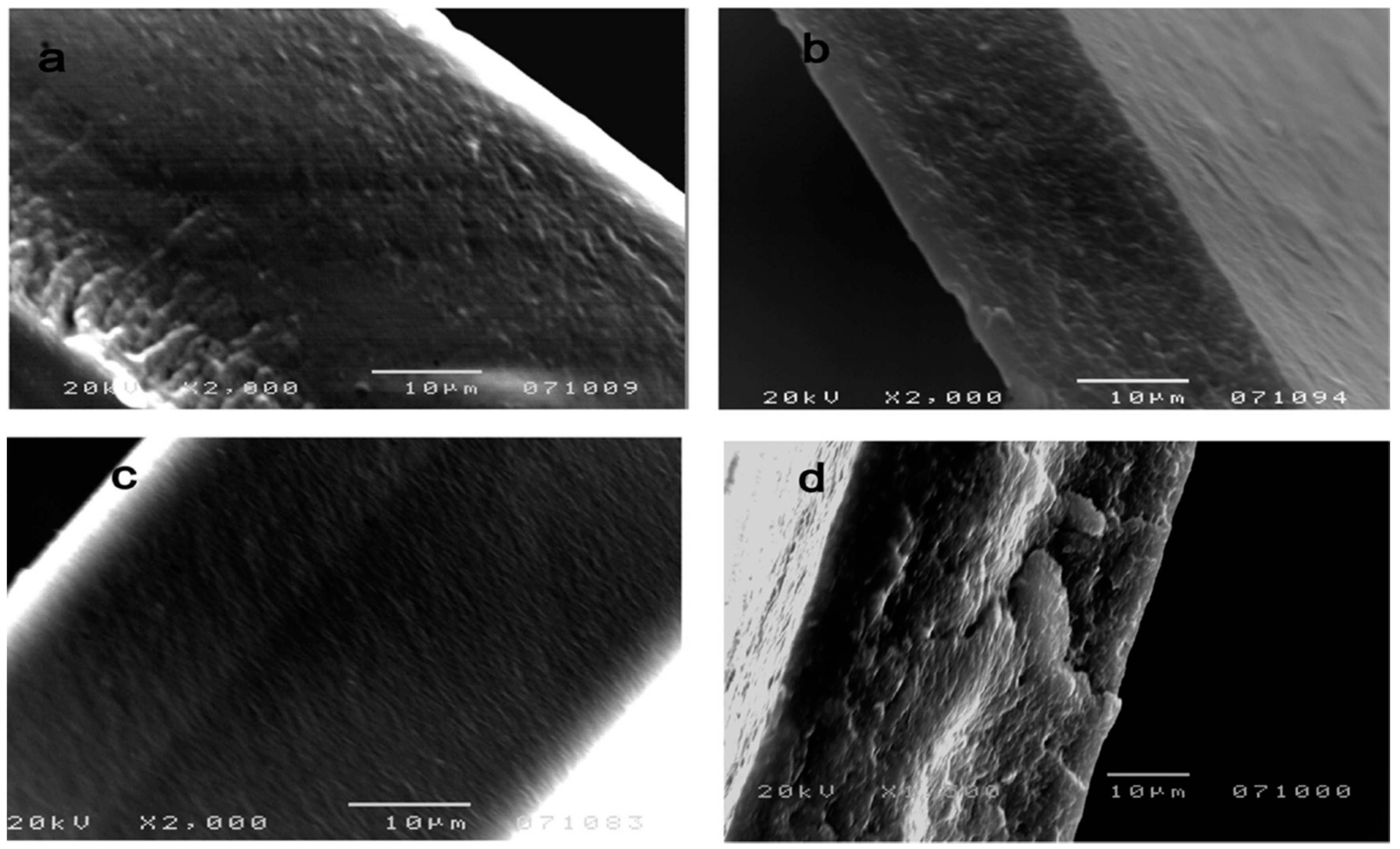

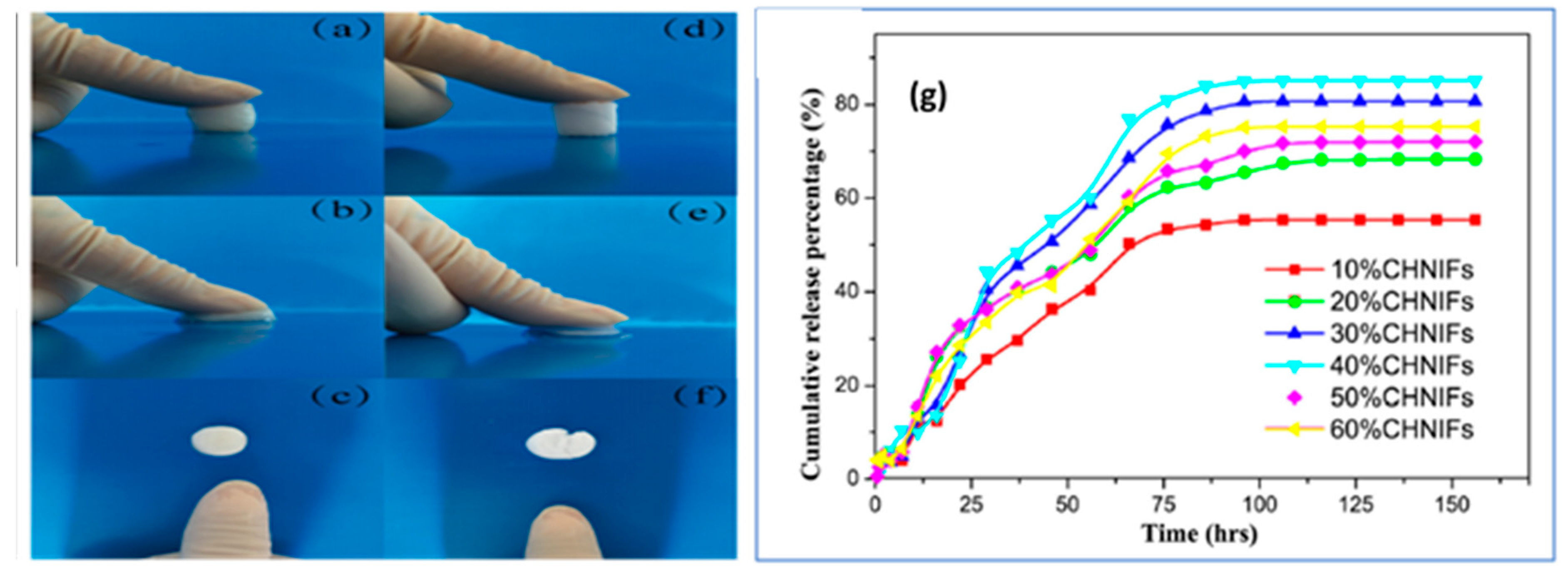
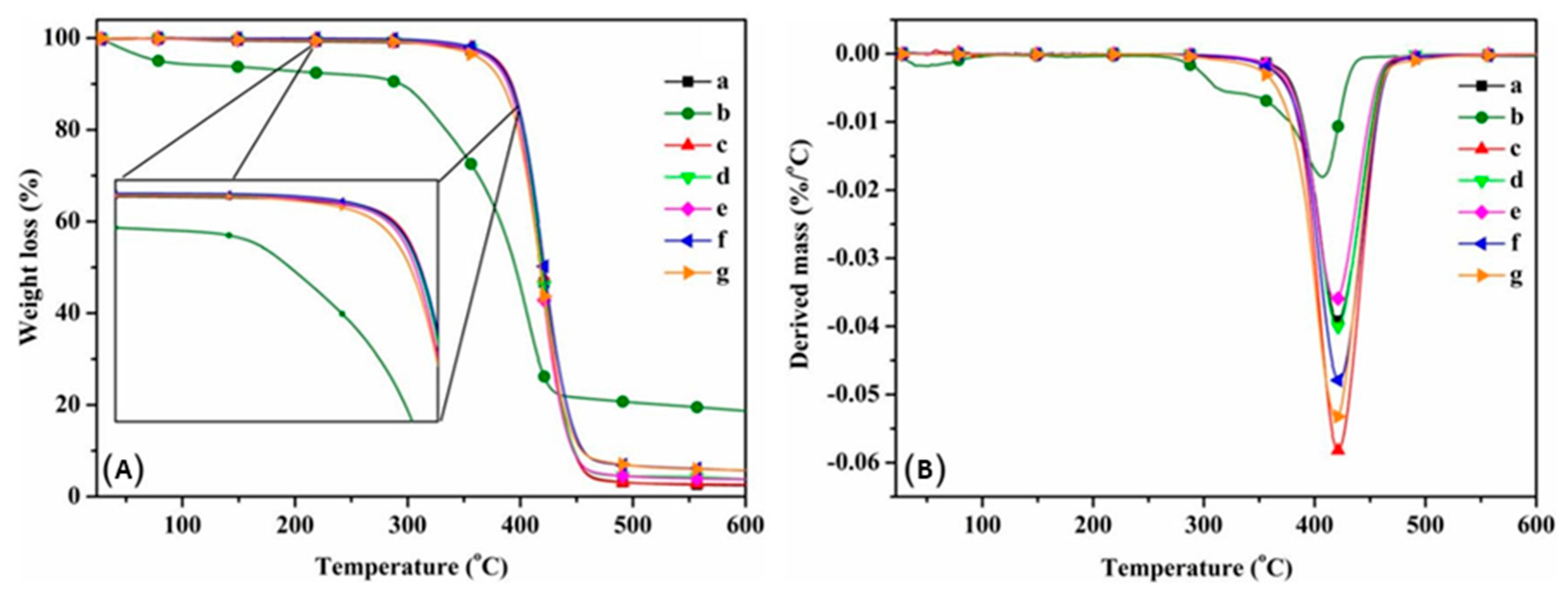



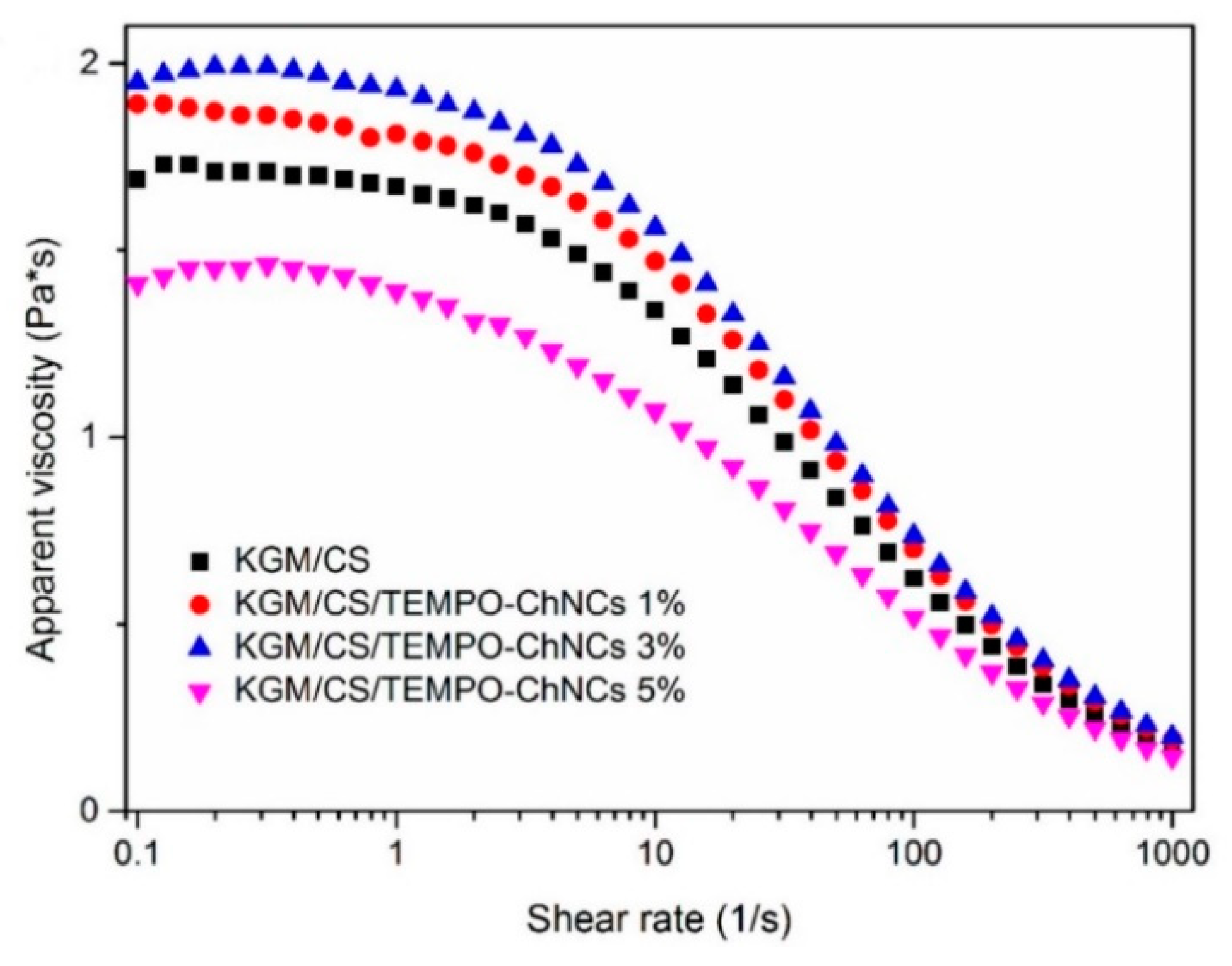


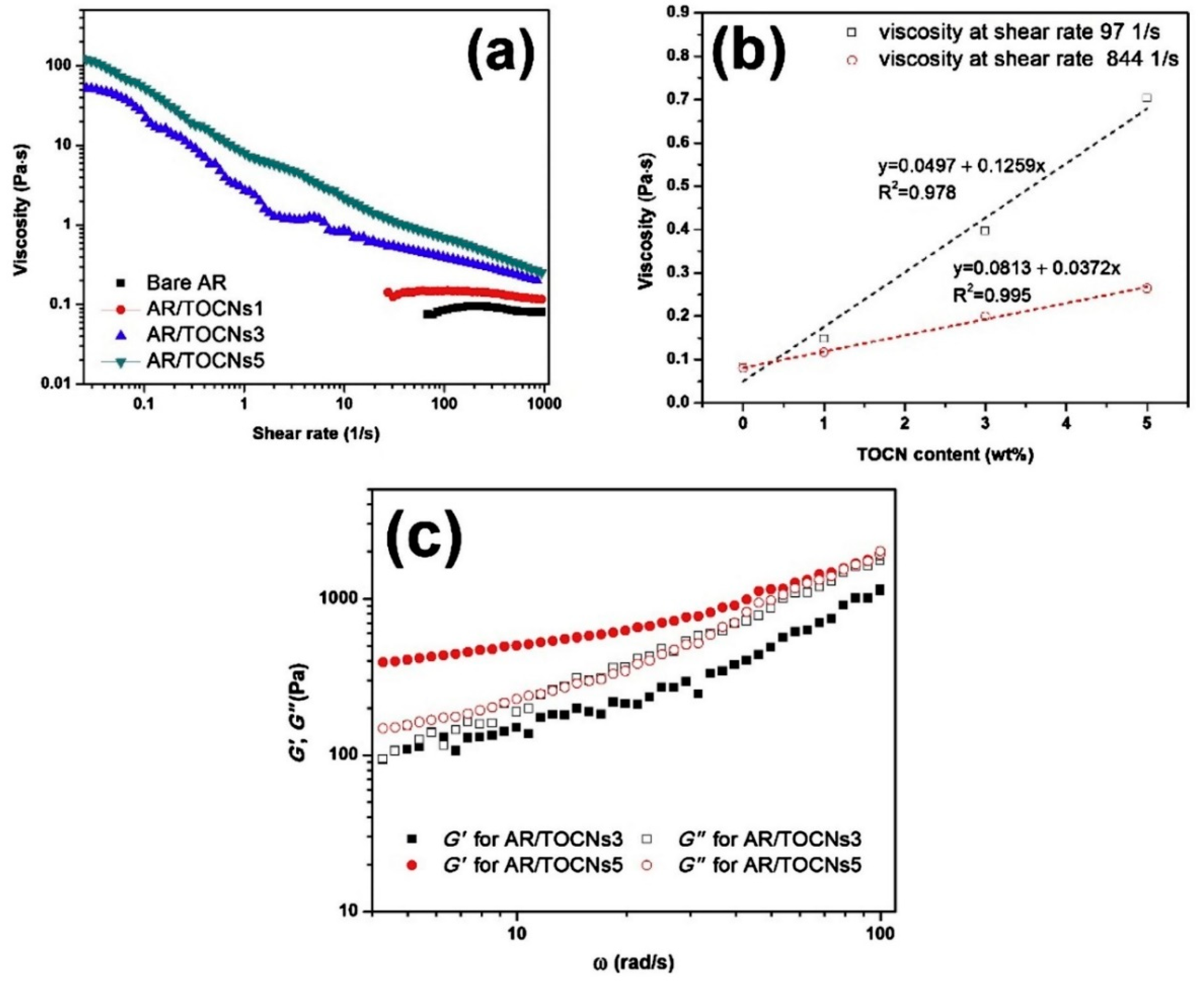

| Form (Whiskers/Crystals/Fibrils) | Matrix/Filler | Form of Composites (Hydrogels/Films/Aerogels) | Applications | References |
|---|---|---|---|---|
| Nanowhiskers | Cellulose | Films | High strength paper for bioassay applications | [118] |
| Nanocrystals | Water-based acrylic resin (WBAR), Biaxially oriented polypropylene (BOPP) | Films | Flexible packaging coatings | [93] |
