A Preliminary Evaluation of the Pro-Chondrogenic Potential of 3D-Bioprinted Poly(ester Urea) Scaffolds
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
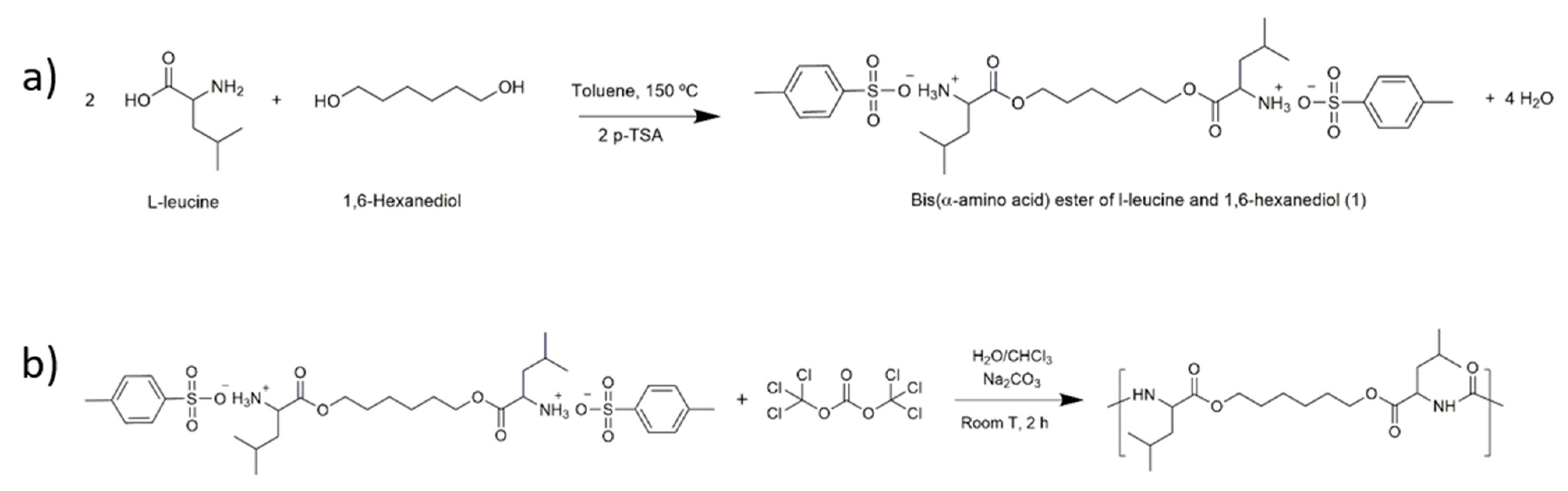
2.2. Synthesis of Poly(Ester Urea) (PEU)
2.3. Scaffold Design and Fabrication
2.4. Preparation of Films from the Scaffolds
2.5. Characterisation of PEU and Scaffolds
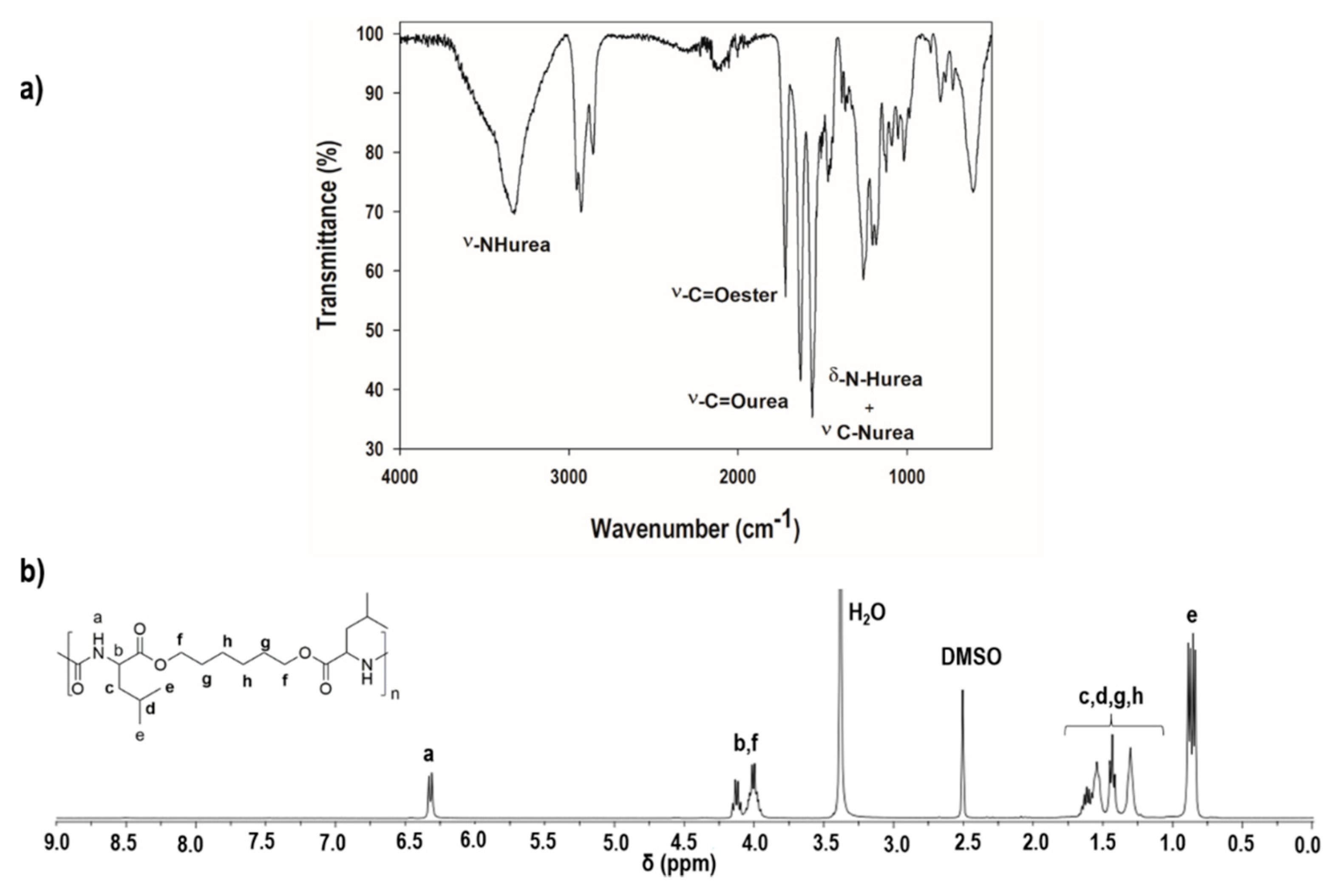
2.5.1. Identification of the Chemical Structure of the Poly(ester Urea) (PEU)
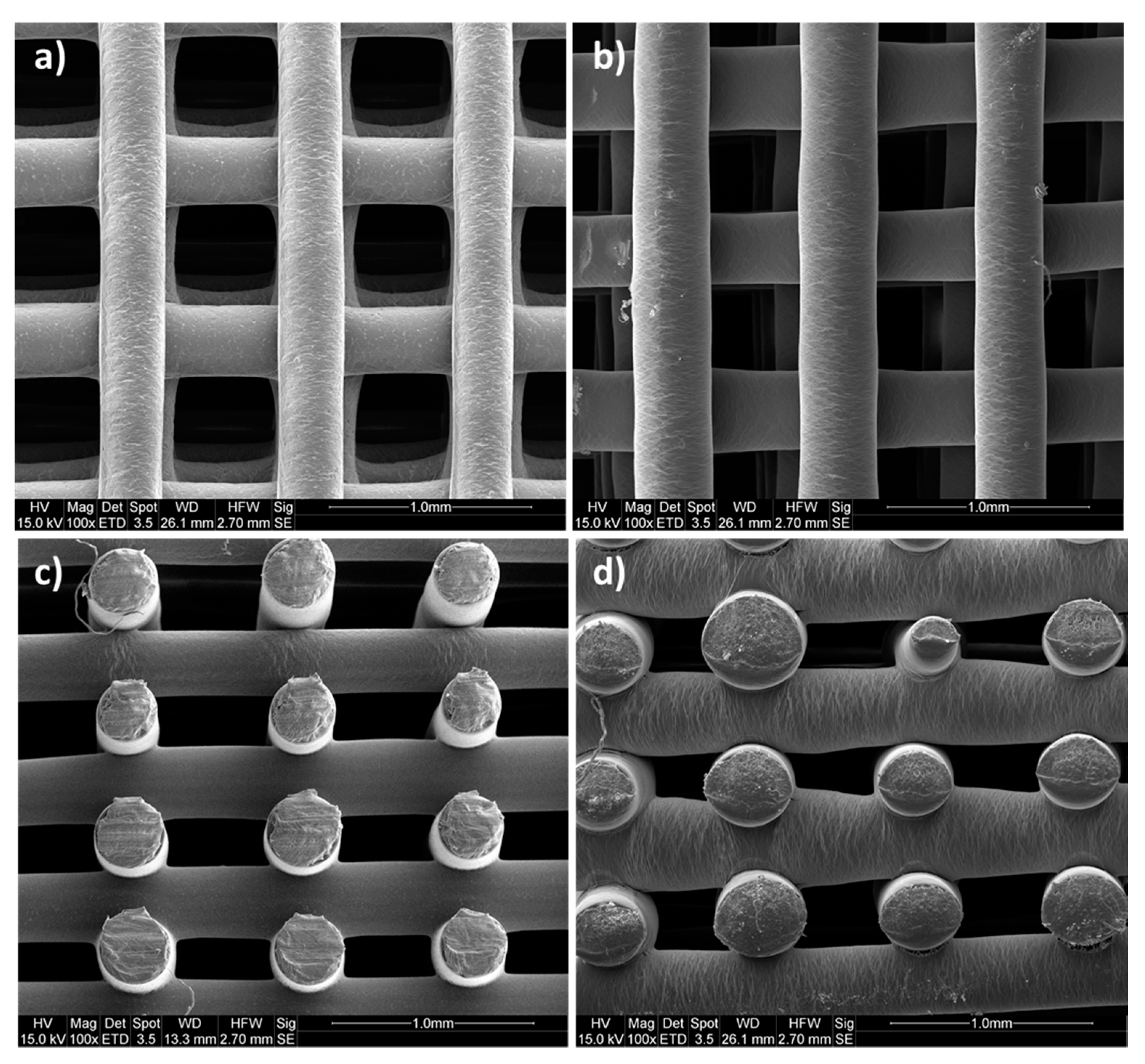
2.5.2. Morphological Analysis
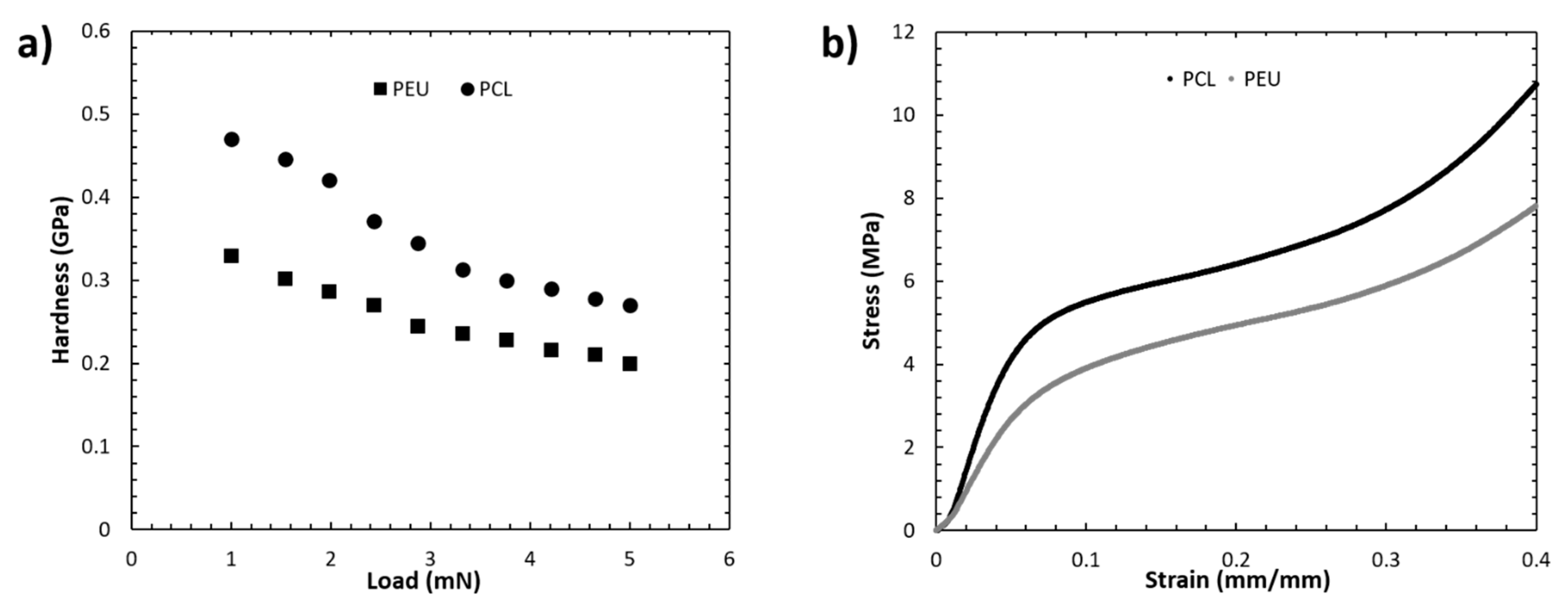
2.5.3. Mechanical Analysis
Nanoindentation Tests
Compression Tests
2.5.4. Water Contact Angle Measurements
2.5.5. Cell Seeding
2.5.6. Confocal Microscopy Imaging
2.5.7. Cell Viability
2.5.8. RT-PCR
2.6. Statistical Analysis
3. Results and Discussion
3.1. Characterisation of the Chemical Structure of the l-Leucine-Based PEU
3.2. Morphological Analysis
3.3. Mechanical Analysis
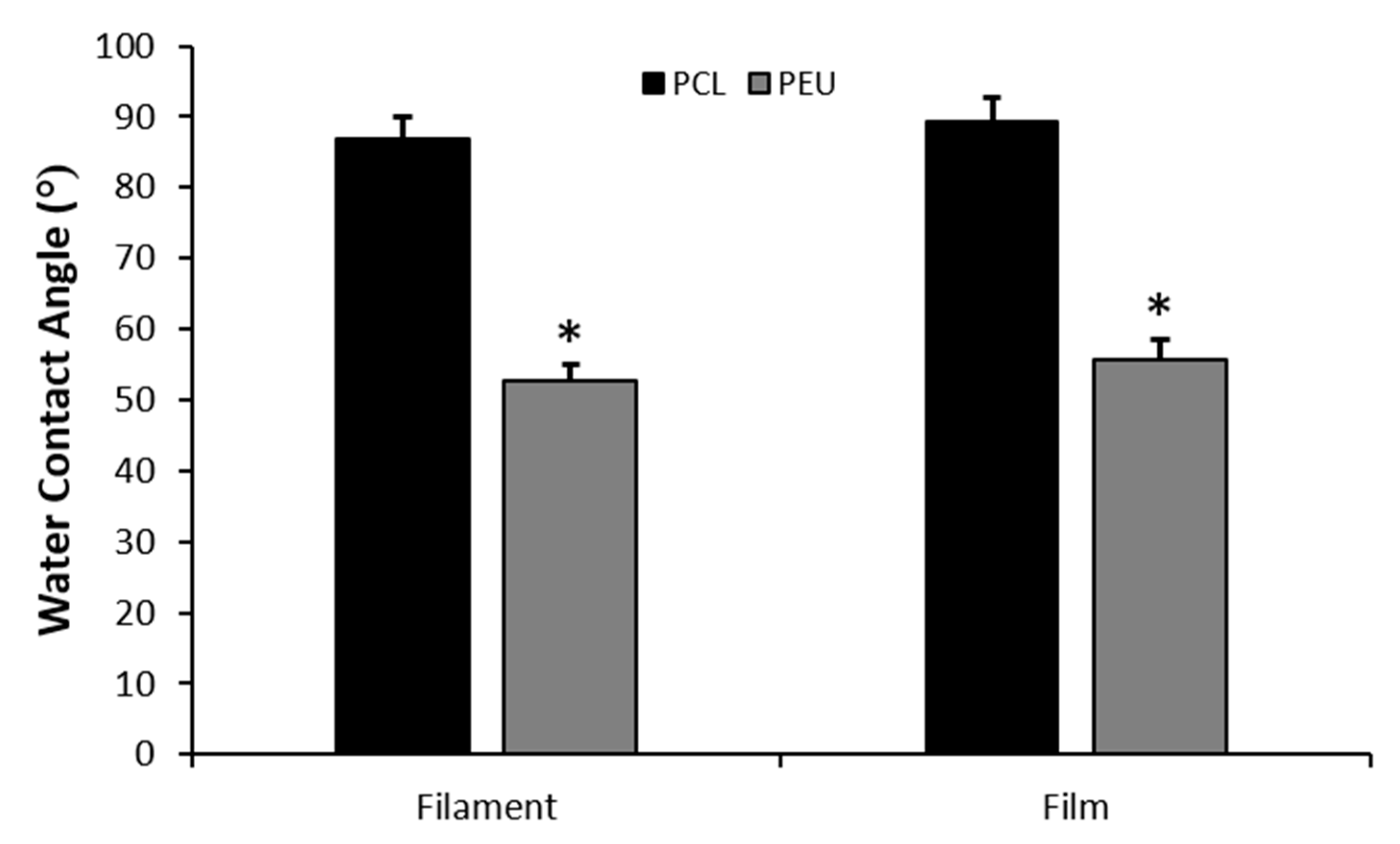
3.4. Water Contact Angle Measurements
3.5. Biological Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Csaki, C.; Schneider, P.R.A.; Shakibaei, M. Mesenchymal Stem Cells as a Potential Pool for Cartilage Tissue Engineering. Ann. Anat. 2008, 190, 395–412. [Google Scholar] [CrossRef] [PubMed]
- Temenoff, J.S.; Mikos, A.G. Review: Tissue Engineering for Regeneration of Articular Cartilage. Biomaterials 2000, 21, 431–440. [Google Scholar] [CrossRef]
- Risbud, M.V.; Sittinger, M. Tissue Engineering: Advances in in Vitro Cartilage Generation. Trends Biotechnol. 2002, 20, 351–356. [Google Scholar] [CrossRef]
- Klein, T.J.; Malda, J.; Sah, R.L.; Hutmacher, D.W. Tissue Engineering of Articular Cartilage with Biomimetic Zones. Tissue Eng. Part B Rev. 2009, 15, 143–157. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, S.M.; Ong, K.L.; Lau, E.; Bozic, K.J. Impact of the Economic Downturn on Total Joint Replacement Demand in the United States. J. Bone Jt. Surgery-Am. Vol. 2014, 96, 624–630. [Google Scholar] [CrossRef] [PubMed]
