New Manufacturing Process of Composites Reinforced with ZnO Nanoparticles Recycled from Alkaline Batteries
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Composites Manufacturing
2.3. Characterization
3. Results and Discussion
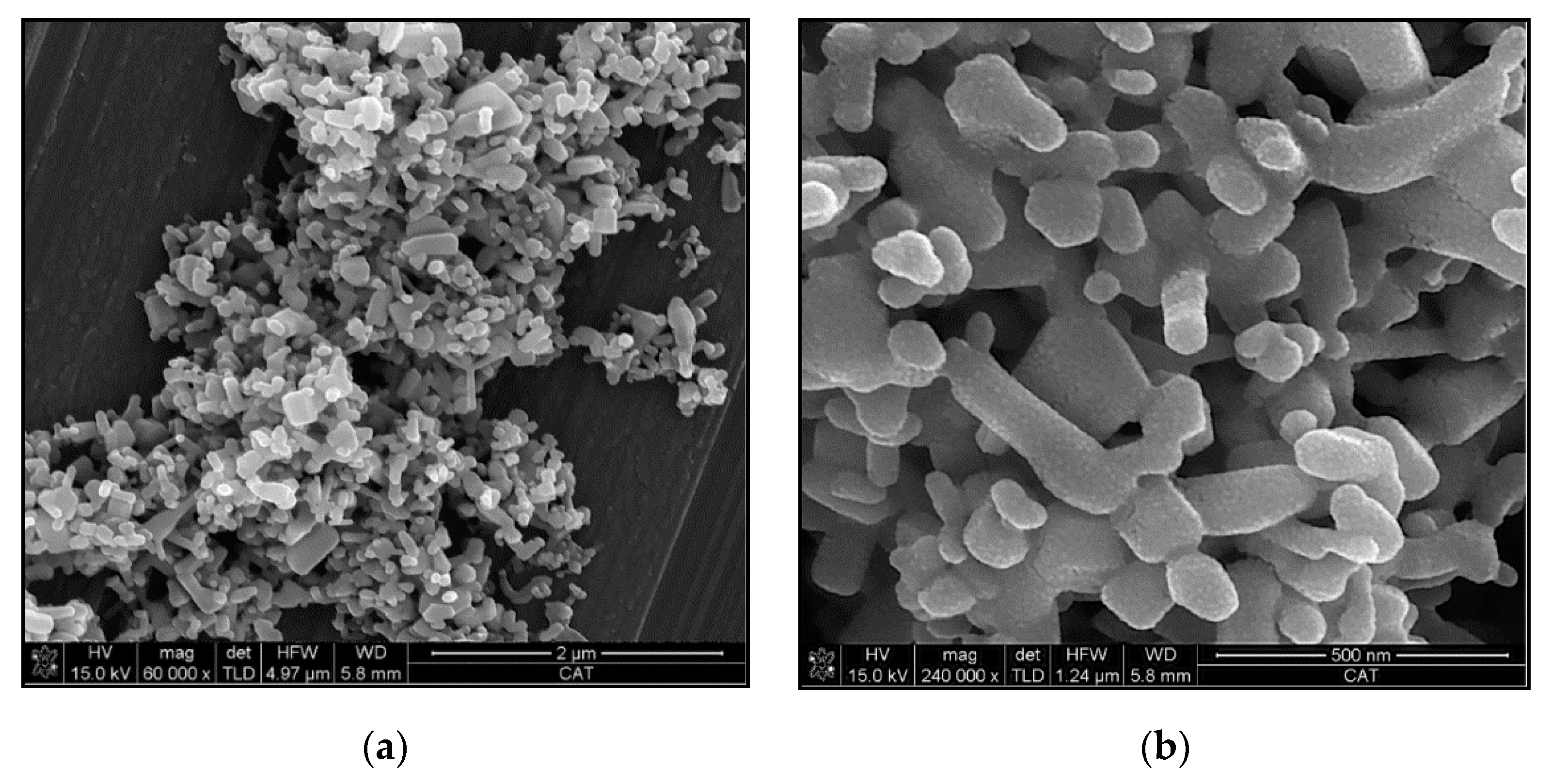
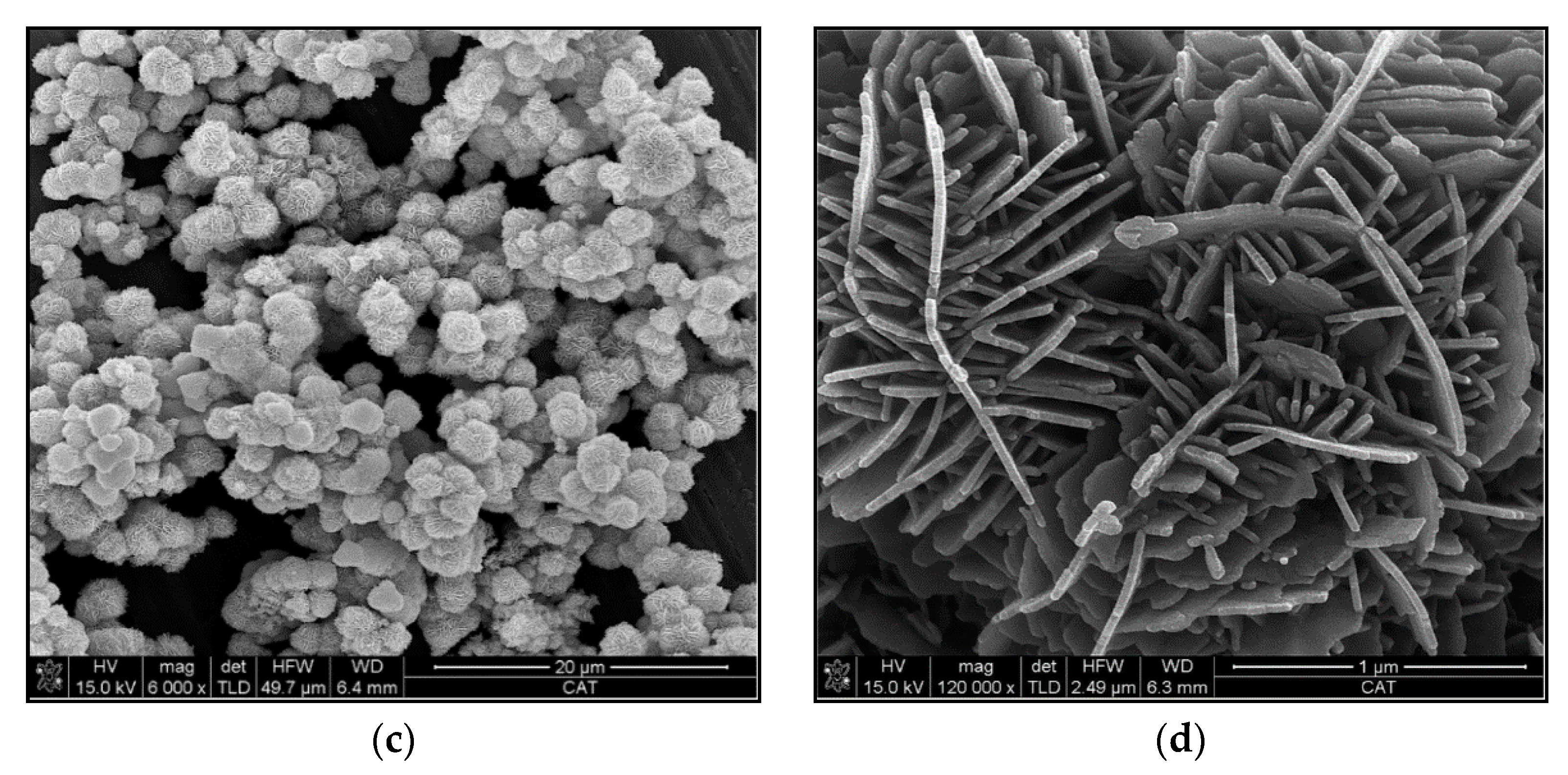
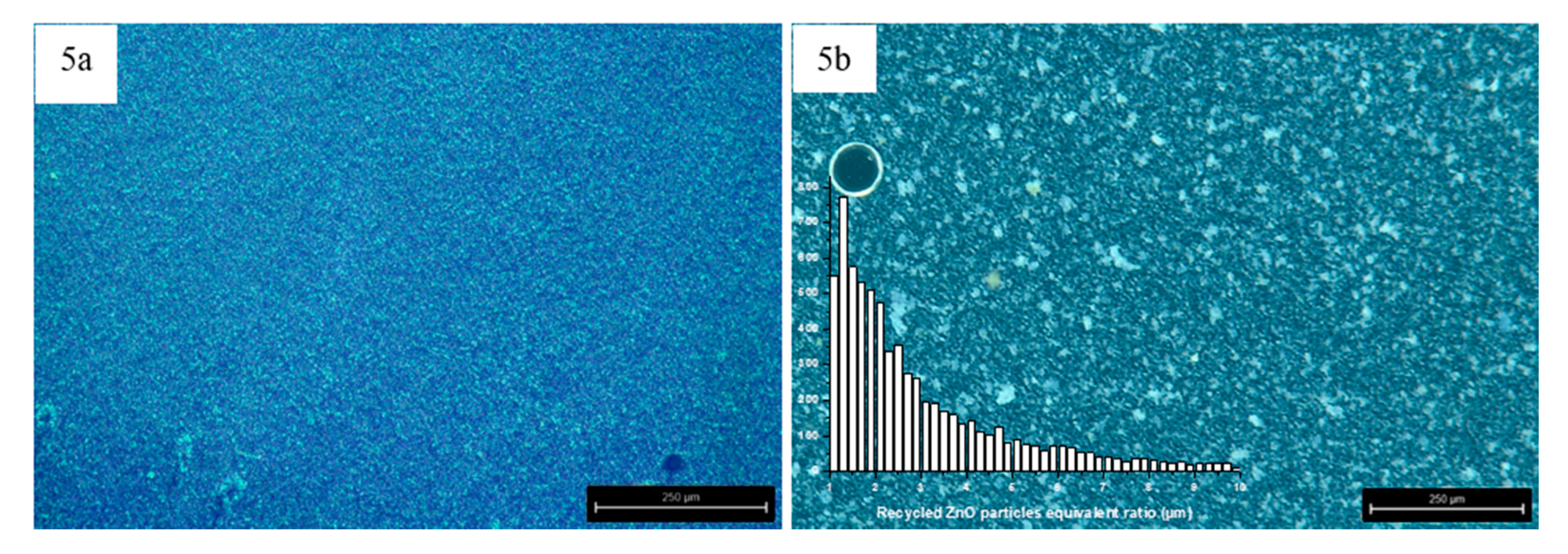
3.1. Characterization of Reinforcements
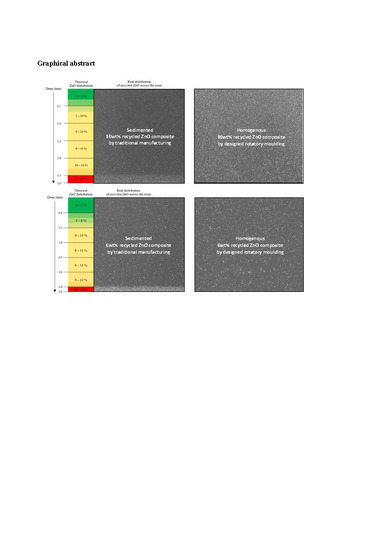
3.2. Sedimentation Analysis and Desing for Rotary Moulding
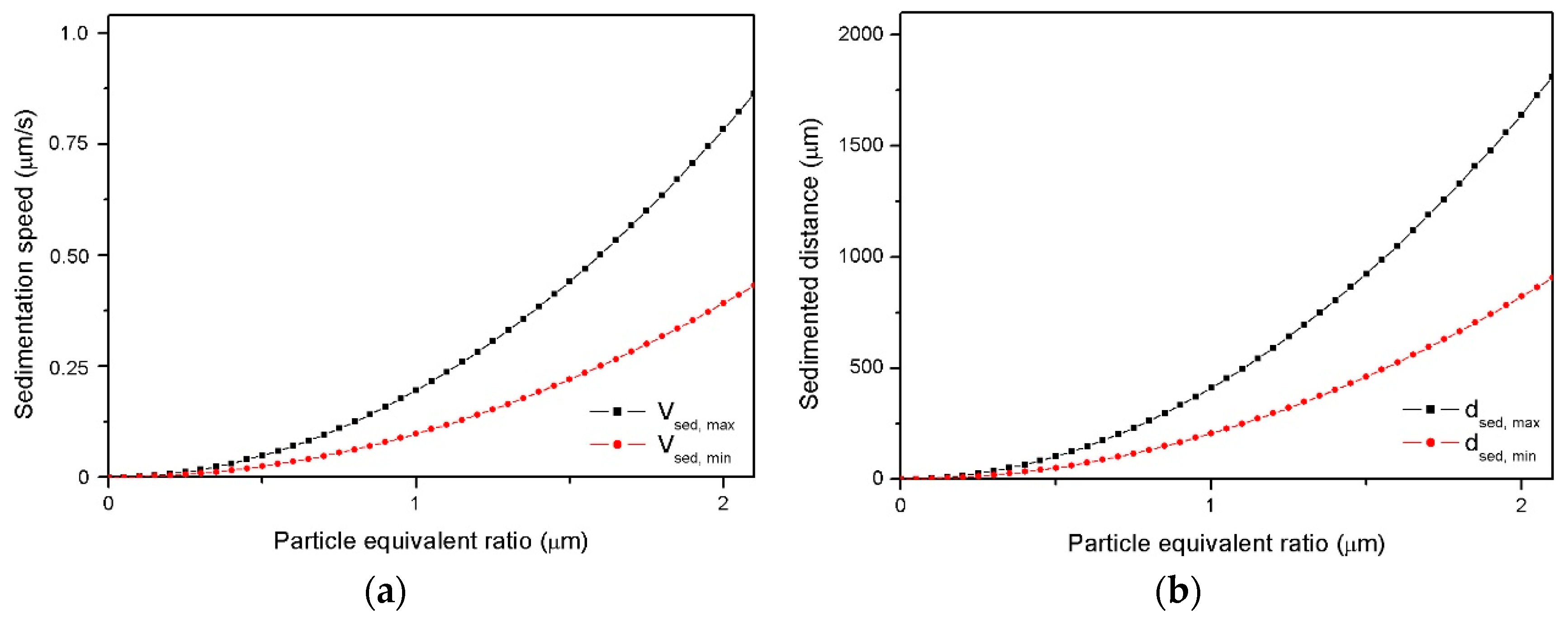
3.2.1. Analysis of Particles Sedimentation
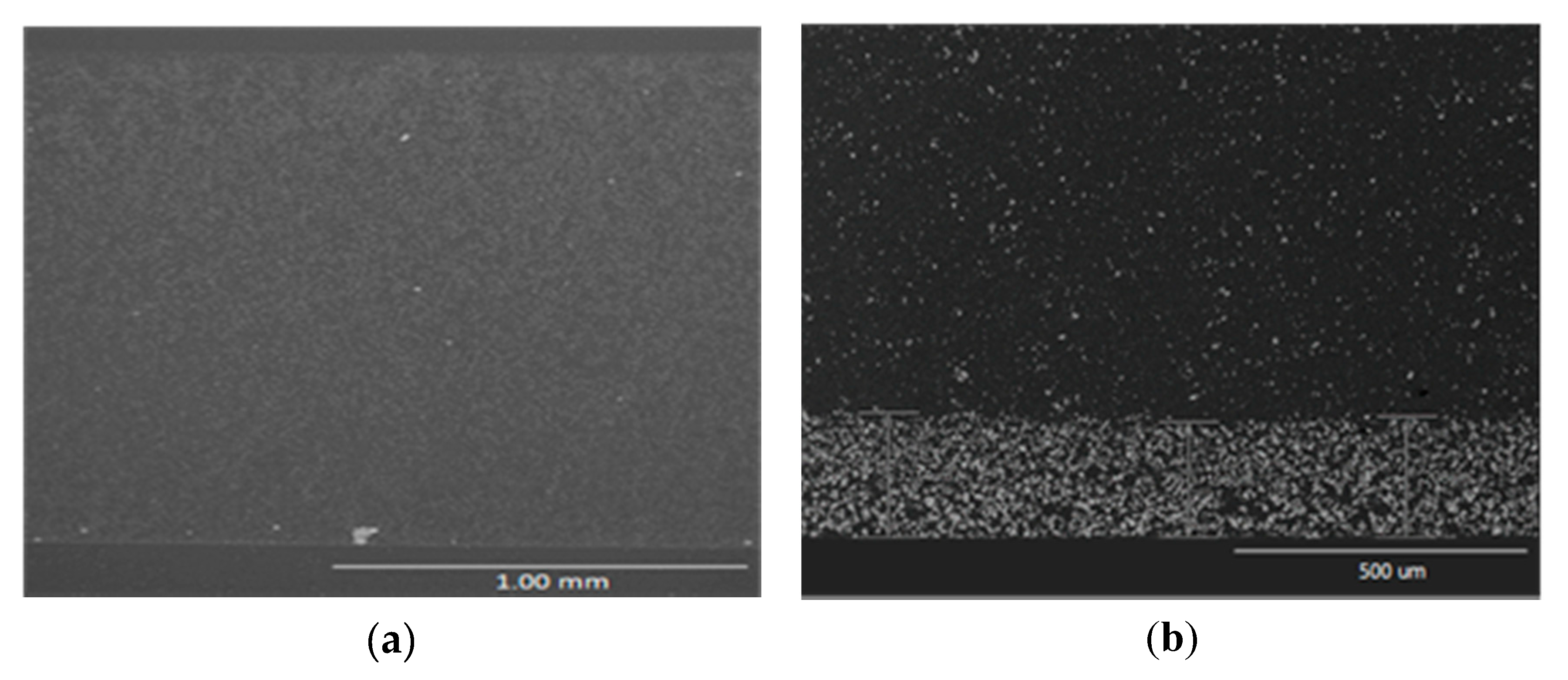
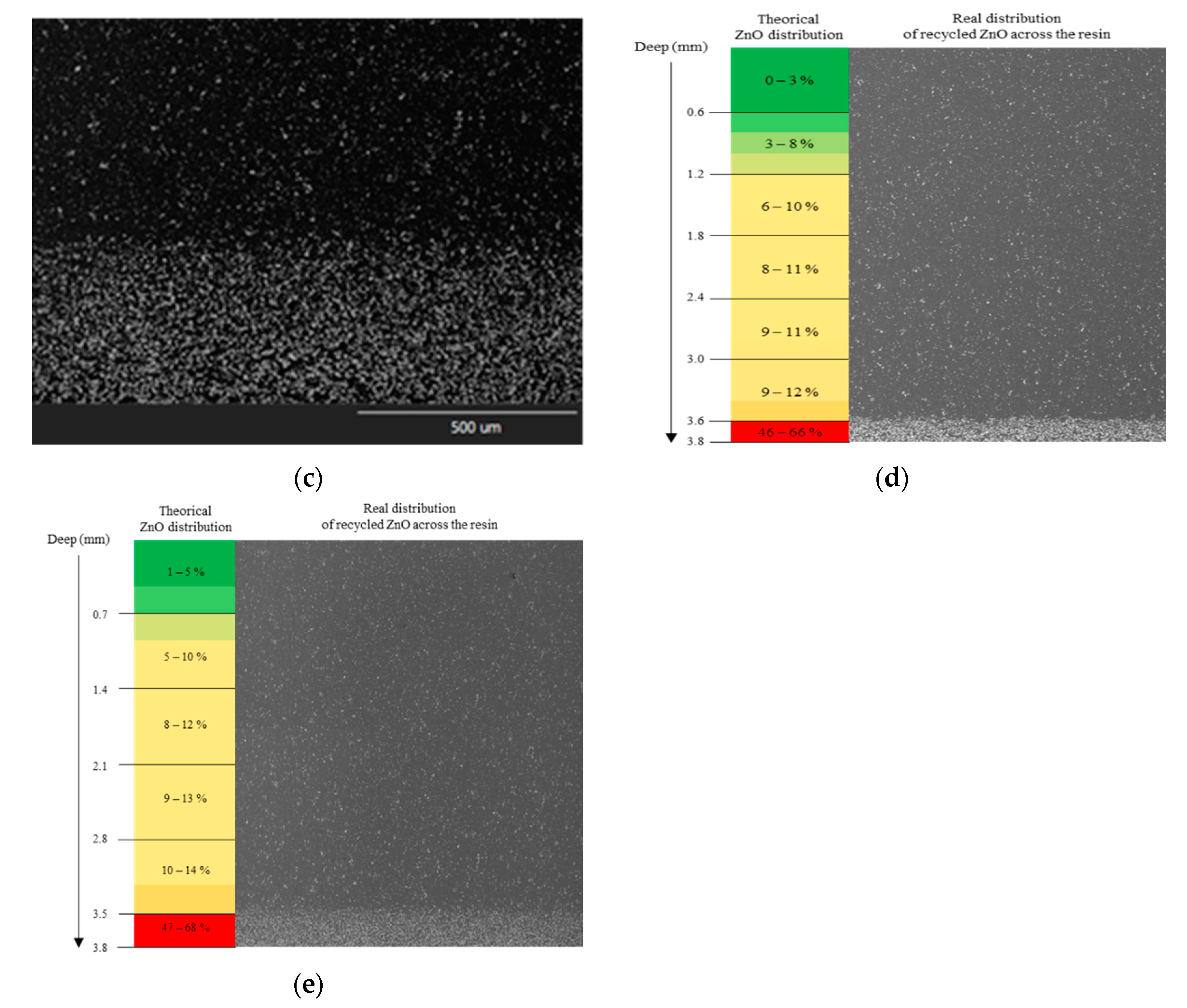
3.2.2. Design of Rotary Molding
3.3. Chracterization of Composites
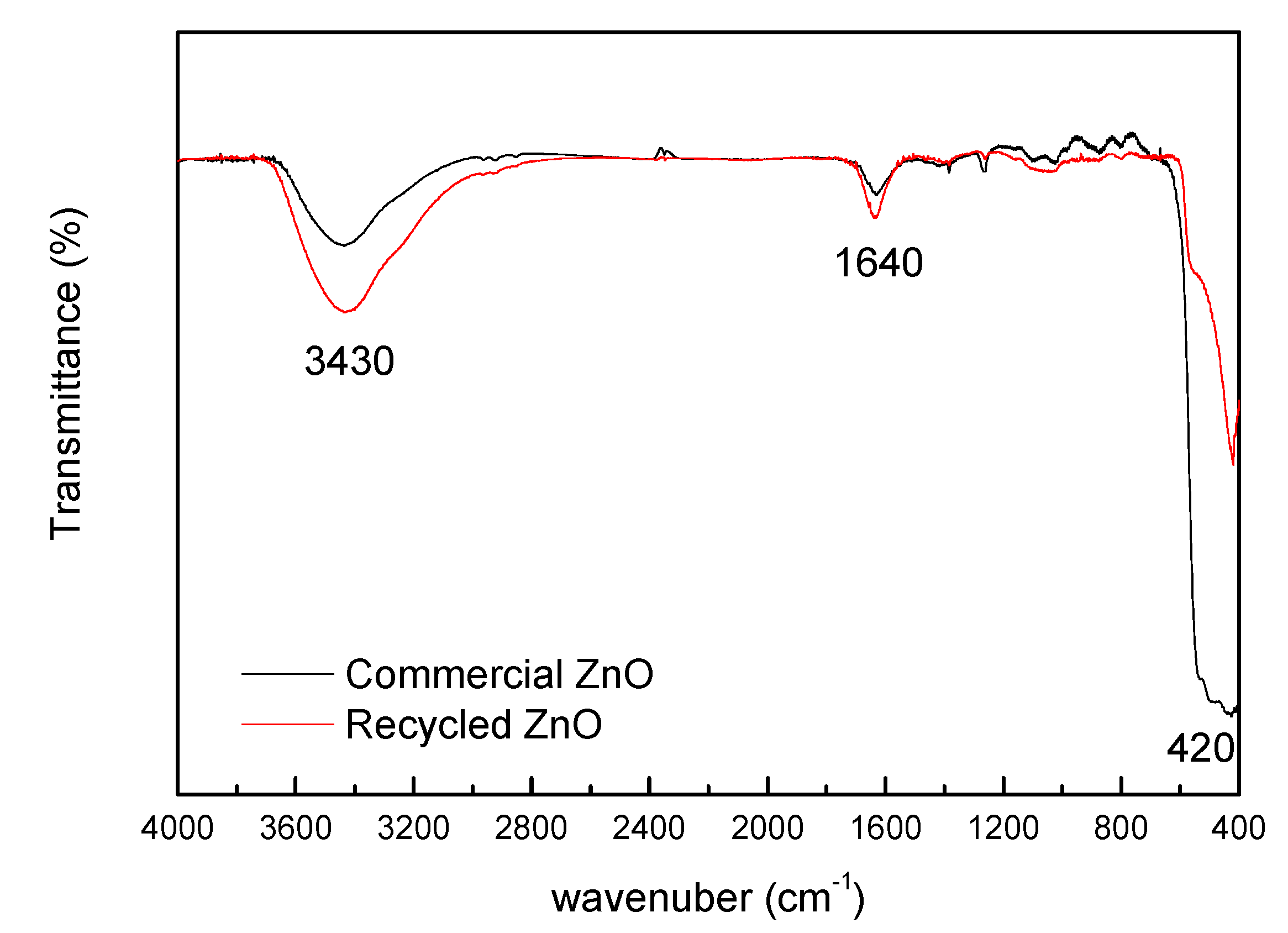
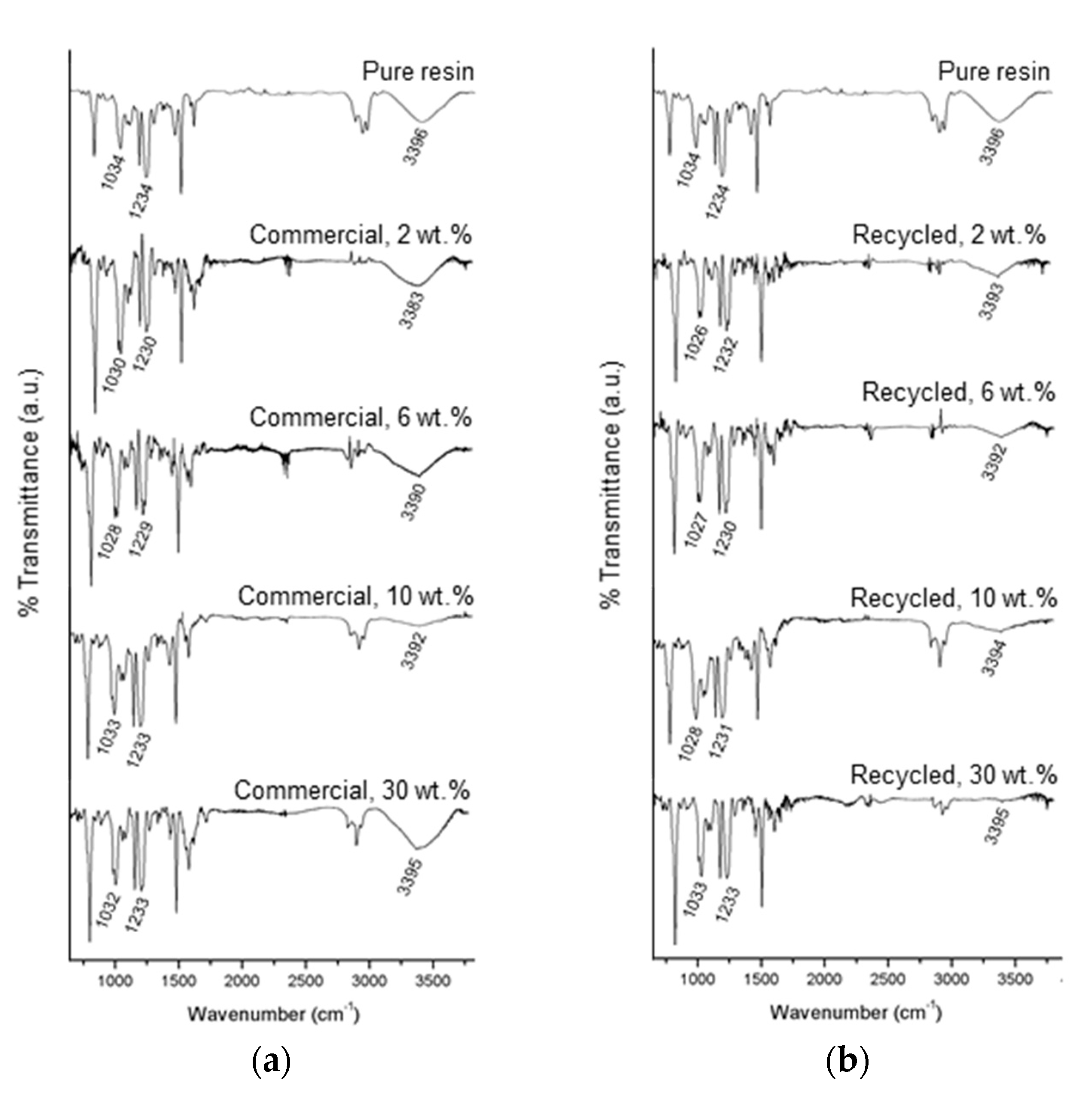
3.3.1. FTIR Analysis
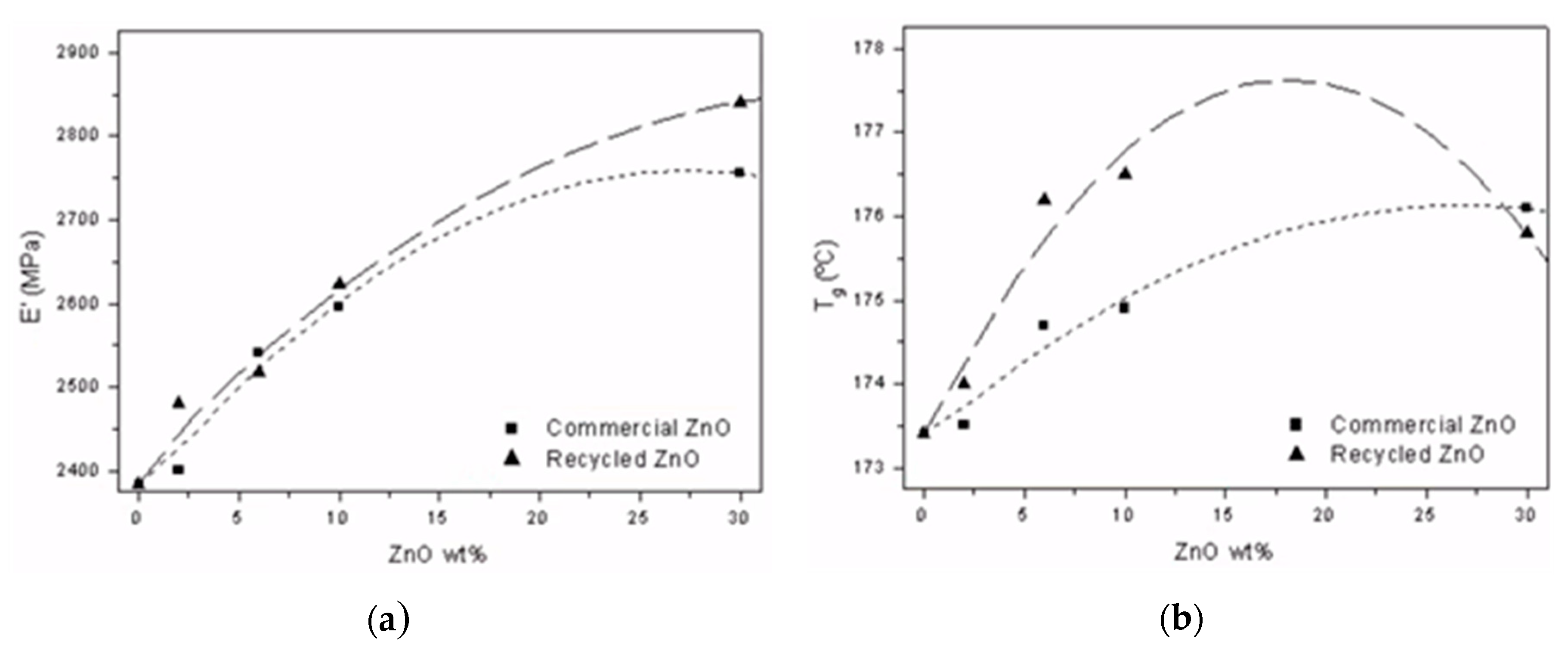
3.3.2. Thermo-Mechanical Behavior
3.3.3. Microhardness
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, X.; Shi, F.; Niu, J.; Jiang, Y.; Wang, Z. Superhydrophobic surfaces: From structural control to functional application. J. Mater. Chem. 2008, 18, 621–633. [Google Scholar] [CrossRef]
- Peng, W.; Gou, X.; Qin, H.; Zhao, M.; Zhao, X.; Guo, Z. Creation of a multifunctional superhydrophobic coating for composite insulators. Chem. Eng. J. 2018, 352, 774–781. [Google Scholar] [CrossRef]
- Han, S.; Yao, T.; Yang, X. Preparation and anti-icing properties of a hydrophobic emulsified asphalt coating. Constr. Build. Mater. 2019, 220, 214–227. [Google Scholar] [CrossRef]
