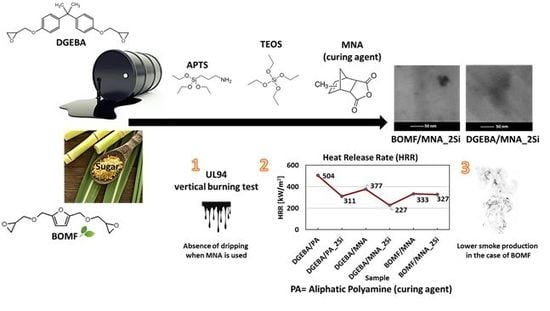
Thermal and Fire Behavior of a Bio-Based Epoxy/Silica Hybrid Cured with Methyl Nadic Anhydride
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Epoxy Resins and Epoxy/Silica Hybrid Nanocomposites
2.3. Methods
3. Results and Discussion
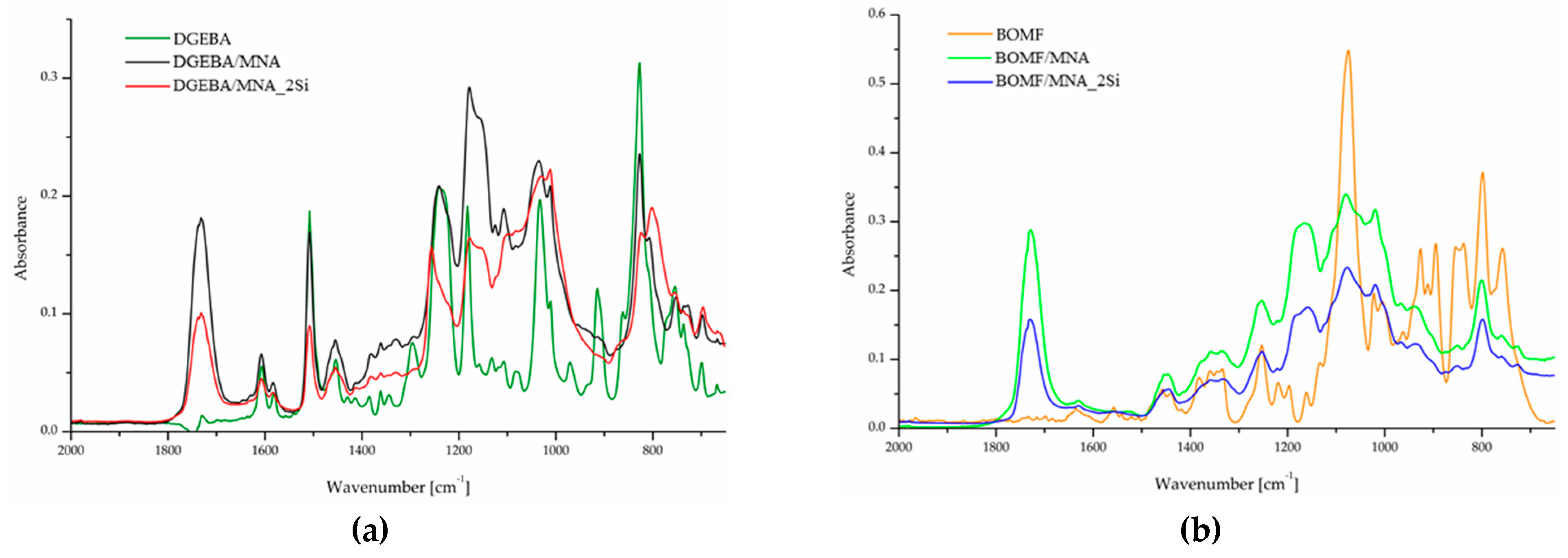
3.1. Characterization of the Epoxy/Silica Hybrid Nanocomposites
3.2. Morphology and Structure of the Obtained Hybrid Systems
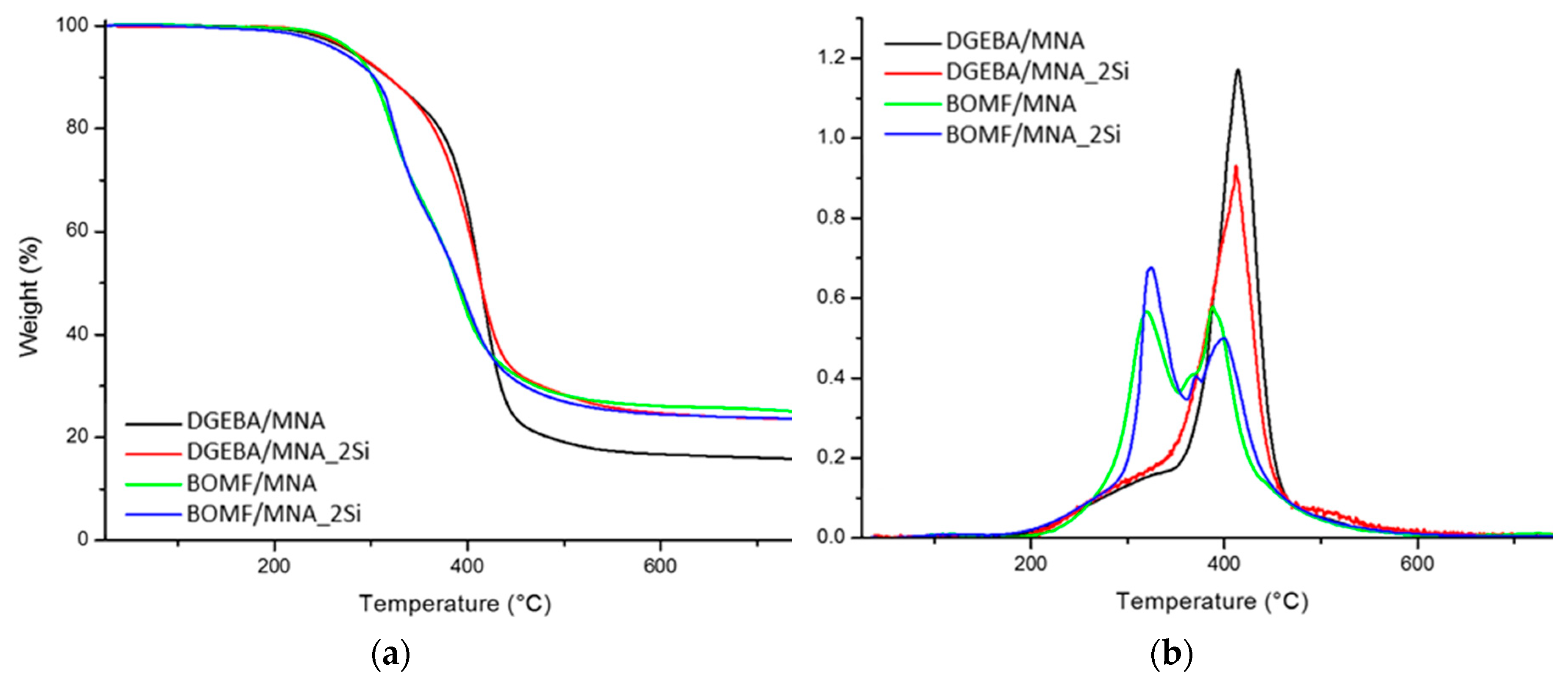
3.3. Thermal Analysis
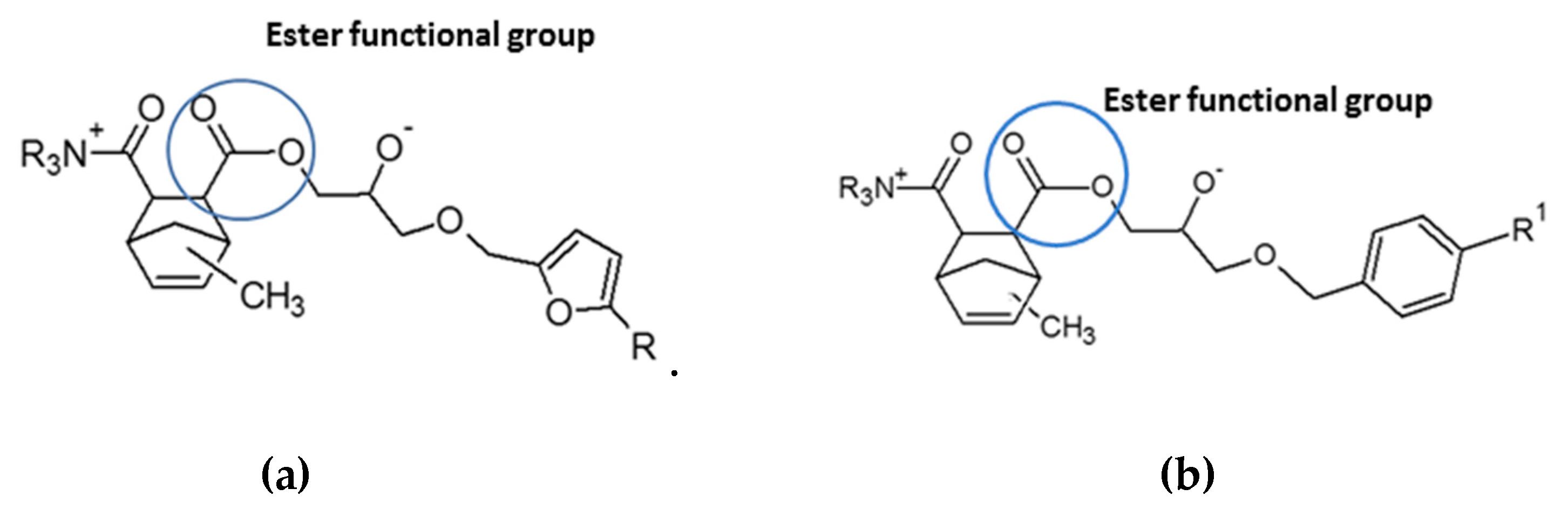
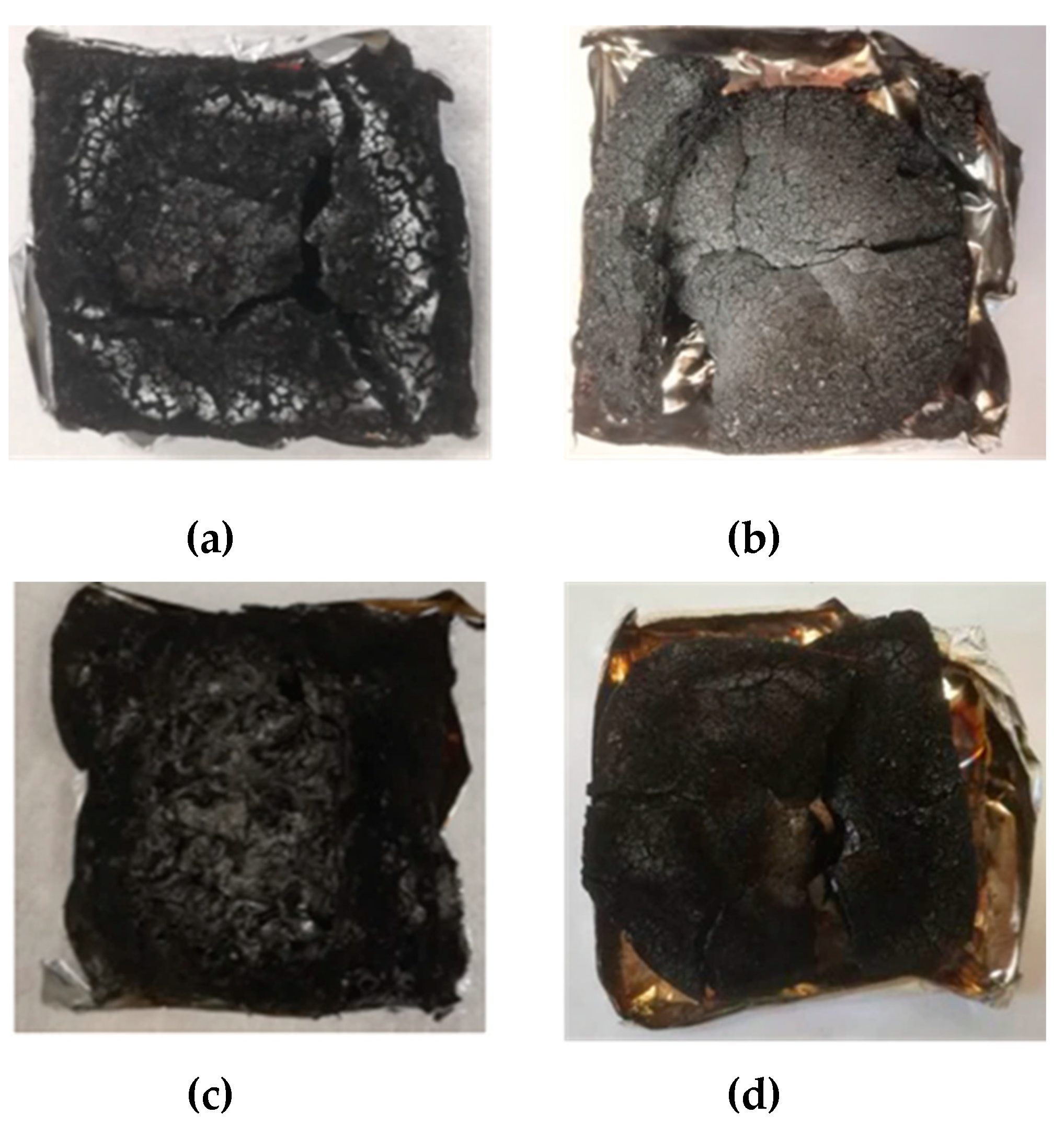
3.4. Flame Retardant Behavior
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Guadagno, L.; Raimondo, M.; Vittoria, V.; Vertuccio, L.; Naddeo, C.; Russo, S.; De Vivo, B.; Lamberti, P.; Spinelli, G.; Tucci, V. Development of epoxy mixtures for application in aeronautics and aerospace. RSC Adv. 2014, 4, 15474–15488. [Google Scholar] [CrossRef]
- Bifulco, A.; Silvestri, B.; Passaro, J.; Boccarusso, L.; Roviello, V.; Branda, F.; Durante, M. A New Strategy to Produce Hemp Fibers through a Waterglass-Based Ecofriendly Process. Materials 2020, 13, 1844. [Google Scholar] [CrossRef] [PubMed]
- Almeida, S.; Raposo, A.; Almeida-González, M.; Carrascosa, C. Bisphenol A: Food exposure and impact on human health. Compr. Rev. Food. Sci. F 2018, 17, 1503–1517. [Google Scholar] [CrossRef] [Green Version]
- Ambs, S.; Neumann, H.G. Acute and chronic toxicity of aromatic amines studied in the isolated perfused rat liver. Toxicol. Appl. Pharm. 1996, 139, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Atta, A.M.; Elsaeed, A.M.; Farag, R.K.; El-Saeed, S.M. Synthesis of unsaturated polyester resins based on rosin acrylic acid adduct for coating applications. React. Funct. Polym. 2007, 67, 549–563. [Google Scholar] [CrossRef]