- Hutmacher, D.W. Scaffolds in Tissue Engineering Bone and Cartilage. Biomater. Silver Jubil. Compend. 2000, 175–189. [Google Scholar] [CrossRef]
- Domingos, M.; Intranuovo, F.; Russo, T.; Santis, R.D.; Gloria, A.; Ambrosio, L.; Ciurana, J.; Bartolo, P. The First Systematic Analysis of 3D Rapid Prototyped Poly(ε-Caprolactone) Scaffolds Manufactured through BioCell Printing: The Effect of Pore Size and Geometry on Compressive Mechanical Behaviour and in Vitro hMSC Viability. Biofabrication 2013, 5, 045004. [Google Scholar] [CrossRef]
- Domingos, M.; Intranuovo, F.; Gloria, A.; Gristina, R.; Ambrosio, L.; Bártolo, P.J.; Favia, P. Improved Osteoblast Cell Affinity on Plasma-Modified 3-D Extruded PCL Scaffolds. Acta Biomater. 2013, 9, 5997–6005. [Google Scholar] [CrossRef]
- Hollister, S.J. Porous Scaffold Design for Tissue Engineering. Nat. Mater. 2005, 4, 518. [Google Scholar] [CrossRef]
- Derby, B. Printing and Prototyping of Tissues and Scaffolds. Science 2012, 338, 921–926. [Google Scholar] [CrossRef]
- Melchels, F.P.W.; Domingos, M.A.N.; Klein, T.J.; Malda, J.; Bartolo, P.J.; Hutmacher, D.W. Additive Manufacturing of Tissues and Organs. Prog. Polym. Sci. 2012, 37, 1079–1104. [Google Scholar] [CrossRef]
- Murphy, S.V.; Atala, A. 3D Bioprinting of Tissues and Organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef] [PubMed]
- Schek, R.M.; Taboas, J.M.; Segvich, S.J.; Hollister, S.J.; Krebsbach, P.H. Solid Free-Form Fabricated Scaffolds. Tissue Eng. 2004, 10, 1376–1385. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Breitenkamp, K.; Finn, M.G.; Lotz, M.; D’Lima, D.D. Direct Human Cartilage Repair Using Three-Dimensional Bioprinting Technology. Tissue Eng. Part A 2012, 18, 1304–1312. [Google Scholar] [CrossRef] [PubMed]
- Holmes, B.; Zhu, W.; Li, J.; Lee, J.D.; Zhang, L.G. Development of Novel Three-Dimensional Printed Scaffolds for Osteochondral Regeneration. Tissue Eng. Part A 2015, 21, 403–415. [Google Scholar] [CrossRef] [PubMed]
