Rheological and Structural Study of Salmon Gelatin with Controlled Molecular Weight
Abstract
1. Introduction
2. Materials and Methods
2.1. Salmon Gelatin Samples
2.2. Proximate Composition
2.3. Molecular Weight by Capillary Viscometry and Electrophoresis
2.4. Amino Acidic Profile by HPLC
2.5. Isoelectric Point by Zeta Potential
2.6. Molecular Configuration by Raman Spectroscopy
2.7. Thermal Properties by DSC
2.8. Viscoelastic Behavior by Rheology
2.8.1. Steady-Shear Flow Measurements
2.8.2. Temperature Sweep Test
2.9. Statistical Analysis
3. Results and Discussions
3.1. Proximate Composition
3.2. Molecular Weight
3.3. Amino Acidic Profile
3.4. Isoelectric Point
3.5. Molecular Configuration
3.6. Thermal Properties
3.7. Rheological Behavior
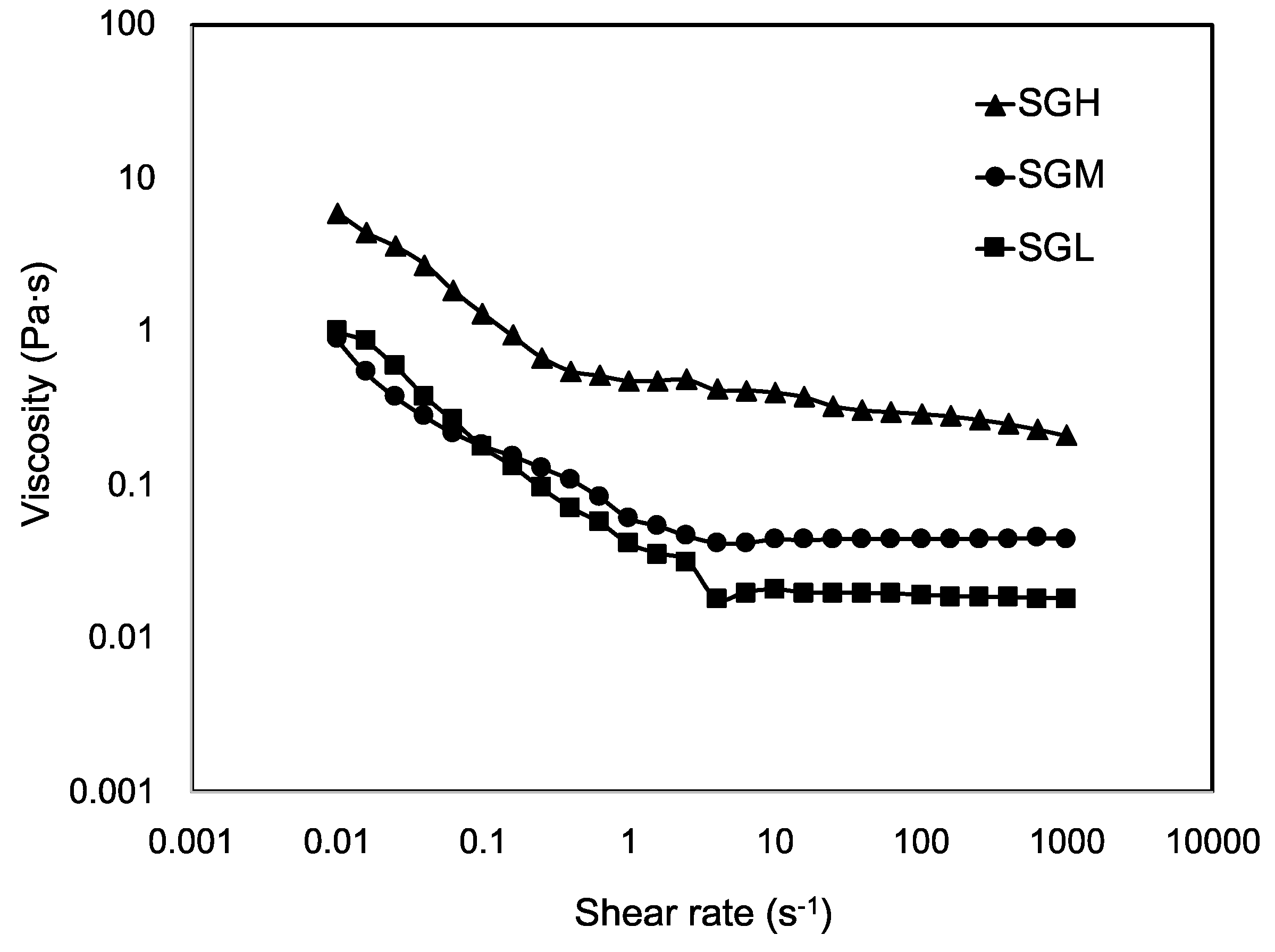
3.7.1. Steady-Shear Flow Measurements
3.7.2. Temperature Sweep Tests
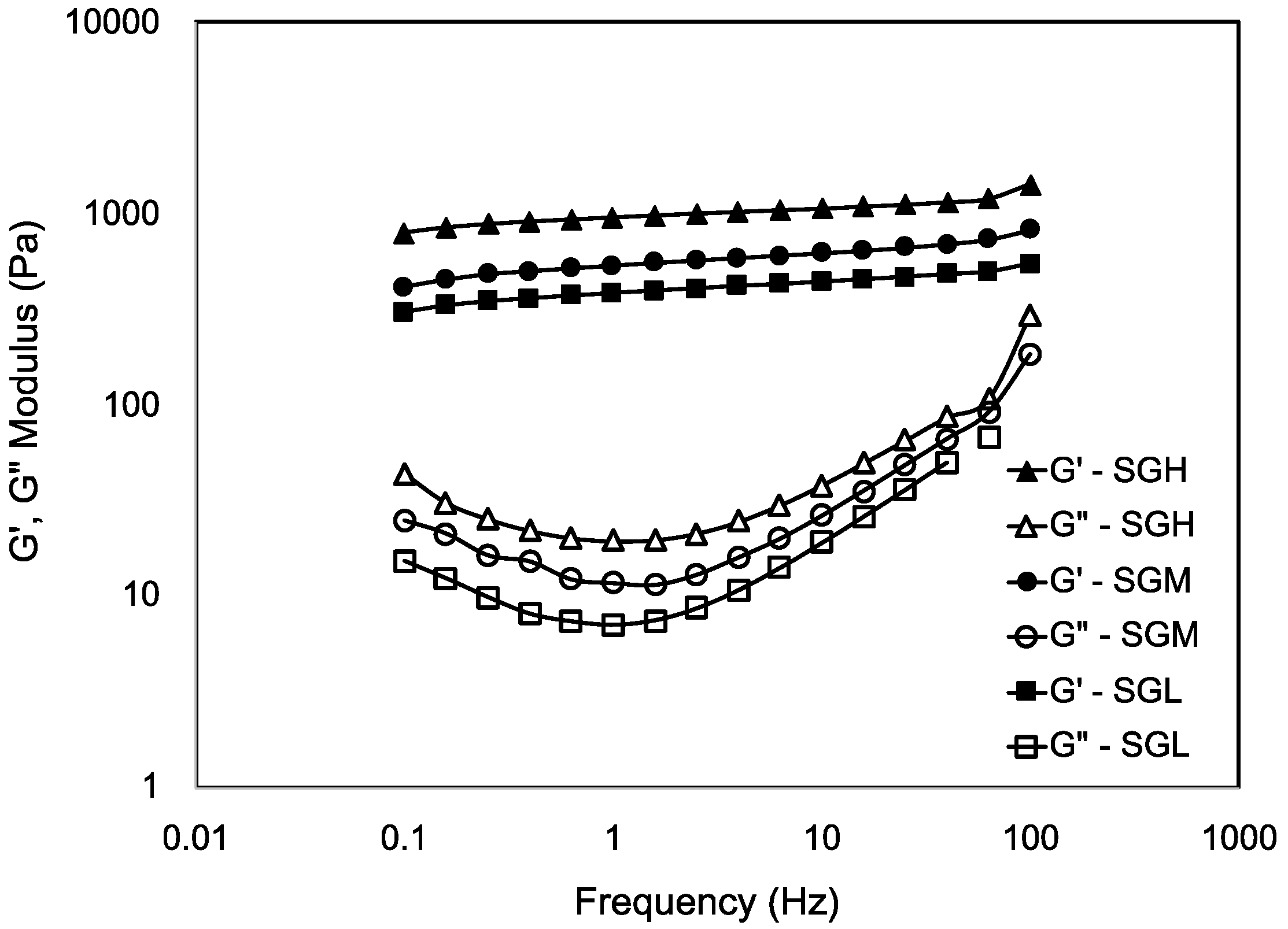
3.7.3. Frequency Sweep Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lin, L.; Regenstein, J.M.; Lv, S.; Lu, J.; Jiang, S. An overview of gelatin derived from aquatic animals: Properties and modification. Trends Food Sci. Technol. 2017, 68, 102–112. [Google Scholar] [CrossRef]
- Sinthusamran, S.; Benjakul, S.; Kishimura, H. Molecular characteristics and properties of gelatin from skin of seabass with different sizes. Int. J. Biol. Macromol. 2015, 73, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Weng, W.; Zheng, H.; Su, W. Characterization of edible films based on tilapia (Tilapia zillii) scale gelatin with different extraction pH. Food Hydrocoll. 2014, 41, 19–26. [Google Scholar] [CrossRef]
- Char, C.; Padilla, C.; Campos, V.; Pepczynska, M.; Paulo, D.; Enrione, J. Characterization and testing of a novel sprayable crosslinked edible coating based on salmon gelatin. Coatings 2019, 9, 595. [Google Scholar] [CrossRef]
- Díaz-Calderón, P.; Flores, E.; González-Muñoz, A.; Pepczynska, M.; Quero, F.; Enrione, J. Influence of extraction variables on the structure and physical properties of salmon gelatin. Food Hydrocoll. 2017, 71, 118–128. [Google Scholar] [CrossRef]
- Gullapalli, R.P.; Mazzitelli, C.L. Gelatin and non-gelatin capsule dosage forms. J. Pharm. Sci. 2017, 106, 1453–1465. [Google Scholar] [CrossRef]
- Pal, A.; Bajpai, J.; Bajpai, A.K. Poly (acrylic acid) grafted gelatin nanocarriers as swelling controlled drug delivery system for optimized release of paclitaxel from modified gelatin. J. Drug Deliv. Sci. Technol. 2018, 45, 323–333. [Google Scholar] [CrossRef]