- Bucknall, C.B.; Partridge, I.K. Phase separation in crosslinked resins containing polymeric modifiers. Polym. Eng. Sci. 1986, 26, 54–62. [Google Scholar] [CrossRef]
- Can, E.; Küsefoğlu, S.; Wool, R.P. Rigid thermosetting liquid molding resins from renewable resources. II. Copolymers of soybean oil monoglyceride maleates with neopentyl glycol and bisphenol A maleates. J. Appl. Polym. Sci. 2002, 83, 972–980. [Google Scholar] [CrossRef]
- Weiss, H. Anhydride curing agents for epoxy resins. Ind. Eng. Chem. 1957, 49, 1089–1090. [Google Scholar] [CrossRef]
- Stöber, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid. Interf. Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Bogush, G.H.; Zukoski Iv, C.F. Uniform silica particle precipitation: An aggregative growth model. J. Colloid. Interf. Sci. 1991, 142, 19–34. [Google Scholar] [CrossRef]
- Branda, F.; Silvestri, B.; Costantini, A.; Luciani, G. Effect of exposure to growth media on size and surface charge of silica based Stöber nanoparticles: A DLS and ζ-potential study. J. Sol.-Gel Sci. Technol. 2015, 73, 54–61. [Google Scholar] [CrossRef]
- Mascia, L.; Prezzi, L.; Lavorgna, M. Peculiarities in the solvent absorption characteristics of epoxy-siloxane hybrids. Polym. Eng. Sci. 2005, 45, 1039–1048. [Google Scholar] [CrossRef]
- Piscitelli, F.; Buonocore, G.G.; Lavorgna, M.; Verdolotti, L.; Pricl, S.; Gentile, G.; Mascia, L. Peculiarities in the structure–Properties relationship of epoxy-silica hybrids with highly organic siloxane domains. Polymer 2015, 63, 222–229. [Google Scholar] [CrossRef] [Green Version]