- Woodfield, T.B.F.; Van Blitterswijk, C.A.; De Wijn, J.; Sims, T.J.; Hollander, A.P.; Riesle, J. Polymer Scaffolds Fabricated with Pore-Size Gradients as a Model for Studying the Zonal Organization within Tissue-Engineered Cartilage Constructs. Tissue Eng. 2005, 11, 1297–1311. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Lembong, J.; Zhang, L.G.; Fisher, J.P. Three-Dimensional Printing Articular Cartilage: Recapitulating the Complexity of Native Tissue. Tissue Eng. Part B Rev. 2017, 23, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Woodruff, M.A.; Hutmacher, D.W. The Return of a Forgotten Polymer - Polycaprolactone in the 21st Century. Prog. Polym. Sci. 2010, 35, 1217–1256. [Google Scholar] [CrossRef]
- Rai, B.; Teoh, S.H.; Hutmacher, D.W.; Cao, T.; Ho, K.H. Novel PCL-Based Honeycomb Scaffolds as Drug Delivery Systems for RhBMP-2. Biomaterials 2005, 26, 3739–3748. [Google Scholar] [CrossRef]
- Caetano, G.; Violante, R.; Sant’Ana, A.B.; Murashima, A.B.; Domingos, M.; Gibson, A.; Bártolo, P.; Frade, M.A. Cellularized versus Decellularized Scaffolds for Bone Regeneration. Mater. Lett. 2016, 182, 318–322. [Google Scholar] [CrossRef]
- Rotbaum, Y.; Puiu, C.; Rittel, D.; Domingos, M. Quasi-Static and Dynamic in Vitro Mechanical Response of 3D Printed Scaffolds with Tailored Pore Size and Architectures. Mater. Sci. Eng. C 2019, 96, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Jeong, C.G.; Hollister, S.J. A Comparison of the Influence of Material on in Vitro Cartilage Tissue Engineering with PCL, PGS, and POC 3D Scaffold Architecture Seeded with Chondrocytes. Biomaterials 2010, 31, 4304–4312. [Google Scholar] [CrossRef] [PubMed]
- Domingos, M.; Gloria, A.; Coelho, J.; Bartolo, P.; Ciurana, J. Three-Dimensional Printed Bone Scaffolds: The Role of Nano/Micro-Hydroxyapatite Particles on the Adhesion and Differentiation of Human Mesenchymal Stem Cells. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2017, 231, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Shor, L.; Güçeri, S.; Chang, R.; Gordon, J.; Kang, Q.; Hartsock, L.; An, Y.; Sun, W. Precision Extruding Deposition (PED) Fabrication of Polycaprolactone (PCL) Scaffolds for Bone Tissue Engineering. Biofabrication 2009, 1, 015003. [Google Scholar] [CrossRef]