- Enrione, J.; Pino, K.; Pepczynska, M.; Brown, D.I.; Ortiz, R.; Sánchez, E.; Acevedo, C.A. A novel biomaterial based on salmon-gelatin and its in vivo evaluation as sterile wound-dressing. Mater. Lett. 2018, 212, 159–164. [Google Scholar] [CrossRef]
- Enrione, J.; Blaker, J.J.; Brown, D.I.; Weinstein-Oppenheimer, C.R.; Pepczynska, M.; Olguín, Y.; Sánchez, E.; Acevedo, C.A. Edible scaffolds based on non-mammalian biopolymers for myoblast growth. Materials 2017, 10, 1404. [Google Scholar] [CrossRef]
- Zaupa, A.; Byres, N.; Dal, C.; Acevedo, C.A.; Angelopoulos, I.; Terraza, C.; Nestle, N.; Abarzúa-illanes, P.N.; Quero, F.; Díaz-Calderón, P.; et al. Cold-adaptation of a methacrylamide gelatin towards the expansion of the biomaterial toolbox for specialized functionalities in tissue engineering. Mater. Sci. Eng. C 2019, 102, 373–390. [Google Scholar] [CrossRef]
- Chiou, B.S.; Avena-Bustillos, R.J.; Bechtel, P.J.; Imam, S.H.; Glenn, G.M.; Orts, W.J. Effects of drying temperature on barrier and mechanical properties of cold-water fish gelatin films. J. Food Eng. 2009, 95, 327–331. [Google Scholar] [CrossRef]
- Quero, F.; Padilla, C.; Campos, V.; Luengo, J.; Caballero, L.; Melo, F.; Li, Q.; Eichhorn, S.J.; Enrione, J. Stress transfer and matrix-cohesive fracture mechanism in microfibrillated cellulose-gelatin nanocomposite films. Carbohydr. Polym. 2018, 195, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Joly-Duhamel, C.; Hellio, D.; Djabourov, M. All gelatin networks: 1. Biodiversity and physical chemistry. Langmuir 2002, 18, 7208–7217. [Google Scholar] [CrossRef]
- Díaz, P.; López, D.; Matiacevich, S.; Osorio, F.; Enrione, J. State diagram of salmon (Salmo salar) gelatin films. J. Sci. Food Agric. 2011, 91, 2558–2565. [Google Scholar] [CrossRef] [PubMed]
- Kwak, H.W.; Shin, M.; Lee, J.Y.; Yun, H.; Song, D.W.; Yang, Y.; Shin, B.S.; Park, Y.H.; Lee, K.H. Fabrication of an ultrafine fish gelatin nanofibrous web from an aqueous solution by electrospinning. Int. J. Biol. Macromol. 2017, 102, 1092–1103. [Google Scholar] [CrossRef] [PubMed]
- Acevedo, C.A.; Orellana, N.; Avarias, K.; Ortiz, R.; Benavente, D.; Prieto, P. Micropatterning technology to design an edible film for in vitro meat production. Food Bioprocess. Technol. 2018, 11, 1267–1273. [Google Scholar] [CrossRef]
- Orellana, N.; Elizabeth, S.; Benavente, D.; Prieto, P.; Enrione, J.; Acevedo, C.A. A new edible film to produce in vitro meat. Foods 2020, 9, 185. [Google Scholar] [CrossRef]
