Detoxification Properties of Guanidinylated Chitosan Against Chemical Warfare Agents and Its Application to Military Protective Clothing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
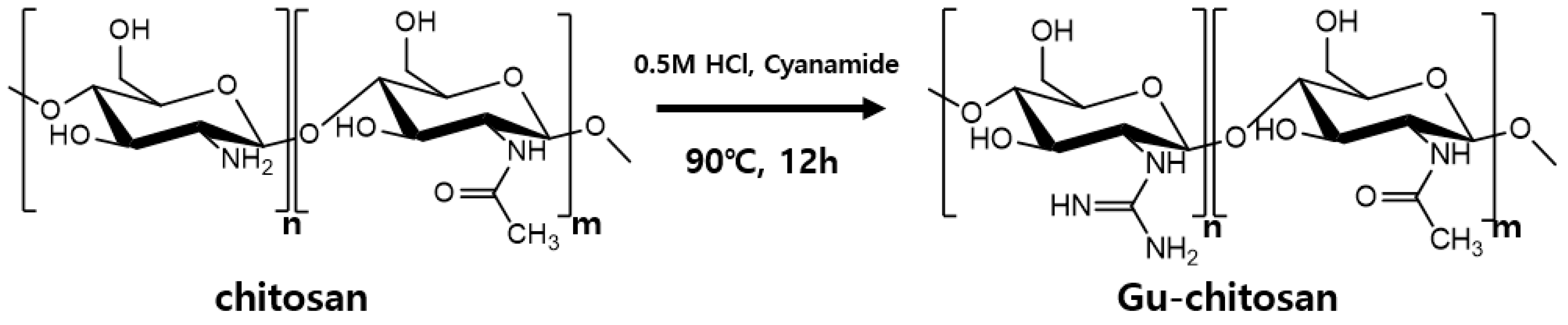
2.2. Chitosan Guanidinylation
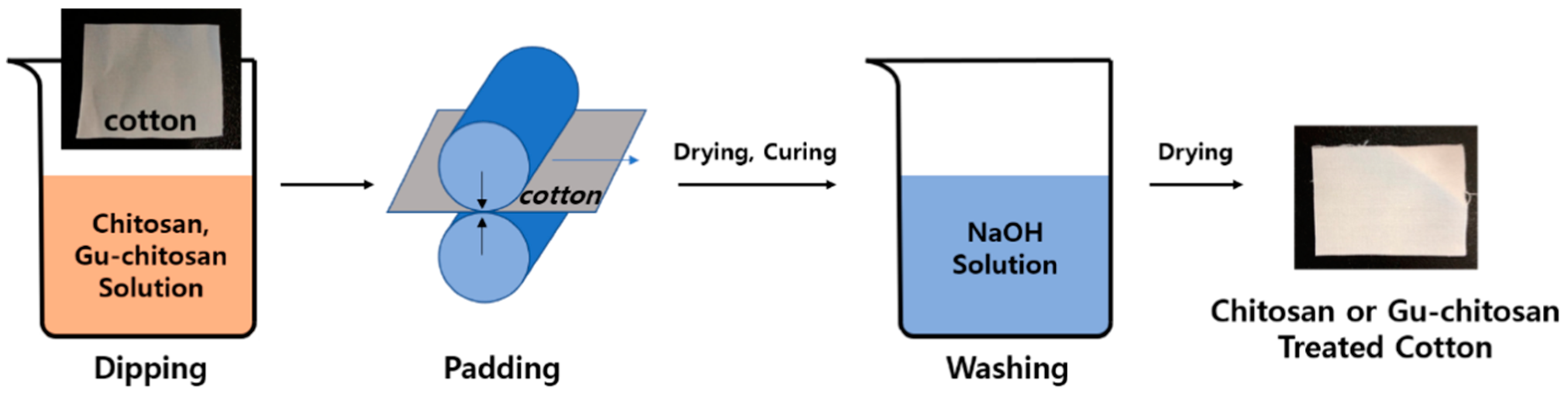
2.3. Preparation of the Chitosan and Guanidinylated Chitosan-Treated Cotton Fabrics