- Ammar, S.; Ramesh, K.; Vengadaesvaran, B.; Ramesh, S.; Arof, A.K. Amelioration of anticorrosion and hydrophobic properties of epoxy/PDMS composite coatings containing nano ZnO particles. Prog. Org. Coat. 2016, 92, 54–65. [Google Scholar] [CrossRef]
- Ramezanzadeh, B.; Attar, M.M.; Farzam, M. A study on the anticorrosion performance of the epoxy-polyamide nanocomposites containing ZnO nanoparticles. Prog. Org. Coat. 2011, 72, 410–422. [Google Scholar] [CrossRef]
- Arribas, C.; Prolongo, M.G.; Sánchez-Cabezudo, M.; Moriche, R.; Prolongo, S.G. Hydrothermal ageing of graphene/carbon nanotubes/epoxy hybrid nanocomposites. Polym. Degrad. Stab. 2019, 170, 109003. [Google Scholar] [CrossRef]
- Psarski, M.; Celichowski, G.; Marczak, J.; Gumowski, K.; Sobieraj, G.B. Superhydrophobic dual-sized filler epoxy composite coatings. Surf. Coatings Technol. 2013, 225, 66–74. [Google Scholar] [CrossRef]
- Zhang, X.; Si, Y.; Mo, J.; Guo, Z. Robust micro-nanoscale flowerlike ZnO/epoxy resin superhydrophobic coating with rapid healing ability. Chem. Eng. J. 2017, 313, 1152–1159. [Google Scholar] [CrossRef]
- Lorwanishpaisarn, N.; Kasemsiri, P.; Srikhao, N.; Jetsrisuparb, K.; Knijnenburg, J.T.N.; Hiziroglu, S.S.; Pongsa, U.; Chindaprasirt, P. Fabrication of durable superhydrophobic epoxy/cashew nut shell liquid based coating containing flower-like zinc oxide for continuous oil/water separation. Surf. Coat. Technol. 2019, 366, 106–113. [Google Scholar] [CrossRef]
- Zhou, H.; Chen, R.; Liu, Q.; Liu, J.; Yu, J.; Wang, C.; Zhang, M.; Liu, P.; Wang, J. Fabrication of ZnO/epoxy resin superhydrophobic coating on AZ31 magnesium alloy. Chem. Eng. J. 2019, 368, 261–272. [Google Scholar] [CrossRef]