- Abdelhedi, O.; Jridi, M.; Nasri, R.; Mora, L.; Toldrá, F.; Nasri, M. Rheological and structural properties of Hemiramphus far skin gelatin: Potential use as an active fish coating agent. Food Hydrocoll. 2019, 87, 331–341. [Google Scholar] [CrossRef]
- Billiet, T.; Gevaert, E.; De Schryver, T.; Cornelissen, M.; Dubruel, P. The 3D printing of gelatin methacrylamide cell-laden tissue-engineered constructs with high cell viability. Biomaterials 2014, 35, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Otoni, C.G.; Avena-Bustillos, R.J.; Chiou, B.S.; Bilbao-Sainz, C.; Bechtel, P.J.; McHugh, T.H. Ultraviolet-B radiation induced cross-linking improves physical properties of cold- and warm-water fish gelatin gels and films. J. Food Sci. 2012, 77, E215–E223. [Google Scholar] [CrossRef]
- Hou, P.Z.; Regenstein, J.M. Optimization of extraction conditions for pollock skin gelatin. J. Food Sci. 2006, 69, C393–C398. [Google Scholar] [CrossRef]
- Association of the Official Analytical Chemists (AOAC). Official Methods of Analysis of AOAC International, 20th ed.; AOAC International: Gaithersbg, MD, USA, 2016. [Google Scholar]
- Harding, S.E. The intrinsic viscosity of biological macromolecules. Progress in measurement, interpretation and application to structure in dilute solution. Prog. Biophys. Mol. Biol. 1997, 68, 207–262. [Google Scholar] [CrossRef]
- Enrione, J.I.; Sáez, C.; López, D.; Skurtys, O.; Acevedo, C.; Osorio, F.; MacNaughtan, W.; Hill, S. Structural relaxation of salmon gelatin films in the Glassy State. Food Bioprocess. Technol. 2012, 5, 2446–2453. [Google Scholar] [CrossRef]
- Rebane, R.; Herodes, K. A sensitive method for free amino acids analysis by liquid chromatography with ultraviolet and mass spectrometric detection using precolumn derivatization with diethyl ethoxymethylenemalonate: Application to the honey analysis. Anal. Chim. Acta 2010, 672, 79–84. [Google Scholar] [CrossRef]
- Neves, A.C.; Harnedy, P.A.; O’Keeffe, M.B.; Alashi, M.A.; Aluko, R.E.; FitzGerald, R.J. Peptide identification in a salmon gelatin hydrolysate with antihypertensive, dipeptidyl peptidase IV inhibitory and antioxidant activities. Food Res. Int. 2017, 100, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Jamilah, B.; Harvinder, K.G. Properties of gelatins from skins of fish-black tilapia (Oreochromis mossambicus) and red tilapia (Oreochromis nilotica). Food Chem. 2002, 77, 81–84. [Google Scholar] [CrossRef]
