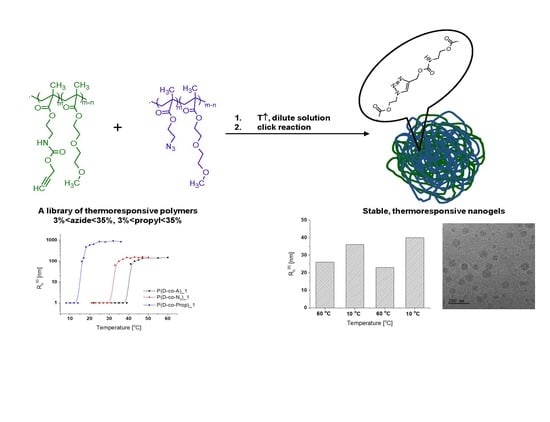
Thermoresponsive Nanogels of Modified Poly((di(ethylene glycol) methyl ether methacrylate)-co-(2-aminoethyl methacrylate))s
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Di(ethylene glycol) Methyl Ether Methacrylate (D) and 2-aminoethyl Methacrylate Hydrochloride (A) Copolymers
2.3. Synthesis of Prop-2-yn-1-yl Carbamate Modified Copolymers
2.4. Synthesis of Azide Modified Copolymers
2.5. Determination of Amine Groups in Copolymers (Kaiser Test)
2.6. Aggregation of Copolymers
2.7. Nanogels Formation
2.8. Proton Nuclear Magnetic Resonance (1H NMR)
2.9. Fourier Transform Infrared Spectroscopy (FTIR)
2.10. Gel Permeation Chromatography (GPC-MALLS)
2.11. High-Performance Reverse-Phase Liquid Chromatography (RP-HPLC)
2.12. Cloud Point Measurement
2.13. Dynamic Light Scattering (DLS)
2.14. Atomic Force Microscopy (AFM)
2.15. Cryogenic Transmission Electron Microscopy (CryoTEM)
3. Results and Discussion
3.1. Synthesis and Characterization of P(D-co-A) Copolymers and Their Modified Derivatives
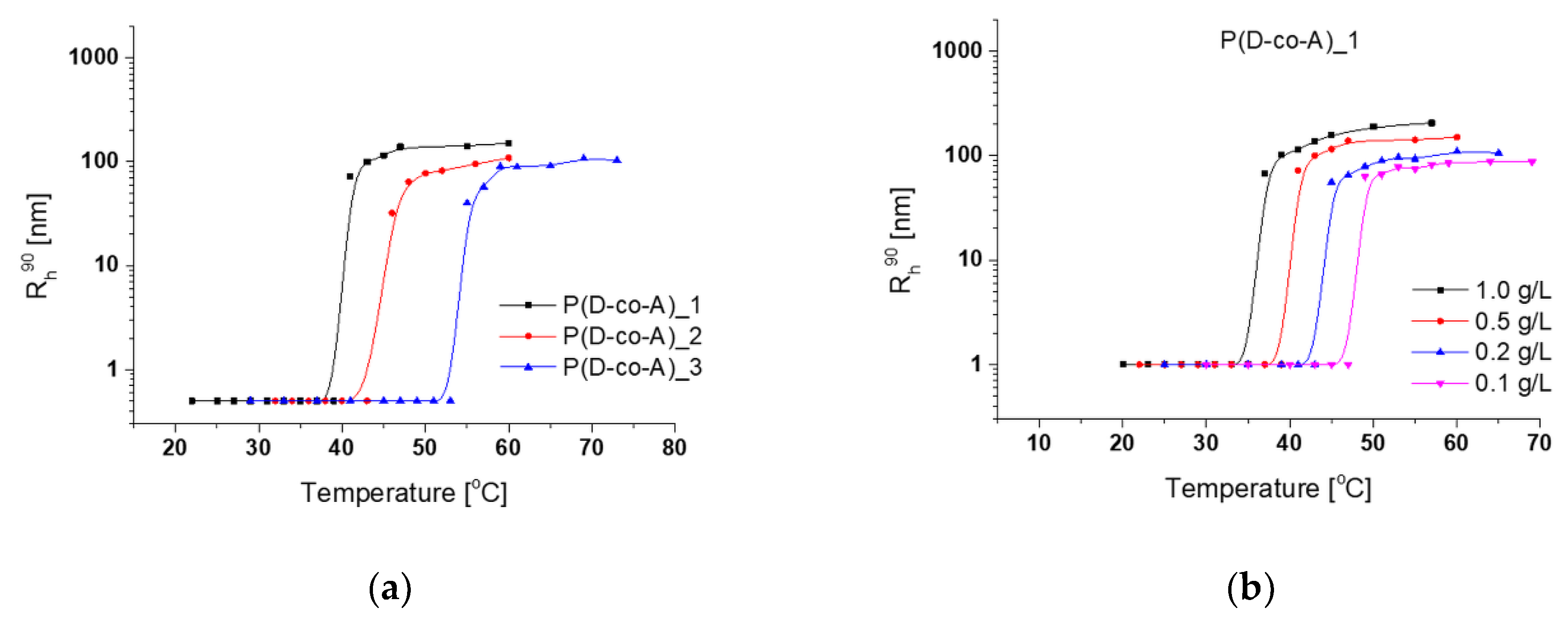
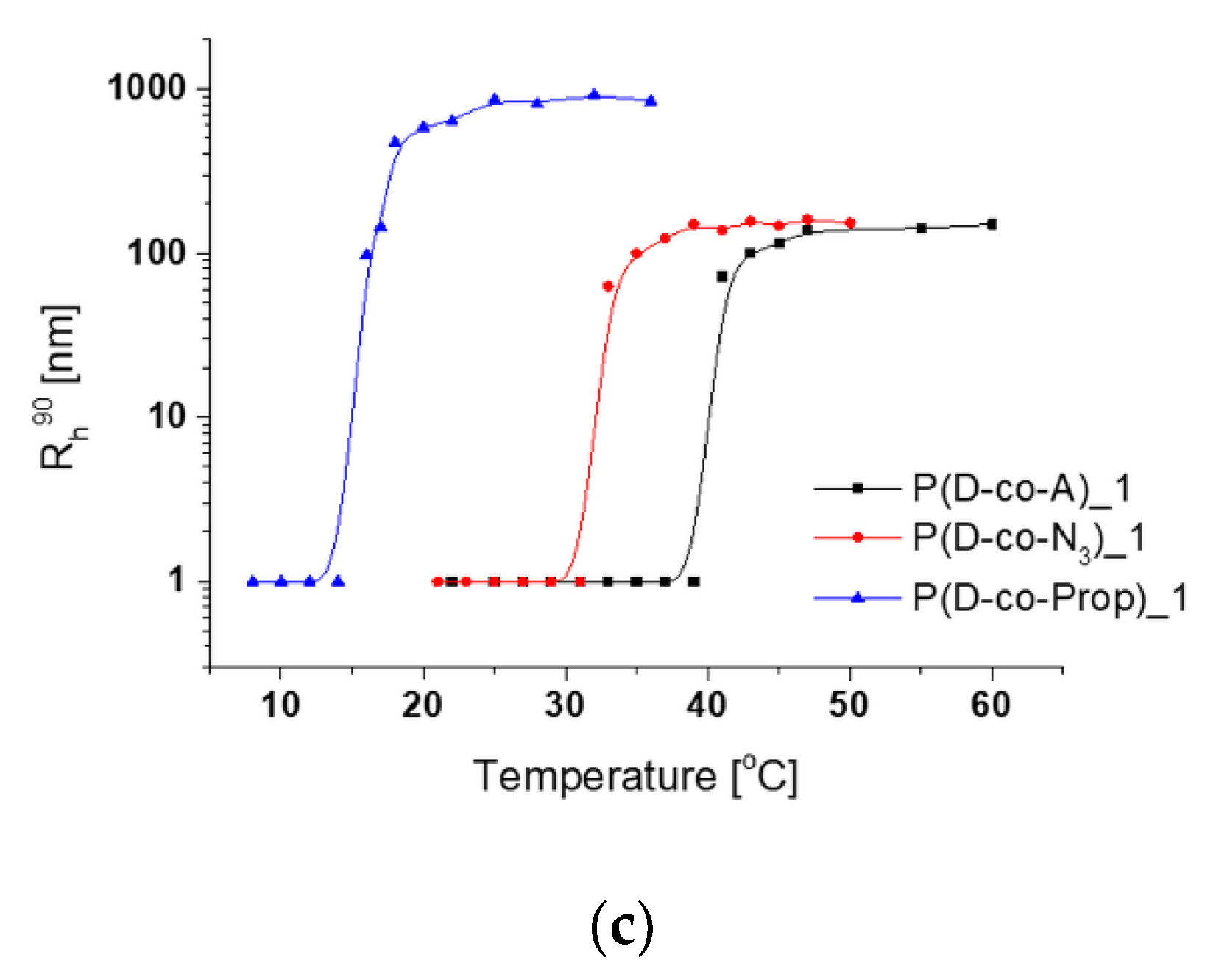
3.2. Thermoresponsiveness of Copolymers
3.3. Aggregation of Thermoresponsive Copolymers
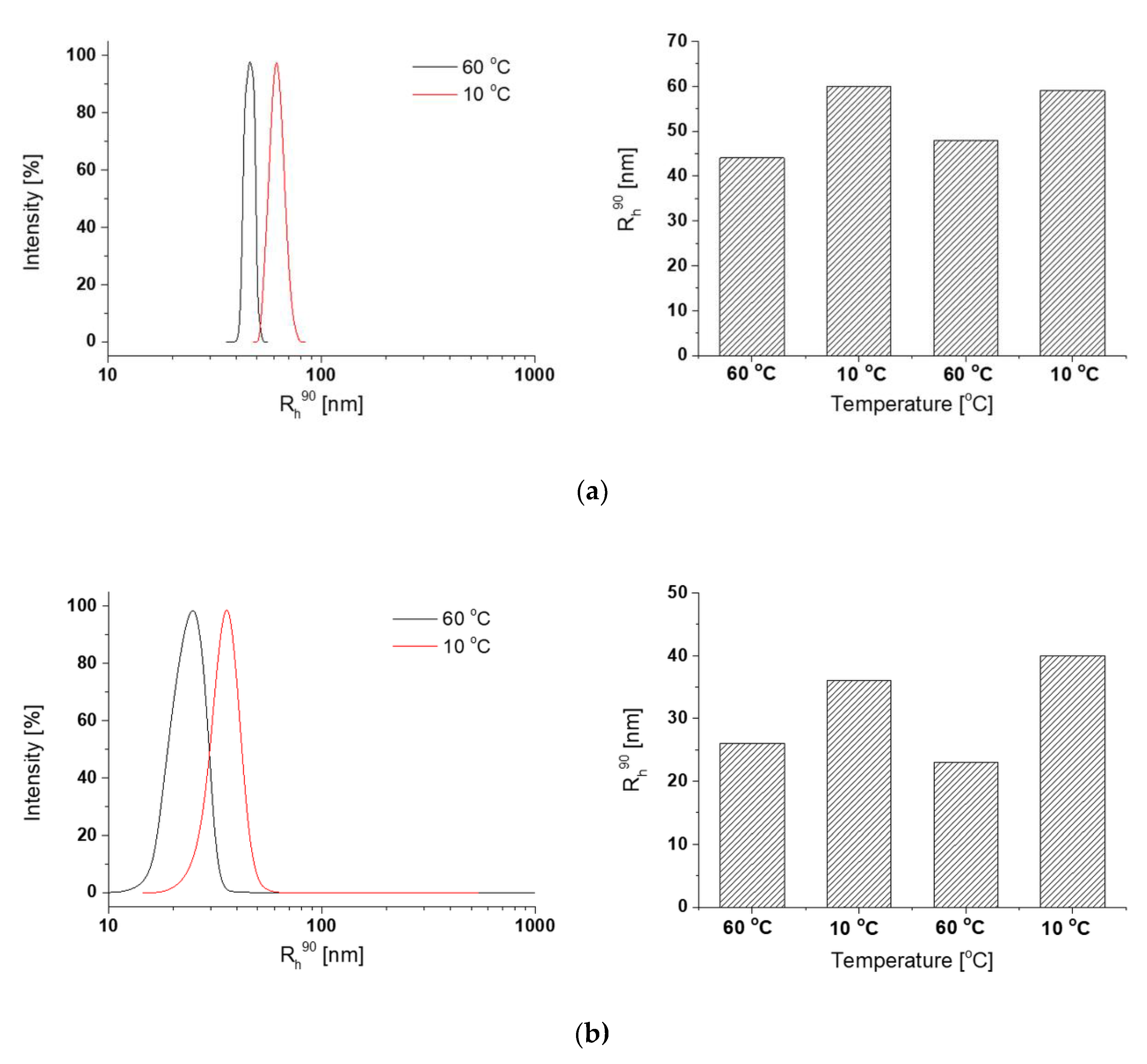
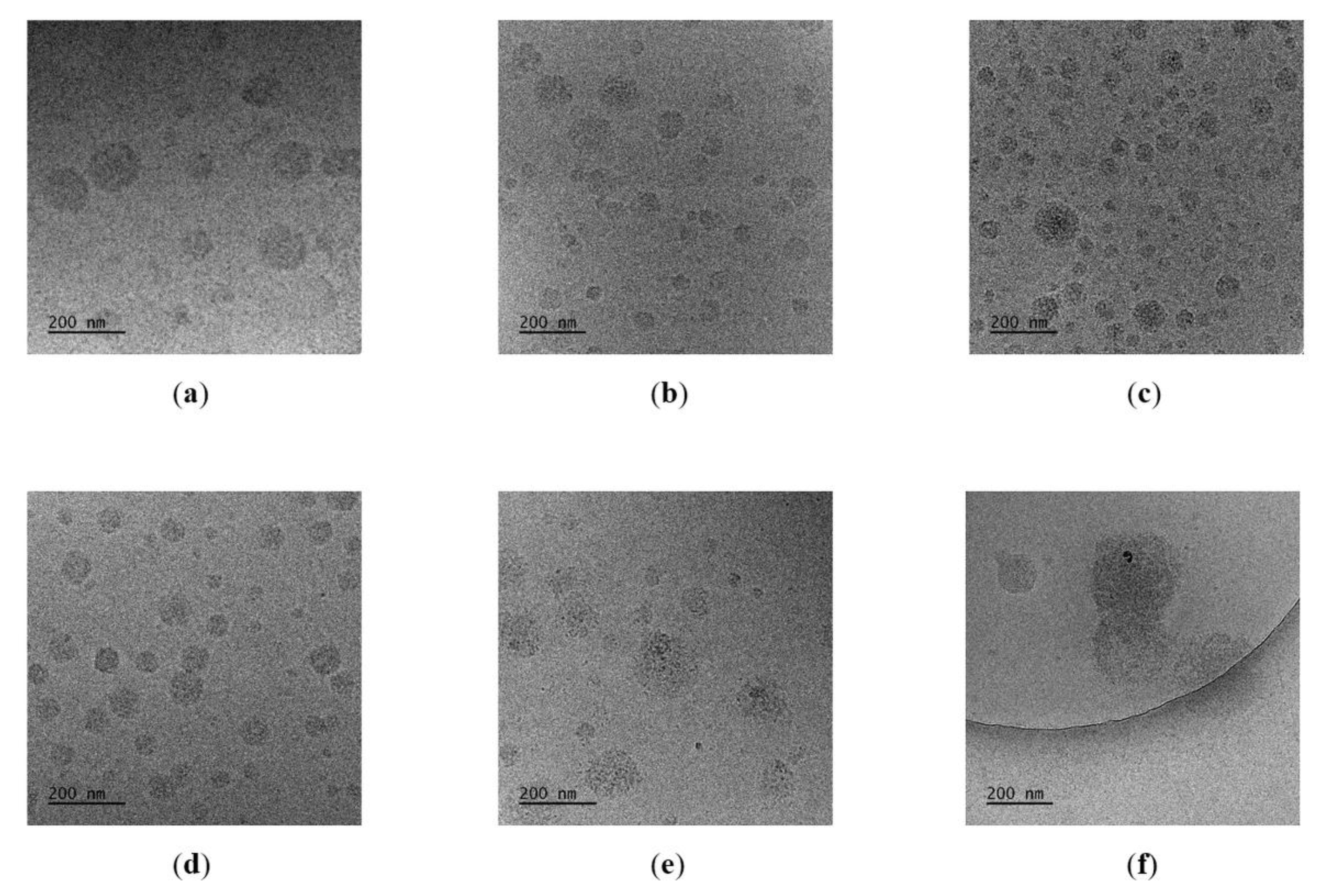
3.4. Nanogel Formation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Soni, K.S.; Desale, S.S.; Bronich, T.K. Nanogels: An overview of properties, biomedical applications and obstacles to clinical translation. J. Control. Release 2016, 240, 109–126. [Google Scholar] [CrossRef]
- Yadav, H.K.; Halabi, N.A.A.; Alsalloum, G.A. Nanogels as novel drug delivery systems—A review. J. Pharm. Pharm. Res. 2017, 1, 1–8. [Google Scholar]
- Neamtu, I.; Rusu, A.G.; Diaconu, A.; Nita, L.E.; Chiriac, A.P. Basic concepts and recent advances in nanogels as carriers for medical applications. Drug Deliv. 2017, 24, 539–557. [Google Scholar] [CrossRef]
- Sabir, F.; Asad, M.I.; Qindeel, M.; Afzal, I.; Dar, M.J.; Shah, K.U.; Zeb, A.; Khan, G.M.; Ahmed, N.; Din, F.-U. Polymeric nanogels as versatile nanoplatforms for biomedical applications. J. Nanomater. 2019, 2019, 1–16. [Google Scholar] [CrossRef]