- Wang, N.; Tang, L.; Cai, Y.; Tong, W.; Xiong, D. Scalable superhydrophobic coating with controllable wettability and investigations of its drag reduction. Colloids Surf. Asp. 2018, 555, 290–295. [Google Scholar] [CrossRef]
- Sari, M.G.; Vahabi, H.; Gabrion, X.; Laheurte, P.; Zarrintaj, P.; Formela, K.; Saeb, M.R. An attempt to mechanistically explain the viscoelastic behavior of transparent epoxy/starch-modified ZnO nanocomposite coatings. Prog. Org. Coat. 2018, 119, 171–182. [Google Scholar] [CrossRef]
- Ponnamma, D.; Cabibihan, J.; Rajan, M.; Pethaiah, S.S.; Deshmukh, K.; Gogoi, J.P.; Pasha, S.K.K.; Ahamed, M.B.; Krishnegowda, J.; Chandrashekar, B.N.; et al. Synthesis, optimization and applications of ZnO/polymer nanocomposites. Mater. Sci. Eng. C 2019, 98, 1210–1240. [Google Scholar] [CrossRef] [PubMed]
- Halder, S.; Prasad, T.; Khan, N.I.; Goyat, M.S.; Chauhan, S.R. Superior mechanical properties of poly vinyl alcohol-assisted ZnO nanoparticle reinforced epoxy composites. Mater. Chem. Phys. 2017, 192, 198–209. [Google Scholar] [CrossRef]
- Antunes, P.V.; Ramalho, A.; Carrilho, E.V.P. Mechanical and wear behaviours of nano and microfilled polymeric composite.Effect of filler fraction and size. Mater. Des. 2014, 61, 50–60. [Google Scholar] [CrossRef]
- Shi, L.; Hu, J.; Lin, X.; Fang, L.; Wu, F.; Xie, J.; Meng, F. A robust superhydrophobic PPS-PTFE/SiO2 composite coating on AZ31 Mg alloy with excellent wear and corrosion resistance properties. J. Alloys Compd. 2017, 721, 157–163. [Google Scholar] [CrossRef]
- Dhoke, S.K.; Khanna, A.S.; Sinha, T.J.M. Effect of nano-ZnO particles on the corrosion behavior of alkyd-based waterborne coatings. Prog. Org. Coat. 2009, 64, 371–382. [Google Scholar] [CrossRef]