- Niu, L.; Zhou, X.; Yuan, C.; Bai, Y.; Lai, K.; Yang, F.; Huang, Y. Characterization of tilapia (Oreochromis niloticus) skin gelatin extracted with alkaline and different acid pretreatments. Food Hydrocoll. 2013, 33, 336–341. [Google Scholar] [CrossRef]
- Voet, D.; Voet, J. Biochemistry, 4th ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2011; ISBN 978-0-470-91410-6. [Google Scholar]
- Sankar, S.; Sekar, S.; Mohan, R.; Rani, S.; Sundaraseelan, J.; Sastry, T.P. Preparation and partial characterization of collagen sheet from fish (Lates calcarifer) scales. Int. J. Biol. Macromol. 2008, 42, 6–9. [Google Scholar] [CrossRef]
- Eysturskard, J.; Haug, I.J.; Elharfaoui, N.; Djabourov, M.; Draget, K.I. Structural and mechanical properties of fish gelatin as a function of extraction conditions. Food Hydrocoll. 2009, 23, 1702–1711. [Google Scholar] [CrossRef]
- Harnedy, P.A.; Parthsarathy, V.; McLaughlin, C.M.; O’Keeffe, M.B.; Allsopp, P.J.; McSorley, E.M.; O’Harte, F.P.M.; FitzGerald, R.J. Atlantic salmon (Salmo salar) co-product-derived protein hydrolysates: A source of antidiabetic peptides. Food Res. Int. 2018, 106, 598–606. [Google Scholar] [CrossRef]
- Liu, D.; Nikoo, M.; Boran, G.; Zhou, P.; Regenstein, J.M. Collagen and gelatin. Annu. Rev. Food Sci. Technol. 2015, 6, 527–557. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.P.; Esquerdo, V.M.; Moura, C.M.; Pinto, L.A.A. Crosslinking agents effect on gelatins from carp and tilapia skins and in their biopolymeric films. Colloids Surf. A Physicochem. Eng. Asp. 2018, 539, 184–191. [Google Scholar] [CrossRef]
- Karim, A.A.; Bhat, R. Fish gelatin: Properties, challenges, and prospects as an alternative to mammalian gelatins. Food Hydrocoll. 2009, 23, 563–576. [Google Scholar] [CrossRef]
- Binsi, P.K.; Shamasundar, B.A.; Dileep, A.O.; Badii, F.; Howell, N.K. Rheological and functional properties of gelatin from the skin of Bigeye snapper (Priacanthus hamrur) fish: Influence of gelatin on the gel-forming ability of fish mince. Food Hydrocoll. 2009, 23, 132–145. [Google Scholar] [CrossRef]
- Zhu, G.; Zhu, X.; Fan, Q.; Wan, X. Raman spectra of amino acids and their aqueous solutions. Spectrochim. Acta-Part A Mol. Biomol. Spectrosc. 2011, 78, 1187–1195. [Google Scholar] [CrossRef]
- Cebi, N.; Durak, M.Z.; Toker, O.S.; Sagdic, O.; Arici, M. An evaluation of Fourier transforms infrared spectroscopy method for the classification and discrimination of bovine, porcine and fish gelatins. Food Chem. 2016, 190, 1109–1115. [Google Scholar] [CrossRef]