- Silvestri, B.; Luciani, G.; Costantini, A.; Tescione, F.; Branda, F.; Pezzella, A. In situ sol gel synthesis and characterization of bioactive pHEMA/SiO2 blend hybrids. J. Biomed. Mater. Res. B 2009, 89, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Bifulco, A.; Tescione, F.; Capasso, A.; Mazzei, P.; Piccolo, A.; Durante, M.; Lavorgna, M.; Malucelli, G.; Branda, F. Effects of post cure treatment in the glass transformation range on the structure and fire behavior of in situ generated silica/epoxy hybrids. J. Sol.-Gel Sci. Technol. 2018, 87, 156–169. [Google Scholar] [CrossRef]
- Passaro, J.; Russo, P.; Bifulco, A.; De Martino, M.T.; Granata, V.; Vitolo, B.; Iannace, G.; Vecchione, A.; Marulo, F.; Branda, F. Water resistant self-extinguishing low frequency soundproofing polyvinylpyrrolidone based electrospun blankets. Polymers 2019, 11, 1205. [Google Scholar] [CrossRef] [Green Version]
- Meng, J.; Zeng, Y.; Zhu, G.; Zhang, J.; Chen, P.; Cheng, Y.; Fang, Z.; Guo, K. Sustainable bio-based furan epoxy resin with flame retardancy. Polym. Chem.-UK 2019, 10, 2370–2375. [Google Scholar] [CrossRef]
- Pan, Y.; Zhan, J.; Pan, H.; Wang, W.; Tang, G.; Song, L.; Hu, Y. Effect of fully biobased coatings constructed via layer-by-layer assembly of chitosan and lignosulfonate on the thermal, flame retardant, and mechanical properties of flexible polyurethane foam. ACS Sustain. Chem. Eng. 2016, 4, 1431–1438. [Google Scholar] [CrossRef]
- Li, C.; Fan, H.; Aziz, T.; Bittencourt, C.; Wu, L.; Wan, D.; Dubois, P. Biobased epoxy resin with low electrical permissivity and flame retardancy: From environmental friendly high-throughput synthesis to properties. ACS Sustain. Chem. Eng. 2018, 6, 8856–8867. [Google Scholar]