- Uppanan, P.; Thavornyutikarn, B.; Kosorn, W.; Kaewkong, P.; Janvikul, W. Enhancement of Chondrocyte Proliferation, Distribution, and Functions within Polycaprolactone Scaffolds by Surface Treatments. J. Biomed. Mater. Res. Part A 2015, 103, 2322–2332. [Google Scholar] [CrossRef]
- Rodriguez-Galan, A.; Franco, L.; Puiggali, J.; Rodriguez-Galan, A.; Franco, L.; Puiggali, J. Degradable Poly(Ester Amide)s for Biomedical Applications. Polymers 2010, 3, 65–99. [Google Scholar] [CrossRef]
- Fonseca, A.C.; Gil, M.H.; Simões, P.N. Biodegradable Poly(Ester Amide)s—A Remarkable Opportunity for the Biomedical Area: Review on the Synthesis, Characterization and Applications. Prog. Polym. Sci. 2014, 39, 1291–1311. [Google Scholar] [CrossRef]
- Huang, S.J.; Bansleben, D.A.; Knox, J.R. Biodegradable Polymers: Chymotrypsin Degradation of a Low Molecular Weight Poly(Ester-Urea) Containing Phenylalanine. J. Appl. Polym. Sci. 1979, 23, 429–437. [Google Scholar] [CrossRef]
- Katsarava, R.; Tugushi, D.; Gomurashvili, Z.D. Poly(Ester Urea) Polymers and Methods of Use. U.S. Patent 8,765,164, 1 July 2014. [Google Scholar]
- Díaz, A.; Del Valle, L.J.; Tugushi, D.; Katsarava, R.; Puiggalí, J. New Poly(Ester Urea) Derived from l-Leucine: Electrospun Scaffolds Loaded with Antibacterial Drugs and Enzymes. Mater. Sci. Eng. C 2015, 46, 450–462. [Google Scholar] [CrossRef]
- Díaz, A.; del Valle, L.; Rodrigo, N.; Casas, M.; Chumburidze, G.; Katsarava, R.; Puiggalí, J. Antimicrobial Activity of Poly(Ester Urea) Electrospun Fibers Loaded with Bacteriophages. Fibers 2018, 6, 33. [Google Scholar] [CrossRef]