- Paul, S.D.; Sharma, H.; Jeswani, G.; Jha, A.K. Chapter 12—Novel gels: Implications for drug delivery. In Nanostructures for Drug Delivery; Andronescu, E., Grumezescu, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 379–412. [Google Scholar]
- Nagel, G.; Sousa-Herves, A.; Wedepohl, S.; Calderón, M. Matrix metalloproteinase-sensitive multistage nanogels promote drug transport in 3d tumor model. Theranostics 2020, 10, 91–108. [Google Scholar] [CrossRef]
- Navarro, L.; Theune, L.E.; Calderón, M. Effect of crosslinking density on thermoresponsive nanogels: A study on the size control and the kinetics release of biomacromolecules. Eur. Polym. J. 2020, 124, 109478. [Google Scholar] [CrossRef]
- Gao, L.; Zabihi, F.; Ehrmann, S.; Hedtrich, S.; Haag, R. Supramolecular nanogels fabricated via host–guest molecular recognition as penetration enhancer for dermal drug delivery. J. Control. Release 2019, 300, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Dey, P.; Bergmann, T.; Cuellar-Camacho, J.L.; Ehrmann, S.; Chowdhury, M.S.; Zhang, M.; Dahmani, I.; Haag, R.; Azab, W. Multivalent flexible nanogels exhibit broad-spectrum antiviral activity by blocking virus entry. ACS Nano 2018, 12, 6429–6442. [Google Scholar] [CrossRef] [PubMed]
- Aseyev, V.; Tenhu, H.; Winnik, F.M. Non-ionic thermoresponsive polymers in water. In Self Organized Nanostructures of Amphiphilic Block Copolymers II; Müller, H.E.A., Borisov, O., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 29–89. [Google Scholar]
- Aseyev, V.O.; Tenhu, H.; Winnik, F.M. Temperature dependence of the colloidal stability of neutral amphiphilic polymers in water. In Conformation-Dependent Design of Sequences in Copolymers II; Khokhlov, A.R., Ed.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 1–85. [Google Scholar]
- Dawson, K.A.; Gorelov, A.V.; Timoshenko, E.G.; Kuznetsov, Y.A.; Du Chesne, A. Formation of mesoglobules from phase separation in dilute polymer solutions: A study in experiment, theory, and applications. Phys. A Stat. Mech. Appl. 1997, 244, 68–80. [Google Scholar] [CrossRef]
- Gorelov, A.V.; Du Chesne, A.; Dawson, K.A. Phase separation in dilute solutions of poly (N-isopropylacrylamide). Phys. A Stat. Mech. Appl. 1997, 240, 443–452. [Google Scholar] [CrossRef]