- Hu, F.; La Scala, J.J.; Sadler, J.M.; Palmese, G.R. Synthesis and characterization of thermosetting furan-based epoxy systems. Macromolecules 2014, 47, 3332–3342. [Google Scholar] [CrossRef]
- Marotta, A.; Ambrogi, V.; Cerruti, P.; Mija, A. Green approaches in the synthesis of furan-based diepoxy monomers. RSC Adv. 2018, 8, 16330–16335. [Google Scholar] [CrossRef] [Green Version]
- Marotta, A.; Faggio, N.; Ambrogi, V.; Cerruti, P.; Gentile, G.; Mija, A. Curing behavior and properties of sustainable furan-based epoxy/anhydride resins. Biomacromolecules 2019, 20, 3831–3841. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Zeng, Y.; Chen, P.; Zhang, J.; Yao, C.; Fang, Z.; Ouyang, P.; Guo, K. Flame Retardancy and Mechanical Properties of Bio-Based Furan Epoxy Resins with High Crosslink Density. Macromol. Mater. Eng. 2019, 305, 1900587. [Google Scholar] [CrossRef]
- Rivero, G.; Villanueva, S.; Manfredi, L.B. Furan resin as a replacement of phenolics: Influence of the clay addition on its thermal degradation and fire behaviour. Fire Mater. 2014, 38, 683–694. [Google Scholar] [CrossRef]
- Rivero, G.; Vázquez, A.; Manfredi, L.B. Synthesis and characterization of nanocomposites based on a furan resin. J. Appl. Polym. Sci. 2010, 117, 1667–1673. [Google Scholar] [CrossRef]
- Yan, H.; Lu, C.; Jing, D.; Hou, X. Chemical degradation of amine-cured DGEBA epoxy resin in supercritical 1-propanol for recycling carbon fiber from composites. Chin. J. Polym. Sci. 2014, 32, 1550–1563. [Google Scholar] [CrossRef]
- Grassie, N.; Guy, M.I.; Tennent, N.H. Degradation of epoxy polymers: Part 1—Products of thermal degradation of bisphenol-A diglycidyl ether. Polym. Degrad. Stab. 1985, 12, 65–91. [Google Scholar] [CrossRef]