- Yu, J.; Xu, Y.; Li, S.; Seifert, G.V.; Becker, M.L. Three-Dimensional Printing of Nano Hydroxyapatite/Poly(Ester Urea) Composite Scaffolds with Enhanced Bioactivity. Biomacromolecules 2017, 18, 4171–4183. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Xu, Y.; Yu, J.; Becker, M.L. Enhanced Osteogenic Activity of Poly(Ester Urea) Scaffolds Using Facile Post-3D Printing Peptide Functionalization Strategies. Biomaterials 2017, 141, 176–187. [Google Scholar] [CrossRef]
- Camarero-Espinosa, S.; Calore, A.; Wilbers, A.; Harings, J.; Moroni, L. Additive Manufacturing of an Elastic Poly(Ester)Urethane for Cartilage Tissue Engineering. Acta Biomater. 2019, 102, 192–204. [Google Scholar] [CrossRef] [PubMed]
- Chkhaidze, E.; Tugushi, D.; Kharadze, D.; Gomurashvili, Z.; Chu, C.-C.; Katsarava, R. New Unsaturated Biodegradable Poly(Ester Amide)s Composed of Fumaric Acid, L-Leucine and α,ω-Alkylene Diols. J. Macromol. Sci. Part A 2011, 48, 544–555. [Google Scholar] [CrossRef]
- Gloria, A.; Causa, F.; Russo, T.; Battista, E.; Della Moglie, R.; Zeppetelli, S.; De Santis, R.; Netti, P.A.; Ambrosio, L. Three-Dimensional Poly(ε-Caprolactone) Bioactive Scaffolds with Controlled Structural and Surface Properties. Biomacromolecules 2012, 13, 3510–3521. [Google Scholar] [CrossRef]
- Wu, X.-F.; Dzenis, Y.A. Droplet on a Fiber: Geometrical Shape and Contact Angle. Acta Mech. 2006, 185, 215–225. [Google Scholar] [CrossRef]
- Chen, H.; Cheng, H.; Jiang, Z.; Qin, D.; Yu, Y.; Tian, G.; Lu, F.; Fei, B.; Wang, G. Contact Angles of Single Bamboo Fibers Measured in Different Environments and Compared with Other Plant Fibers and Bamboo Strips. BioResources 2013, 8, 2827–2838. [Google Scholar] [CrossRef]
- Ebenstein, D.M.; Pruitt, L.A. Nanoindentation of Biological Materials. Nano Today 2006, 1, 26–33. [Google Scholar] [CrossRef]
- Zhou, J.; Komvopoulos, K. Surface and Interface Viscoelastic Behaviors of Thin Polymer Films Investigated by Nanoindentation. J. Appl. Phys. 2006, 100, 114329. [Google Scholar] [CrossRef]