- Sandhya, P.K.; Sreekala, M.S.; Padmanabhan, M.; Thomas, S. Mechanical and thermal properties of ZnO anchored GO reinforced phenol formaldehyde resin. Diam. Relat. Mater. 2020, 108, 107961. [Google Scholar] [CrossRef]
- Ghamsari, M.S.; Alamdari, S.; Razzaghi, D.; Pirlar, M.A. ZnO nanocrystals with narrow-band blue emission. J. Lumin. 2019, 205, 508–518. [Google Scholar] [CrossRef]
- Hawkins, S.A.; Yao, H.; Wang, H.; Sue, H.J. Tensile properties and electrical conductivity of epoxy composite thin films containing zinc oxide quantum dots and multi-walled carbon nanotubes. Carbon N. Y. 2017, 115, 18–27. [Google Scholar] [CrossRef]
- Kumar, A.M.; Khan, A.; Khan, M.Y.; Suleiman, R.K.; Jose, J.; Dafalla, H. Hierarchical graphitic carbon nitride-ZnO nanocomposite: Viable reinforcement for the improved corrosion resistant behavior of organic coatings. Mater. Chem. Phys. 2020, 251, 122987. [Google Scholar] [CrossRef]
- Zabihi, O.; Mostafavi, S.M.; Ravari, F.; Khodabandeh, A.; Hooshafza, A.; Zare, K.; Shahizadeh, M. The effect of zinc oxide nanoparticles on thermo-physical properties of diglycidyl ether of bisphenol A/2,2′-Diamino-1,1′-binaphthalene nanocomposites. Thermochim. Acta 2011, 521, 49–58. [Google Scholar] [CrossRef]
- Karasinski, E.N.; da Luz, M.G.; Lepienski, C.M.; Coelho, L.A.F. Nanostructured coating based on epoxy/metal oxides: Kinetic curing and mechanical properties. Thermochim. Acta 2013, 569, 167–176. [Google Scholar] [CrossRef]
- Ghaffari, M.; Ehsani, M.; Vandalvand, M.; Avazverdi, E.; Askari, A.; Goudarzi, A. Studying the effect of micro- and nano-sized ZnO particles on the curing kinetic of epoxy/polyaminoamide system. Prog. Org. Coat. 2015, 89, 277–283. [Google Scholar] [CrossRef]