- Plummer, R.; Hill, D.J.T.; Whittaker, A.K. Solution properties of star and linear poly(N-isopropylacrylamide). Macromolecules 2006, 39, 8379–8388. [Google Scholar] [CrossRef]
- Xu, J.; Liu, S. Synthesis of well-defined 7-arm and 21-arm poly(N-isopropylacrylamide) star polymers with β-cyclodextrin cores via click chemistry and their thermal phase transition behavior in aqueous solution. J. Polym. Sci. A Polym. Chem. 2009, 47, 404–419. [Google Scholar] [CrossRef]
- Yuan, W.; Yuan, J.; Zhou, M.; Sui, X. Synthesis, characterization, and thermal properties of dendrimer-star, block-comb copolymers by ring-opening polymerization and atom transfer radical polymerization. J. Polym. Sci. A Polym. Chem. 2006, 44, 6575–6586. [Google Scholar] [CrossRef]
- Lambermont-Thijs, H.M.L.; Hoogenboom, R.; Fustin, C.-A.; Bomal-D’Haese, C.; Gohy, J.-F.; Schubert, U.S. Solubility behavior of amphiphilic block and random copolymers based on 2-ethyl-2-oxazoline and 2-nonyl-2-oxazoline in binary water–ethanol mixtures. J. Polym. Sci. A Polym. Chem. 2009, 47, 515–522. [Google Scholar] [CrossRef]
- Seno, K.-I.; Tsujimoto, I.; Kikuchi, T.; Kanaoka, S.; Aoshima, S. Thermosensitive gradient copolymers by living cationic polymerization: Semibatch precision synthesis and stepwise dehydration-induced micellization and physical gelation. J. Polym. Sci. A Polym. Chem. 2008, 46, 6151–6164. [Google Scholar] [CrossRef]
- Meeussen, F.; Nies, E.; Berghmans, H.; Verbrugghe, S.; Goethals, E.; Du Prez, F. Phase behaviour of poly(N-vinyl caprolactam) in water. Polymer 2000, 41, 8597–8602. [Google Scholar] [CrossRef]
- Van Durme, K.; Van Assche, G.; Van Mele, B. Kinetics of demixing and remixing in poly(N-isopropylacrylamide)/water studied by modulated temperature dsc. Macromolecules 2004, 37, 9596–9605. [Google Scholar] [CrossRef]
- Zhang, Y.; Cremer, P.S. Interactions between macromolecules and ions: The hofmeister series. Curr. Opin. Chem. Biol. 2006, 10, 658–663. [Google Scholar] [CrossRef]
- Zhang, Y.; Furyk, S.; Bergbreiter, D.E.; Cremer, P.S. Specific ion effects on the water solubility of macromolecules: Pnipam and the hofmeister series. J. Am. Chem. Soc. 2005, 127, 14505–14510. [Google Scholar] [CrossRef]
- Laschewsky, A.; Rekaï, E.D. Photochemical modification of the lower critical solution temperature of cinnamoylated poly(N-2-hydroxypropylmethacrylamide) in water. Macromol. Rapid Commun. 2000, 21, 937–940. [Google Scholar] [CrossRef]