- Kyriakidou, K.; Lucarini, G.; Zizzi, A.; Salvolini, E.; Mattioli Belmonte, M.; Mollica, F.; Gloria, A.; Ambrosio, L. Dynamic Co-Seeding of Osteoblast and Endothelial Cells on 3D Polycaprolactone Scaffolds for Enhanced Bone Tissue Engineering. J. Bioact. Compat. Polym. 2008, 23, 227–243. [Google Scholar] [CrossRef]
- Zhu, B.; Bailey, S.R.; Mauli Agrawal, C. Calcification of Primary Human Osteoblast Cultures under Flow Conditions Using Polycaprolactone Scaffolds for Intravascular Applications. J. Tissue Eng. Regen. Med. 2012, 6, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Mavis, B.; Demirtaş, T.T.; Gümüşderelioğlu, M.; Gündüz, G.; Çolak, Ü. Synthesis, Characterization and Osteoblastic Activity of Polycaprolactone Nanofibers Coated with Biomimetic Calcium Phosphate. Acta Biomater. 2009, 5, 3098–3111. [Google Scholar] [CrossRef] [PubMed]
- Campbell, S.E.; Ferguson, V.L.; Hurley, D.C. Nanomechanical Mapping of the Osteochondral Interface with Contact Resonance Force Microscopy and Nanoindentation. Acta Biomater. 2012, 8, 4389–4396. [Google Scholar] [CrossRef]
- Moxon, S.R.; Cooke, M.E.; Cox, S.C.; Snow, M.; Jeys, L.; Jones, S.W.; Smith, A.M.; Grover, L.M. Suspended Manufacture of Biological Structures. Adv. Mater. 2017, 29, 1605594. [Google Scholar] [CrossRef]
- Cox, T.R.; Erler, J.T. Remodeling and Homeostasis of the Extracellular Matrix: Implications for Fibrotic Diseases and Cancer. Dis. Model. Mech. 2011, 4, 165–178. [Google Scholar] [CrossRef]
- Steward, A.J.; Wagner, D.R.; Kelly, D.J. The Pericellular Environment Regulates Cytoskeletal Development and the Differentiation of Mesenchymal Stem Cells and Determines Their Response to Hydrostatic Pressure. Eur. Cells Mater. 2012, 25, 167–178. [Google Scholar] [CrossRef]
- Taylor, M.; Urquhart, A.J.; Zelzer, M.; Davies, M.C.; Alexander, M.R. Picoliter Water Contact Angle Measurement on Polymers. Langmuir 2007, 23, 6875–6878. [Google Scholar] [CrossRef]
- Park, G.E.; Pattison, M.A.; Park, K.; Webster, T.J. Accelerated Chondrocyte Functions on NaOH-Treated PLGA Scaffolds. Biomaterials 2005, 26, 3075–3082. [Google Scholar] [CrossRef]