- Jena, K.K.; Alhassan, S.M.; Arora, N. Facile and rapid synthesis of efficient epoxy-novolac acrylate/MWCNTs-APTES-ZnO hybrid coating films by UV irradiation: Thermo-mechanical, shape stability, swelling, hydrophobicity and antibacterial properties. Polymer (Guildf) 2019, 179, 121621. [Google Scholar] [CrossRef]
- Ma, J.; An, W.; Xu, Q.; Fan, Q.; Wang, Y. Antibacterial casein-based ZnO nanocomposite coatings with improved water resistance crafted via double in situ route. Prog. Org. Coat. 2019, 134, 40–47. [Google Scholar] [CrossRef]
- Xu, J.; Song, R.; Dai, Y.; Yang, S.; Li, J.; Wei, R. Characterization of zinc oxide nanoparticles-epoxy resin composite and its antibacterial effects on spoilage bacteria derived from silvery pomfret (Pampus argenteus). Food Packag. Shelf Life 2019, 22, 100418. [Google Scholar] [CrossRef]
- Malucelli, G.; Fioravanti, A.; Francioso, L.; de Pascali, C.; Signore, M.A.; Carotta, M.C.C.; Bonanno, A.; Duraccio, D. Preparation and characterization of UV-cured composite films containing ZnO nanostructures: Effect of filler geometric features on piezoelectric response. Prog. Org. Coat. 2017, 109. [Google Scholar] [CrossRef]
- Guo, L.; Zhang, Z.; Kang, R.; Chen, Y.; Hou, X.; Wu, Y.; Wang, M.; Wang, B.; Cui, J.; Jiang, N.; et al. Enhanced thermal conductivity of epoxy composites filled with tetrapod-shaped ZnO. RSC Adv. 2018, 8, 12337–12343. [Google Scholar] [CrossRef]
- Yuan, F.Y.; Zhang, H.B.; Li, X.; Li, X.Z.; Yu, Z.Z. Synergistic effect of boron nitride flakes and tetrapod-shaped ZnO whiskers on the thermal conductivity of electrically insulating phenol formaldehyde composites. Compos. Part A Appl. Sci. Manuf. 2013, 53, 137–144. [Google Scholar] [CrossRef]