- Trzebicka, B.; Weda, P.; Utrata-Wesołek, A.; Dworak, A.; Tsvetanov, C. Mesoglobules of random copolyethers as templates for nanoparticles. J. Polym. Sci. A Polym. Chem. 2010, 48, 4074–4083. [Google Scholar] [CrossRef]
- Dworak, A.; Lipowska, D.; Szweda, D.; Suwinski, J.; Trzebicka, B.; Szweda, R. Degradable polymeric nanoparticles by aggregation of thermoresponsive polymers and “click” chemistry. Nanoscale 2015, 7, 16823–16833. [Google Scholar] [CrossRef]
- Lutz, J.-F. Polymerization of oligo(ethylene glycol) (meth)acrylates: Toward new generations of smart biocompatible materials. J. Polym. Sci. A Polym. Chem. 2008, 46, 3459–3470. [Google Scholar] [CrossRef]
- Lutz, J.-F.; Weichenhan, K.; Akdemir, Ö.; Hoth, A. About the phase transitions in aqueous solutions of thermoresponsive copolymers and hydrogels based on 2-(2-methoxyethoxy)ethyl methacrylate and oligo(ethylene glycol) methacrylate. Macromolecules 2007, 40, 2503–2508. [Google Scholar] [CrossRef]
- Siu, M.; He, C.; Wu, C. Formation of mesoglobular phase of amphiphilic copolymer chains in dilute solution: Effect of comonomer distribution. Macromolecules 2003, 36, 6588–6592. [Google Scholar] [CrossRef]
- Agarwal, S.; Kumar, R.; Kissel, T.; Reul, R. Synthesis of degradable materials based on caprolactone and vinyl acetate units using radical chemistry. Polym. J. 2009, 41, 650. [Google Scholar] [CrossRef]
- Jamróz-Piegza, M.; Utrata-Wesołek, A.; Trzebicka, B.; Dworak, A. Hydrophobic modification of high molar mass polyglycidol to thermosensitive polymers. Eur. Polym. J. 2006, 42, 2497–2506. [Google Scholar] [CrossRef]
- Jerca, F.A.; Anghelache, A.M.; Ghibu, E.; Cecoltan, S.; Stancu, I.-C.; Trusca, R.; Vasile, E.; Teodorescu, M.; Vuluga, D.M.; Hoogenboom, R.; et al. Poly(2-isopropenyl-2-oxazoline) hydrogels for biomedical applications. Chem. Mater. 2018, 30, 7938–7949. [Google Scholar] [CrossRef]
- Jerca, F.A.; Jerca, V.V.; Anghelache, A.M.; Vuluga, D.M.; Hoogenboom, R. Poly(2-isopropenyl-2-oxazoline) as a versatile platform towards thermoresponsive copolymers. Polym. Chem. 2018, 9, 3473–3478. [Google Scholar] [CrossRef]