- Ma, Z.; Gao, C.; Gong, Y.; Shen, J. Chondrocyte Behaviors on Poly-l-Lactic Acid (PLLA) Membranes Containing Hydroxyl, Amide or Carboxyl Groups. Biomaterials 2003, 24, 3725–3730. [Google Scholar] [CrossRef]
- Cui, Y.L.; Di Qi, A.; Liu, W.G.; Wang, X.H.; Wang, H.; Ma, D.M.; De Yao, K. Biomimetic Surface Modification of Poly(l-Lactic Acid) with Chitosan and Its Effects on Articular Chondrocytes in Vitro. Biomaterials 2003, 24, 3859–3868. [Google Scholar] [CrossRef]
- Zhang, H.; Du, G.; Zhang, J. Assay of Mitochondrial Functions by Resazurin in Vitro. Acta Pharmacol. Sin. 2004, 25, 385–389. [Google Scholar] [PubMed]
- Mhanna, R.; Kashyap, A.; Palazzolo, G.; Vallmajo-Martin, Q.; Becher, J.; Möller, S.; Schnabelrauch, M.; Zenobi-Wong, M. Chondrocyte Culture in Three Dimensional Alginate Sulfate Hydrogels Promotes Proliferation While Maintaining Expression of Chondrogenic Markers. Tissue Eng. Part A 2014, 20, 1454–1464. [Google Scholar] [CrossRef] [PubMed]
- Pustlauk, W.; Paul, B.; Gelinsky, M.; Bernhardt, A. Jellyfish Collagen and Alginate: Combined Marine Materials for Superior Chondrogenesis of HMSC. Mater. Sci. Eng. C 2016, 64, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Nalluri, S.M.; Krishnan, G.R.; Cheah, C.; Arzumand, A.; Yuan, Y.; Richardson, C.A.; Yang, S.; Sarkar, D. Hydrophilic Polyurethane Matrix Promotes Chondrogenesis of Mesenchymal Stem Cells. Mater. Sci. Eng. C 2015, 54, 182–195. [Google Scholar] [CrossRef]
- Cheon, Y.W.; Lee, W.J.; Baek, H.S.; Lee, Y.D.; Park, J.-C.; Park, Y.H.; Ki, C.S.; Chung, K.-H.; Rah, D.K. Enhanced Chondrogenic Responses of Human Articular Chondrocytes Onto Silk Fibroin/Wool Keratose Scaffolds Treated With Microwave-Induced Argon Plasma. Artif. Organs 2010, 34, 384–392. [Google Scholar] [CrossRef]
- Loty, S.; Forest, N.; Boulekbache, H.; Sautier, J.M. Cytochalasin D Induces Changes in Cell Shape and Promotes in Vitro Chondrogenesis: A Morphological Study. Biol. Cell 1995, 83, 149–161. [Google Scholar] [CrossRef]