- Duconseille, A.; Gaillard, C.; Santé-Lhoutellier, V.; Astruc, T. Molecular and structural changes in gelatin evidenced by Raman microspectroscopy. Food Hydrocoll. 2018, 77, 777–786. [Google Scholar] [CrossRef]
- Celedón, A.; Aguilera, J.M. Applications of microprobe Raman spectroscopy in food science. Food Sci. Technol. Int. 2002, 8, 101–108. [Google Scholar] [CrossRef]
- Ellepola, S.W.; Choi, S.M.; Phillips, D.L.; Ma, C.Y. Raman spectroscopic study of rice globulin. J. Cereal Sci. 2006, 43, 85–93. [Google Scholar] [CrossRef]
- Nakabayashi, T.; Kosugi, K.; Nishi, N. Liquid structure of acetic acid studied by Raman spectroscopy and Ab initio molecular orbital calculations. J. Phys. Chem. A 1999, 103, 8595–8603. [Google Scholar] [CrossRef]
- Elharfaoui, N.; Djabourov, M.; Babel, W. Molecular weight influence on gelatin gels: Structure, enthalpy and rheology. Macromol. Symp. 2007, 256, 149–157. [Google Scholar] [CrossRef]
- Badii, F.; MacNaughtan, W.; Mitchell, J.R.; Farhat, I. The effect of drying temperature on physical properties of thin gelatin films. Dry. Technol. 2014, 32, 30–38. [Google Scholar] [CrossRef]
- Chandra, M.V.; Shamasundar, B.A. Texture profile analysis and functional properties of gelatin from the skin of three species of fresh water fish. Int. J. Food Prop. 2015, 18, 572–584. [Google Scholar] [CrossRef]
- Casanovas, A.; Hernández, M.J.; Martí-Bonmatí, E.; Dolz, M. Cluster classification of dysphagia-oriented products considering flow, thixotropy and oscillatory testing. Food Hydrocoll. 2011, 25, 851–859. [Google Scholar] [CrossRef]
- Mezger, T.G. Applied Rheology, 6th ed.; Anton Paar GmbH: Graz, Austria, 2019; ISBN 978-3-9504016-0-8. [Google Scholar]
- Huang, T.; Tu, Z.; Wang, H.; Zhang, L. Rheological and structural properties of fish scales gelatin: Effects of conventional and ultrasound- assisted extraction. Int. J. Food Prop. 2017, 20, 1210–1220. [Google Scholar] [CrossRef]
- Gornall, J.L.; Terentjev, E.M. Helix–coil transition of gelatin: Helical morphology and stability. Soft Matter 2008, 4, 544–549. [Google Scholar] [CrossRef]
- Guo, L.; Colby, R.H.; Lusignan, C.P.; Whitesides, T.H. Kinetics of triple helix formation in semidilute gelatin solutions. Macromolecules 2003, 36, 9999–10008. [Google Scholar] [CrossRef]
- Haug, I.J.; Draget, K.I.; Smidsrød, O. Physical and rheological properties of fish gelatin compared to mammalian gelatin. Food Hydrocoll. 2004, 18, 203–213. [Google Scholar] [CrossRef]