- Zhang, G.; Wu, C. Folding and formation of mesoglobules in dilute copolymer solutions. In Conformation-Dependent Design of Sequences in Copolymers I; Khokhlov, A.R., Ed.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 101–176. [Google Scholar]
- Aseyev, V.; Hietala, S.; Laukkanen, A.; Nuopponen, M.; Confortini, O.; Du Prez, F.E.; Tenhu, H. Mesoglobules of thermoresponsive polymers in dilute aqueous solutions above the LCST. Polymer 2005, 46, 7118–7131. [Google Scholar] [CrossRef]
- Rangelov, S.; Simon, P.; Toncheva-Moncheva, N.; Dimitrov, P.; Gajewska, B.; Tsvetanov, C.B. Nanosized colloidal particles from thermosensitive poly(methoxydiethyleneglycol methacrylate)s in aqueous media. Polym. Bull. 2012, 68, 2175–2185. [Google Scholar] [CrossRef]
- Trzebicka, B.; Szweda, D.; Rangelov, S.; Kowalczuk, A.; Mendrek, B.; Utrata-Wesołek, A.; Dworak, A. (Co)polymers of oligo(ethylene glycol) methacrylates—temperature-induced aggregation in aqueous solution. J. Polym. Sci. A Polym. Chem. 2013, 51, 614–623. [Google Scholar] [CrossRef]
- Wu, C.; Li, W.; Zhu, X.X. Viscoelastic effect on the formation of mesoglobular phase in dilute solutions. Macromolecules 2004, 37, 4989–4992. [Google Scholar] [CrossRef]
- Junk, M.J.N.; Li, W.; Schlüter, A.D.; Wegner, G.; Spiess, H.W.; Zhang, A.; Hinderberger, D. Formation of a mesoscopic skin barrier in mesoglobules of thermoresponsive polymers. J. Am. Chem. Soc. 2011, 133, 10832–10838. [Google Scholar] [CrossRef]
- Kujawa, P.; Aseyev, V.; Tenhu, H.; Winnik, F.M. Temperature-sensitive properties of poly(N-isopropylacrylamide) mesoglobules formed in dilute aqueous solutions heated above their demixing point. Macromolecules 2006, 39, 7686–7693. [Google Scholar] [CrossRef]
- Barker, I.C.; Cowie, J.M.G.; Huckerby, T.N.; Shaw, D.A.; Soutar, I.; Swanson, L. Studies of the “smart” thermoresponsive behavior of copolymers of N-isopropylacrylamide and N,N-dimethylacrylamide in dilute aqueous solution. Macromolecules 2003, 36, 7765–7770. [Google Scholar] [CrossRef]
- Nuopponen, M.; Ojala, J.; Tenhu, H. Aggregation behaviour of well defined amphiphilic diblock copolymers with poly(N-isopropylacrylamide) and hydrophobic blocks. Polymer 2004, 45, 3643–3650. [Google Scholar] [CrossRef]