- Crompton, T.R. Characteristics and Analysis of Non-Flammable Polymers; Smithers Rapra: Shawbury, UK, 2013. [Google Scholar]
- Nikishin, G.I.; Starostin, E.K.; Golovin, B.A. Thermal decomposition of esters of peroxydicarboxylic acids. Bull. Acad. Sci. USSR Div. Chem. Sci. 1973, 22, 801–806. [Google Scholar] [CrossRef]
- Zhang, W.; He, X.; Song, T.; Jiao, Q.; Yang, R. The influence of the phosphorus-based flame retardant on the flame retardancy of the epoxy resins. Polym. Degrad. Stabil. 2014, 109, 209–217. [Google Scholar] [CrossRef]
- Miao, J.T.; Yuan, L.; Guan, Q.; Liang, G.; Gu, A. Biobased heat resistant epoxy resin with extremely high biomass content from 2, 5-furandicarboxylic acid and eugenol. ACS Sustain. Chem. Eng. 2017, 5, 7003–7011. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y.; Lu, X.D.; Yu, J. Study on melting behavior of polymers during burning. Fire Sci. Technol. 2005, 8, 637–646. [Google Scholar] [CrossRef]
- Bourbigot, S.; Duquesne, S. Fire retardant polymers: Recent developments and opportunities. J. Mater. Chem. 2007, 17, 2283–2300. [Google Scholar] [CrossRef]
- Bifulco, A.; Parida, D.; Salmeia, K.A.; Nazir, R.; Lehner, S.; Stämpfli, R.; Markus, H.; Malucelli, G.; Branda, F.; Gaan, S. Fire and mechanical properties of DGEBA-based epoxy resin cured with a cycloaliphatic hardener: Combined action of silica, melamine and DOPO-derivative. Mater. Des. 2020, 108862. [Google Scholar] [CrossRef]
- Schartel, B. Phosphorus-based flame retardancy mechanisms—Old hat or a starting point for future development? Materials 2010, 3, 4710–4745. [Google Scholar] [CrossRef] [Green Version]