- Jiang, Y.; Sun, R.; Zhang, H.B.; Min, P.; Yang, D.; Yu, Z.Z. Graphene-coated ZnO tetrapod whiskers for thermally and electrically conductive epoxy composites. Compos. Part A Appl. Sci. Manuf. 2017, 94, 104–112. [Google Scholar] [CrossRef]
- Deka, B.K.; Hazarika, A.; Kwon, O.; Kim, D.Y.; Park, Y.B.; Park, H.W. Multifunctional enhancement of woven carbon fiber/ZnO nanotube-based structural supercapacitor and polyester resin-domain solid-polymer electrolytes. Chem. Eng. J. 2017, 325, 672–680. [Google Scholar] [CrossRef]
- Yari, H.; Rostami, M. Enhanced weathering performance of epoxy/ZnO nanocomposite coatings via functionalization of ZnO UV blockers with amino and glycidoxy silane coupling agents. Prog. Org. Coat. 2020, 147, 105773. [Google Scholar] [CrossRef]
- Xavier, L.H.; Giese, E.C.; Ribeiro-Duthie, A.C.; Lins, F.A.F. Sustainability and the circular economy: A theoretical approach focused on e-waste urban mining. Resour. Policy 2019, 101467. [Google Scholar] [CrossRef]
- López, F.A.; Cebriano, T.; García-Díaz, I.; Fernández, P.; Rodríguez, O.; Fernández, A.L. Synthesis and microstructural properties of zinc oxide nanoparticles prepared by selective leaching of zinc from spent alkaline batteries using ammoniacal ammonium carbonate. J. Clean. Prod. 2017, 148, 795–803. [Google Scholar] [CrossRef]
- Cebriano, T.; García-Díaz, I.; Fernández, A.L.; Fernández, P.; López, F.A. Synthesis and characterization of ZnO micro- and nanostructures grown from recovered ZnO from spent alkaline batteries. J. Environ. Chem. Eng. 2017, 5, 2903–2911. [Google Scholar] [CrossRef]