| Nanocrystals | Carboxymethyl cellulose | Films | Active food packaging materials and biomedical applications | [84] |
| Partly deacetylated chitin nanofibre (PDCNF) | TEMPO-oxidized cellulose nanofibre (TOCNF) | Hydrogels and aerogels | Green renewable high-efficiency adsorbent for water purification | [60] |
| Oxidised chitin nanocrystals | Chitosan | Films | Seafood spoilage monitoring | [121] |
| Nanocrystals | Manganese oxide | Powder | Hybrid sorbent for heavy metal ions | [122] |
| Partially deacetylated α-chitin nanofibers (α-DECHNs) | TEMPO-oxidized cellulose nanofibers (TOCNFs) | Wires | Conductive material applications | [85] |
| Oxidised chitin nanocrystals | Gelatin | Films | Fish freshness monitoring | [97] |
| Surface-deacetylated chitin nanofibers | Sacran polysaccharide | Freeze-dried pellets | Extended-release excipient for tetrahydrocurcumin (THC) for wound healing | [123] |
| Nanofibers | Cellulose nanocrystals (CNC) | Spray coating | Enhancement of oxygen permeability of PLA films | [107] |
| Nanofibers | Pectin + Nanolignocellulose | Improvement of probiotic survival in fruit juice and under gastrointestinal conditions | [124] | |
| Nanofibrils (Surface-deacetylated cationic NFs) | Cellulose nanofibrils | Hydrogels | Biomimetic scaffolds for bone tissue engineering | [116] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joseph, B.; Mavelil Sam, R.; Balakrishnan, P.; J. Maria, H.; Gopi, S.; Volova, T.; C. M. Fernandes, S.; Thomas, S. Extraction of Nanochitin from Marine Resources and Fabrication of Polymer Nanocomposites: Recent Advances. Polymers 2020, 12, 1664. https://doi.org/10.3390/polym12081664
Joseph B, Mavelil Sam R, Balakrishnan P, J. Maria H, Gopi S, Volova T, C. M. Fernandes S, Thomas S. Extraction of Nanochitin from Marine Resources and Fabrication of Polymer Nanocomposites: Recent Advances. Polymers. 2020; 12(8):1664. https://doi.org/10.3390/polym12081664
Chicago/Turabian StyleJoseph, Blessy, Rubie Mavelil Sam, Preetha Balakrishnan, Hanna J. Maria, Sreeraj Gopi, Tatiana Volova, Susana C. M. Fernandes, and Sabu Thomas. 2020. "Extraction of Nanochitin from Marine Resources and Fabrication of Polymer Nanocomposites: Recent Advances" Polymers 12, no. 8: 1664. https://doi.org/10.3390/polym12081664
APA StyleJoseph, B., Mavelil Sam, R., Balakrishnan, P., J. Maria, H., Gopi, S., Volova, T., C. M. Fernandes, S., & Thomas, S. (2020). Extraction of Nanochitin from Marine Resources and Fabrication of Polymer Nanocomposites: Recent Advances. Polymers, 12(8), 1664. https://doi.org/10.3390/polym12081664