- Kashiwagi, T.; Du, F.; Winey, K.I.; Groth, K.M.; Shields, J.R.; Bellayer, S.P.; Kim, H.; Douglas, J.F. Flammability properties of polymer nanocomposites with single-walled carbon nanotubes: Effects of nanotube dispersion and concentration. Polymer 2005, 46, 471–481. [Google Scholar] [CrossRef]
- Kashiwagi, T.; Du, F.; Douglas, J.F.; Winey, K.I.; Harris, R.H.; Shields, J.R. Nanoparticle Networks Reduce the Flammability of Polymer Nanocomposites. Nat. Mater. 2005, 4, 928–933. [Google Scholar] [CrossRef]
- Jian, R.; Wang, P.; Duan, W.; Wang, J.; Zheng, X.; Weng, J. Synthesis of a novel P/N/S-containing flame retardant and its application in epoxy resin: Thermal property, flame retardance, and pyrolysis behavior. Ind. Eng. Chem. Res. 2016, 55, 11520–11527. [Google Scholar] [CrossRef]
- Visakh, P.M.; Arao, Y. Flame Retardants: Polymer Blends, Composites and Nanocomposites; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Omidvarborna, H.; Kumar, A.; Kim, D.S. Recent studies on soot modeling for diesel combustion. Renew. Sust. Energy. Rev. 2015, 48, 635–647. [Google Scholar] [CrossRef]
- Zhang, W.; Fina, A.; Ferraro, G.; Yang, R. FTIR and GCMS analysis of epoxy resin decomposition products feeding the flame during UL 94 standard flammability test. Application to the understanding of the blowing-out effect in epoxy/polyhedral silsesquioxane formulations. J. Anal. Appl. Pyrol. 2018, 135, 271–280. [Google Scholar] [CrossRef] [Green Version]
- Kandola, B.K.; Ebdon, J.R.; Chowdhury, K.P. Flame retardance and physical properties of novel cured blends of unsaturated polyester and furan resins. Polymers 2015, 7, 298–315. [Google Scholar] [CrossRef] [Green Version]
- Toan, M.; Park, J.W.; Kim, H.J.; Shin, S. Synthesis and characterization of a new phosphorus-containing furan-based epoxy curing agent as a flame retardant. Fire Mater. 2019, 43, 717–724. [Google Scholar] [CrossRef]