- Toncheva-Moncheva, N.; Dimitrov, P.; Tsvetanov, C.B.; Robak, B.; Trzebicka, B.; Dworak, A.; Rangelov, S. Formation of mesoglobules in aqueous media from thermo-sensitive poly(ethoxytriethyleneglycol acrylate). Polym. Bull. 2011, 67, 1335–1346. [Google Scholar] [CrossRef]
- Lipowska-Kur, D.; Szweda, R.; Trzebicka, B.; Dworak, A. Preparation and characterization of doxorubicin nanocarriers based on thermoresponsive oligo(ethylene glycol) methyl ether methacrylate polymer-drug conjugates. Eur. Polym. J. 2018, 109, 391–401. [Google Scholar] [CrossRef]
- Libera, M.; Trzebicka, B.; Kowalczuk, A.; Wałach, W.; Dworak, A. Synthesis and thermoresponsive properties of four arm, amphiphilic poly(tert-butyl-glycidylether)-block-polyglycidol stars. Polymer 2011, 52, 250–257. [Google Scholar] [CrossRef]
- Libera, M.; Wałach, W.; Trzebicka, B.; Rangelov, S.; Dworak, A. Thermosensitive dendritic stars of tert-butyl-glycidylether and glycidol—Synthesis and encapsulation properties. Polymer 2011, 52, 3526–3536. [Google Scholar] [CrossRef]
- Haladjova, E.; Rangelov, S.; Tsvetanov, C.; Simon, P. Preparation of polymeric nanocapsules via nano-sized poly(methoxydiethyleneglycol methacrylate) colloidal templates. Polymer 2014, 55, 1621–1627. [Google Scholar] [CrossRef]
- Haladjova, E.; Simeonova, M.; Rangelov, S.; Tsvetanov, C.; Lalev, G. Template-assisted approach for preparation of poly(butyl-2-cyanoacrylate) nanocapsules. Nanosci. Nanotechnol. 2016, 16. [Google Scholar]
- Ivanova, T.; Haladjova, E.; Mees, M.; Momekova, D.; Rangelov, S.; Momekov, G.; Hoogenboom, R. Characterization of polymer vector systems based on partially hydrolyzed polyoxazoline for gene transfection. Pharmacia 2016, 63, 3–8. [Google Scholar]
- Trzcinska, R.; Szweda, D.; Rangelov, S.; Suder, P.; Silberring, J.; Dworak, A.; Trzebicka, B. Bioactive mesoglobules of poly(di(ethylene glycol) monomethyl ether methacrylate)–peptide conjugate. J. Polym. Sci. A Polym. Chem. 2012, 50, 3104–3115. [Google Scholar] [CrossRef]
- Trzebicka, B.; Haladjova, E.; Otulakowski, Ł.; Oleszko, N.; Wałach, W.; Libera, M.; Rangelov, S.; Dworak, A. Hybrid nanoparticles obtained from mixed mesoglobules. Polymer 2015, 68, 65–73. [Google Scholar] [CrossRef]
- Trzebicka, B.; Robak, B.; Trzcinska, R.; Szweda, D.; Suder, P.; Silberring, J.; Dworak, A. Thermosensitive pnipam-peptide conjugate—Synthesis and aggregation. Eur. Polym. J. 2013, 49, 499–509. [Google Scholar] [CrossRef]