- Sperling, L.H. Dilute solution thermodynamics, molecular weights, and sizes. In Introduction to Physical Polymer Science; Sperling, L., Ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2005. [Google Scholar] [CrossRef]
- Boran, G.; Mulvaney, S.J.; Regenstein, J.M. Rheological properties of gelatin from silver carp skin compared to commercially available gelatins from different sources. J. Food Sci. 2010, 75, E565–E571. [Google Scholar] [CrossRef]
- Ikeda, S.; Nishinari, K. “Weak Gel” -type rheological properties of aqueous dispersions of nonaggregated K-carrageenan helices. J. Agric. Food Chem. 2001, 49, 4436–4441. [Google Scholar] [CrossRef]
- Sarbon, N.M.; Badii, F.; Howell, N.K. Preparation and characterisation of chicken skin gelatin as an alternative to mammalian gelatin. Food Hydrocoll. 2013, 30, 143–151. [Google Scholar] [CrossRef]
- Chandra, M.V.; Shamasundar, B.A. Rheological properties of gelatin prepared from the swim bladders of freshwater fish Catla catla. Food Hydrocoll. 2015, 48, 47–54. [Google Scholar] [CrossRef]
- Clark, A.H.; Ross-Murphy, S.B. Structural and mechanical properties of biopolymer gels. Adv. Polym. Sci. 1987, 83, 57–192. [Google Scholar] [CrossRef]
- Arnesen, J.A.; Gildberg, A. Extraction and characterisation of gelatine from Atlantic salmon (Salmo salar) skin. Bioresour. Technol. 2007, 98, 53–57. [Google Scholar] [CrossRef]
- Gudmundsson, M. Rheological properties of fish gelatins. J. Food Sci. 2002, 67, 2171–2176. [Google Scholar] [CrossRef]
- Eysturskard, J.; Haug, I.J.; Ulset, A.S.; Draget, K.I. Mechanical properties of mammalian and fish gelatins based on their weight average molecular weight and molecular weight distribution. Food Hydrocoll. 2009, 23, 2315–2321. [Google Scholar] [CrossRef]




| Component | SGL | SGM | SGH |
|---|---|---|---|
| Moisture (%, wb) 1 | 6.6 (0.0) a | 4.0 (1.5) b | 3.9 (0.2) c |
| Protein (%, db) 2 | 86.9 (4.2) a | 93.9 (0.4) b | 99.4 (0.07) c |
| Non-nitrogenous Extract (%, db) 3 | 12.3 (4.1) a | 5.5 (0.4) b | 0.0 (0.0) c |
| Fat (%, db) | ND 4 | ND | ND |
| Ash (%, db) | 0.8 (0.08) a | 0.6 (0.01) b | 0.6 (0.07) b |
| Sample | MW (kDa) | pI | Tgelling (°C, by DSC) | ΔH (J g−1dry sample) | Viscosity (Pa·s) | Tgelling (°C, by Rheology) | |
|---|---|---|---|---|---|---|---|
| 4 °C | 25 °C | ||||||
| SGL | 64.6 (14) a | 9.7 (0.5) a | 3.3 (0.5) a | −1.8 (0.7) a | 1.6 (1.2) a | 0.02 (0.003) a | 2.8 (0.01) a |