| Molecule Structure | Nomenclature |
|---|---|
 | Diglycidyl ether of bisphenol A (DGEBA) |
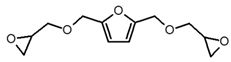 | 2,5-Bis[(oxiran-2-ylmethoxy)methyl]furan (BOMF) |
 | Tetraethyl orthosilicate (TEOS) |
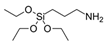 | 3-Aminopropyl)triethoxysilane (APTS) |
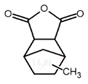 | Methyl nadic anhydride (MNA) |
 | 2-methyl imidazole (2-MI) |
| Sample | DGEBA (g) | BOMF (g) | TEOS (g) | APTS (g) | EtOH (g) | H2O (g) | 2-MI (g) | MNA (g) |
|---|---|---|---|---|---|---|---|---|
| DGEBA/MNA | 43.8 | --- | --- | --- | --- | --- | 0.32 | 20.7 |
| DGEBA/MNA_2Si | 45.0 | --- | 3.89 | 2.00 | 0.50 | 1.73 | 0.31 | 21.3 |
| BOMF/MNA | --- | 36.8 | --- | --- | --- | --- | 0.32 | 27.3 |
| BOMF/MNA_2Si | --- | 45.0 | 3.89 | 2.00 | 0.50 | 1.73 | 0.39 | 32.6 |
| Sample | Tg (°C) | T5 (°C) | T10 (°C) | T50 (°C) | R700 (wt. %) |
|---|---|---|---|---|---|
| DGEBA/MNA | 65 | 279 | 317 | 414 | 16 |
| DGEBA/MNA_2Si | 72 | 278 | 316 | 410 | 24 |
| BOMF/MNA | 53 | 281 | 302 | 389 | 26 |
| BOMF/MNA_2Si | 43 | 268 | 303 | 391 | 24 |
| Sample. | TTI (s) | HRR (kW/m2) | pkHRR (kW/m2) | THR (MJ/m2) | Residue Mass (%) |
|---|---|---|---|---|---|
| DGEBA/PA * | 54 ± 3 | 504 ± 23 | 1971 ± 384 | 84 ± 3 | 2 ± 0.7 |
| DGEBA/PA_2Si * | 37 ± 4 | 311 ± 12 | 991 ± 73 | 67 ± 9 | 6 ± 0.5 |
| DGEBA/MNA | 43 ± 3 | 377 ± 9 | 1119 ± 39 | 94 ± 6 | 8 ± 1 |
| DGEBA/MNA_2Si | 54 ± 3 | 227 ± 9 | 625 ± 33 | 96 ± 1 | 11 ± 1 |
| BOMF/MNA | 49 ± 3 | 333 ± 4 | 1216 ± 37 | 75 ± 6 | 10 ± 1 |
| BOMF/MNA_2Si | 45 ± 2 | 327 ± 4 | 983 ± 11 | 76 ± 2 | 11 ± 1 |
| Sample | TSR (m2/m2) | SEA (m2/kg) | CO Yield (kg/kg) | CO2 Yield (kg/kg) | CO/CO2ratio |