- Weda, P.; Trzebicka, B.; Dworak, A.; Tsvetanov, C.B. Thermosensitive nanospheres of low-density core—An approach to hollow nanoparticles. Polymer 2008, 49, 1467–1474. [Google Scholar] [CrossRef]
- Osváth, Z.; Iván, B. The dependence of the cloud point, clearing point, and hysteresis of poly(N-isopropylacrylamide) on experimental conditions: The need for standardization of thermoresponsive transition determinations. Macromol. Chem. Phys. 2017, 218, 1600470. [Google Scholar] [CrossRef]
- Virtanen, J.; Tenhu, H. Thermal properties of poly(N-isopropylacrylamide)-g-poly(ethylene oxide) in aqueous solutions: Influence of the number and distribution of the grafts. Macromolecules 2000, 33, 5970–5975. [Google Scholar] [CrossRef]
- Xu, X.D.; Chen, C.S.; Wang, Z.C.; Wang, G.R.; Cheng, S.X.; Zhang, X.Z.; Zhuo, R.X. “Click” chemistry for in situ formation of thermoresponsive p(NIPAM-co-HEMA)-based hydrogels. J. Polym. Sci. A Polym. Chem. 2008, 46, 5263–5277. [Google Scholar] [CrossRef]
- Vasilevskaya, V.V.; Khalatur, P.G.; Khokhlov, A.R. Conformational polymorphism of amphiphilic polymers in a poor solvent. Macromolecules 2003, 36, 10103–10111. [Google Scholar] [CrossRef]



| Copolymer | Molar Ratio D:A 1 | Mtheor. [g/mol] 2 | D:A Conversion [%] 3 | DP from Conversion [D:A] 3 | Mn,conv [g/mol] 4 | Mn [g/mol] 5 | Mw/Mn 5 | Amount of A via Kaiser Test | |
|---|---|---|---|---|---|---|---|---|---|
| [%mol] | Per Chain | ||||||||
| P(D-co-A)_1 | 276:24 | 55 800 | 59:58 | 163:14 | 33 300 | 32 900 | 1.10 | 3 | 5 |
| P(D-co-A)_2 | 270:30 | 55 700 | 56:60 | 151:18 | 32 000 | 34 900 | 1.15 | 8 | 17 |
| P(D-co-A)_3 | 255:45 | 55 400 | 40:42 | 102:19 | 23 000 | 34 400 | 1.12 | 10 | 21 |
| P(D-co-A)_4 | 240:60 | 55 100 | 50:45 | 120:27 | 28 000 | 41 000 | 1.14 | 24 | 60 |
| P(D-co-A)_5 | 225:75 | 54 700 | 50:42 | 113:31 | 27 000 | 30 500 | 1.14 | 35 | 66 |
| Amount of A in Initial P(D-co-A) | TCP [°C] | ||||||
|---|---|---|---|---|---|---|---|
| [%mol] | Per Chain | P(D-co-A) | P(D-co-N3) | P(D-co-Prop) | |||
| UV-Vis | DLS | UV-Vis | DLS | UV-Vis | DLS | ||
| 3 | 5 | 32.2 | 36.6 | 31.8 | 30.7 | 16.6 | 17.9 |
| 8 | 17 | 35.0 | 42.7 | 34.4 | 29.7 | 13.6 | 15.8 |