- Glowacki, J.; Trepman, E.; Folkman, J. Cell Shape and Phenotypic Expression in Chondrocytes. Proc. Soc. Exp. Biol. Med. 1983, 172, 93–98. [Google Scholar] [CrossRef]
- Cheng, A.; Cain, S.A.; Tian, P.; Baldwin, A.K.; Uppanan, P.; Kielty, C.M.; Kimber, S.J. Recombinant Extracellular Matrix Protein Fragments Support Human Embryonic Stem Cell Chondrogenesis. Tissue Eng. Part A 2018, 24, 968. [Google Scholar] [CrossRef] [PubMed]

| Process Parameters | |||||
|---|---|---|---|---|---|
| DV (mm/s) | ST (μm) | LT (°C) | EP (bar) | SRV (rpm) | |
| PCL | 20 | 280 | 90 | 5 | 11 |
| PEU | 22 | 280 | 125 | 5 | 8 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moxon, S.R.; Ferreira, M.J.S.; Santos, P.d.; Popa, B.; Gloria, A.; Katsarava, R.; Tugushi, D.; Serra, A.C.; Hooper, N.M.; Kimber, S.J.; et al. A Preliminary Evaluation of the Pro-Chondrogenic Potential of 3D-Bioprinted Poly(ester Urea) Scaffolds. Polymers 2020, 12, 1478. https://doi.org/10.3390/polym12071478
Moxon SR, Ferreira MJS, Santos Pd, Popa B, Gloria A, Katsarava R, Tugushi D, Serra AC, Hooper NM, Kimber SJ, et al. A Preliminary Evaluation of the Pro-Chondrogenic Potential of 3D-Bioprinted Poly(ester Urea) Scaffolds. Polymers. 2020; 12(7):1478. https://doi.org/10.3390/polym12071478
Chicago/Turabian StyleMoxon, Samuel R., Miguel J.S. Ferreira, Patricia dos Santos, Bogdan Popa, Antonio Gloria, Ramaz Katsarava, David Tugushi, Armenio C. Serra, Nigel M. Hooper, Susan J. Kimber, and et al. 2020. "A Preliminary Evaluation of the Pro-Chondrogenic Potential of 3D-Bioprinted Poly(ester Urea) Scaffolds" Polymers 12, no. 7: 1478. https://doi.org/10.3390/polym12071478
APA StyleMoxon, S. R., Ferreira, M. J. S., Santos, P. d., Popa, B., Gloria, A., Katsarava, R., Tugushi, D., Serra, A. C., Hooper, N. M., Kimber, S. J., Fonseca, A. C., & Domingos, M. A. N. (2020). A Preliminary Evaluation of the Pro-Chondrogenic Potential of 3D-Bioprinted Poly(ester Urea) Scaffolds. Polymers, 12(7), 1478. https://doi.org/10.3390/polym12071478