| SGM | 94.5 (2.5) b | 9.6 (0.7) a | 6.9 (0.8) b | −4.0 (0.7) b | 9.7 (0.1) b | 0.05 (0.001) b | 7.0 (0.41) b |
| SGH | 172.7 (26) c | 9.8 (0.1) a | 10.3 (0.6) c | −5.8 (2.1) b | 36.2 (4.5) c | 0.28 (0.013) c | 10.3 (0.73) c |
| g/100 gprotein | |||
|---|---|---|---|
| Amino Acid | SGL | SGM | SGH |
| alanine | 6.04 (0.6) a | 6.27 (0.1) a | 6.02 (1.4) a |
| arginine | 5.36 (0.7) a | 6.13 (0.4) b | 7.06 (0.2) c |
| aspartic acid | 4.35 (0.3) a | 5.08 (0.2) b | 3.98 (0.3) a |
| glutamic acid | 8.13 (1.1) a | 8.15 (0.0) a | 9.61 (0.0) b |
| glycine | 18.88 (3.2) a | 18.54 (0.3) a | 25.23 (2.1) b |
| hydroxiproline | 5.87 (0.8) a | 5.94 (0.1) a | 8.06 (1.1) b |
| hysitidine | 0.33 (0.1) a | 0.59 (0.1) a | 0.26 (0.4) a |
| isoleucine | 0.65 (0.0) a | 0.73 (0.0) b | 0.52 (0.3) a,b |
| leucine | 1.33 (0.0) a | 1.47 (0.0) b | 1.03 (0.6) a,b |
| lysine | 2.94 (0.4) a | 3.01 (0.0) a | 2.87 (0.7) a |
| methionine | 1.25 (0.1) a | 1.38 (0.0) a | 1.24 (0.5) a |
| phenylalanine | 1.18 (0.0) a | 1.28 (0.0) b | 1.31 (0.0) b |
| proline | 6.76 (1.2) a | 6.93 (0.2) a | 10.57 (1.4) b |
| serine | 3.05 (0.4) a | 3.14 (0.0) a | 4.00 (0.3) a |
| treonine | 1.33 (0.1) a | 1.44 (0.0) a | 1.52 (0.1) a |
| valine | 0.61 (0.1) a | 0.94 (0.2) a | 0.47 (0.7) a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Enrione, J.; Char, C.; Pepczynska, M.; Padilla, C.; González-Muñoz, A.; Olguín, Y.; Quinzio, C.; Iturriaga, L.; Díaz-Calderón, P. Rheological and Structural Study of Salmon Gelatin with Controlled Molecular Weight. Polymers 2020, 12, 1587. https://doi.org/10.3390/polym12071587
Enrione J, Char C, Pepczynska M, Padilla C, González-Muñoz A, Olguín Y, Quinzio C, Iturriaga L, Díaz-Calderón P. Rheological and Structural Study of Salmon Gelatin with Controlled Molecular Weight. Polymers. 2020; 12(7):1587. https://doi.org/10.3390/polym12071587
Chicago/Turabian StyleEnrione, Javier, Cielo Char, Marzena Pepczynska, Cristina Padilla, Adrian González-Muñoz, Yusser Olguín, Claudia Quinzio, Laura Iturriaga, and Paulo Díaz-Calderón. 2020. "Rheological and Structural Study of Salmon Gelatin with Controlled Molecular Weight" Polymers 12, no. 7: 1587. https://doi.org/10.3390/polym12071587
APA StyleEnrione, J., Char, C., Pepczynska, M., Padilla, C., González-Muñoz, A., Olguín, Y., Quinzio, C., Iturriaga, L., & Díaz-Calderón, P. (2020). Rheological and Structural Study of Salmon Gelatin with Controlled Molecular Weight. Polymers, 12(7), 1587. https://doi.org/10.3390/polym12071587