|---|---|---|---|---|---|
| DGEBA/PA * | 3066 ± 206 | 940 ± 36 | 0.06 ± 0.03 | 2.1 ± 0.06 | 0.028 |
| DGEBA/PA_2Si * | 2604 ± 291 | 941 ± 38 | 0.06 ± 0.04 | 1.9 ± 0.03 | 0.031 |
| DGEBA/MNA | 3619 ± 292 | 835 ± 8 | 0.05 ± 0.01 | 1.9 ± 0.1 | 0.026 |
| DGEBA/MNA_2Si | 3864 ± 309 | 808 ± 5 | 0.06 ± 0.01 | 1.8 ± 0.2 | 0.033 |
| BOMF/MNA | 2086 ± 242 | 479 ± 9 | 0.05 ± 0.01 | 1.8 ± 0.1 | 0.027 |
| BOMF/MNA_2Si | 1802 ± 147 | 444 ± 8 | 0.05 ± 0.01 | 1.9 ± 0.1 | 0.026 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bifulco, A.; Marotta, A.; Passaro, J.; Costantini, A.; Cerruti, P.; Gentile, G.; Ambrogi, V.; Malucelli, G.; Branda, F. Thermal and Fire Behavior of a Bio-Based Epoxy/Silica Hybrid Cured with Methyl Nadic Anhydride. Polymers 2020, 12, 1661. https://doi.org/10.3390/polym12081661
Bifulco A, Marotta A, Passaro J, Costantini A, Cerruti P, Gentile G, Ambrogi V, Malucelli G, Branda F. Thermal and Fire Behavior of a Bio-Based Epoxy/Silica Hybrid Cured with Methyl Nadic Anhydride. Polymers. 2020; 12(8):1661. https://doi.org/10.3390/polym12081661
Chicago/Turabian StyleBifulco, Aurelio, Angela Marotta, Jessica Passaro, Aniello Costantini, Pierfrancesco Cerruti, Gennaro Gentile, Veronica Ambrogi, Giulio Malucelli, and Francesco Branda. 2020. "Thermal and Fire Behavior of a Bio-Based Epoxy/Silica Hybrid Cured with Methyl Nadic Anhydride" Polymers 12, no. 8: 1661. https://doi.org/10.3390/polym12081661
APA StyleBifulco, A., Marotta, A., Passaro, J., Costantini, A., Cerruti, P., Gentile, G., Ambrogi, V., Malucelli, G., & Branda, F. (2020). Thermal and Fire Behavior of a Bio-Based Epoxy/Silica Hybrid Cured with Methyl Nadic Anhydride. Polymers, 12(8), 1661. https://doi.org/10.3390/polym12081661