| 10 | 21 | 37.3 | 43.0 | -1 | 38.4 | 11.6 | 11.2 |
| 24 | 60 | 42.6 | 47.8 | -1 | -1 | -2 | -2 |
| 35 | 66 | 48.0 | 48.5 | -1 | -1 | -2 | -2 |
| Amount of A in Initial P(D-co-A) | ||||
|---|---|---|---|---|
| [%mol] | Per Chain | P(D-co-A) | P(D-co-N3) | P(D-co-Prop) |
| 3 | 5 | 120 | 132 | 599 |
| 8 | 17 | 77 | 118 | 158 |
| 10 | 21 | 79 | 76 | 68 |
| 24 | 60 | -1 | -1 | -2 |
| 35 | 66 | -1 | -1 | -2 |
| Swelling Ratio V10/V60 4 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Before Reaction | After Reaction | |||||||||
| Polymer Mixture | Amount of Functional Groups Per Chain | 60 °C | 60 °C | 10 °C | ||||||
| N3 1 | Prop 1 | N3 2 | ||||||||
| R1 | 5 | 5 | 0 | 44.0 | 0.037 | 44.5 | 0.054 | 60.5 | 0.133 | 2.5 |
| R2 | 17 | 17 | 0 | 41.0 | 0.116 | 42.5 | 0.299 | 51.0 | 0.018 | 1.7 |
| R3 | 21 | 21 | 0 | 26/91.0 | 0.237 3 | 42/167 | 0.152 3 | 219.5 | 0.199 | 2.3 |
| R4 | 21 | 17 | 4 | 21.5 | 0.300 | 25.5 | 0.145 | 36.0 | 0.269 | 2.8 |
| R5 | 17 | 5 | 12 | 44.0 | 0.190 | 47.5 | 0.187 | 68.5 | 0.244 | 3.0 |
| R6 | 21 | 5 | 16 | 30/89.5 | 0.332 3 | 151.5 | 0.100 | 176.5 | 0.084 | 1.6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lipowska-Kur, D.; Otulakowski, Ł.; Trzebicka, B.; Utrata-Wesołek, A.; Dworak, A. Thermoresponsive Nanogels of Modified Poly((di(ethylene glycol) methyl ether methacrylate)-co-(2-aminoethyl methacrylate))s. Polymers 2020, 12, 1645. https://doi.org/10.3390/polym12081645
Lipowska-Kur D, Otulakowski Ł, Trzebicka B, Utrata-Wesołek A, Dworak A. Thermoresponsive Nanogels of Modified Poly((di(ethylene glycol) methyl ether methacrylate)-co-(2-aminoethyl methacrylate))s. Polymers. 2020; 12(8):1645. https://doi.org/10.3390/polym12081645
Chicago/Turabian StyleLipowska-Kur, Daria, Łukasz Otulakowski, Barbara Trzebicka, Alicja Utrata-Wesołek, and Andrzej Dworak. 2020. "Thermoresponsive Nanogels of Modified Poly((di(ethylene glycol) methyl ether methacrylate)-co-(2-aminoethyl methacrylate))s" Polymers 12, no. 8: 1645. https://doi.org/10.3390/polym12081645
APA StyleLipowska-Kur, D., Otulakowski, Ł., Trzebicka, B., Utrata-Wesołek, A., & Dworak, A. (2020). Thermoresponsive Nanogels of Modified Poly((di(ethylene glycol) methyl ether methacrylate)-co-(2-aminoethyl methacrylate))s. Polymers, 12(8), 1645. https://doi.org/10.3390/polym12081645