- Song, S.Y.; Park, M.S.; Lee, D.; Lee, J.W.; Yun, J.S. Optimization and characterization of high-viscosity ZrO2 ceramic nanocomposite resins for supportless stereolithography. Mater. Des. 2019, 180, 107960. [Google Scholar] [CrossRef]
- Paul, N.; Biggs, S.; Shiels, J.; Hammond, R.B.; Edmondson, M.C.; Maxwell, L.; Harbottle, D.; Hunter, T.N. Influence of shape and surface charge on the sedimentation of spheroidal, cubic and rectangular cuboid particles. Powder Technol. 2017, 322, 75–83. [Google Scholar] [CrossRef]
- Nolte, H.; Schilde, C.; Kwade, A. Production of highly loaded nanocomposites by dispersing nanoparticles in Epoxy Resin. Chem. Eng. Technol. 2010, 33, 1447–1455. [Google Scholar] [CrossRef]
- González-Campo, A.; Orchard, K.L.; Sato, N.; Shaffer, M.S.P.; Williams, C.K. One-pot, in situ synthesis of ZnO-carbon nanotube–epoxy resin hybrid nanocomposites. Chem. Commun. 2009, 4034–4036. [Google Scholar] [CrossRef][Green Version]
- Mills, P.; Snabre, P. Settling of a suspension of hard spheres. Europhys. Lett. 1994, 25, 651–656. [Google Scholar] [CrossRef]
- Valverde, J.M.; Quintanilla, M.A.S.; Castellanos, A.; Mills, P. The settling of fine cohesive powders. Europhys. Lett. 2001, 54, 329–334. [Google Scholar] [CrossRef]
- Mostafaei, A.; Zolriasatein, A. Synthesis and characterization of conducting polyaniline nanocomposites containing ZnO nanorods. Prog. Nat. Sci. Mater. Int. 2012, 22, 273–280. [Google Scholar] [CrossRef]
- Ghule, K.; Ghule, A.V.; Chen, B.J.; Ling, Y.C. Preparation and characterization of ZnO nanoparticles coated paper and its antibacterial activity study. Green Chem. 2006, 8, 1034–1041. [Google Scholar] [CrossRef]
- Ahmadi, Z. Nanostructured epoxy adhesives: A review. Prog. Org. Coat. 2019, 135, 449–453. [Google Scholar] [CrossRef]

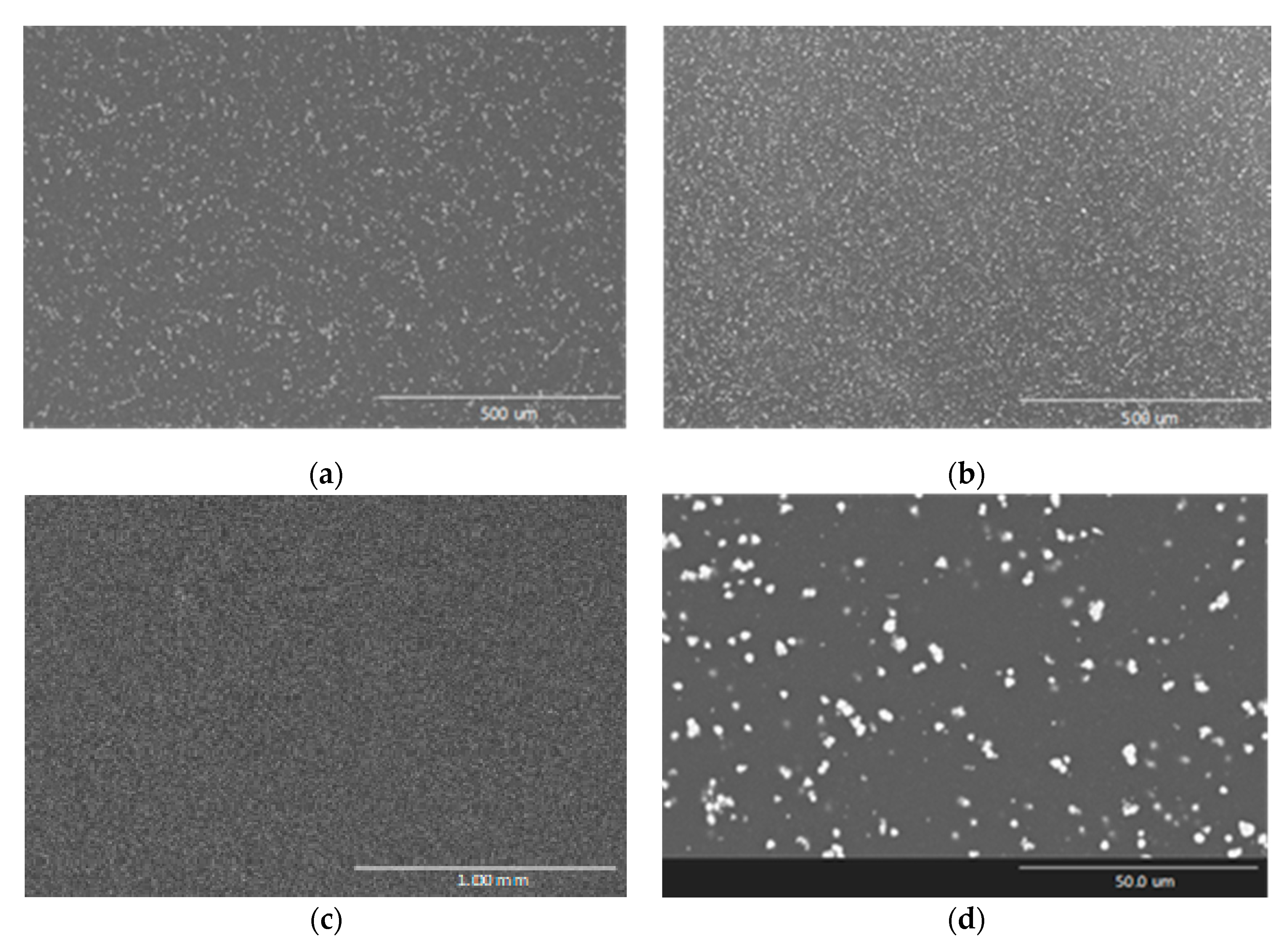
| MgO | Al2O3 | SiO2 | P2O5 | Cl | K2O | CaO | TiO2 | MnO | Fe2O3 | NiO | CuO | ZnO |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.4 | 0.09 | 0.16 | 1.48 | 0.03 | 4.20 | 0.34 | 0.03 | 0.02 | 0.10 | 0.11 | 0.17 | 92.73 |
| 0 wt % | 2 wt % | 6 wt % | 10 wt % | 30 wt % | |
|---|---|---|---|---|---|
| Recycled ZnO | 22.0 ± 1.4 | 22.6 ± 3.0 | 22.4 ± 1.0 | 23.2 ± 1.9 | 40.1 ± 6.9 |
| Commercial ZnO | 22.0 ± 1.4 | 22.0 ± 0.8 | 22.0 ± 0.7 | 24.3 ± 2.8 | 37.3 ± 8.7 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lorero, I.; Campo, M.; Del Rosario, G.; López, F.A.; Prolongo, S.G. New Manufacturing Process of Composites Reinforced with ZnO Nanoparticles Recycled from Alkaline Batteries. Polymers 2020, 12, 1619. https://doi.org/10.3390/polym12071619
Lorero I, Campo M, Del Rosario G, López FA, Prolongo SG. New Manufacturing Process of Composites Reinforced with ZnO Nanoparticles Recycled from Alkaline Batteries. Polymers. 2020; 12(7):1619. https://doi.org/10.3390/polym12071619
Chicago/Turabian StyleLorero, Isaac, Mónica Campo, Gilberto Del Rosario, Félix Antonio López, and Silvia González Prolongo. 2020. "New Manufacturing Process of Composites Reinforced with ZnO Nanoparticles Recycled from Alkaline Batteries" Polymers 12, no. 7: 1619. https://doi.org/10.3390/polym12071619
APA StyleLorero, I., Campo, M., Del Rosario, G., López, F. A., & Prolongo, S. G. (2020). New Manufacturing Process of Composites Reinforced with ZnO Nanoparticles Recycled from Alkaline Batteries. Polymers, 12(7), 1619. https://doi.org/10.3390/polym12071619