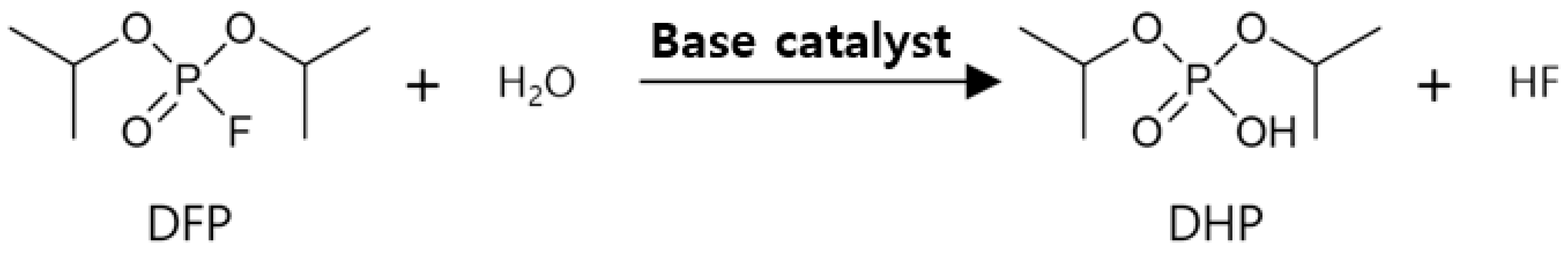
2.4. Test of the Detoxification Properties of the Chitosan, Guanidinylated Chitosan, and Prepared Fabrics
2.5. Characterization and Evaluation of the Guanidinylated Chitosan and Prepared Fabrics
3. Results and Discussion
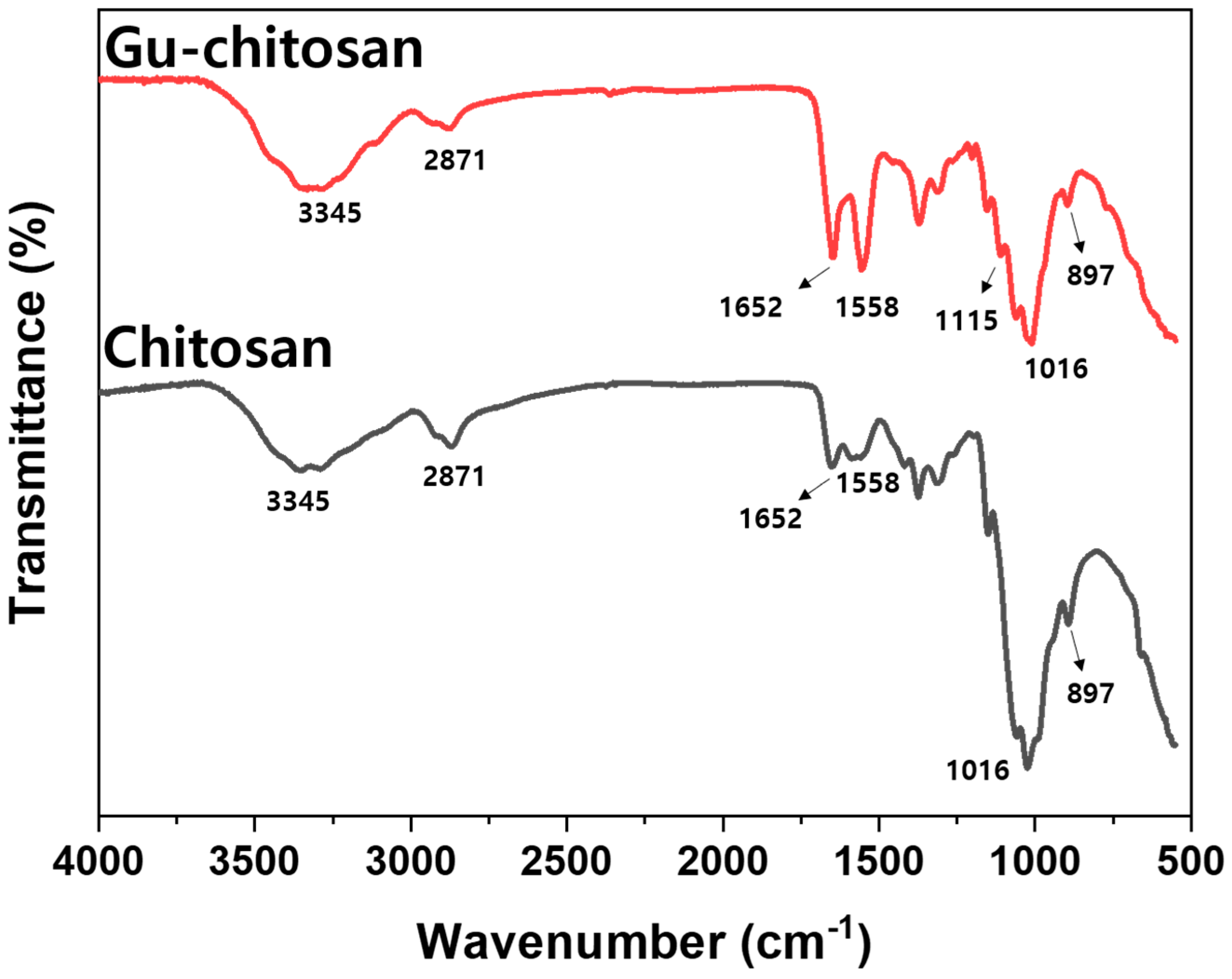
3.1. Chemical Structure Characterization of the Guanidinylated Chitosan
3.2. Chemical and Morphological Changes of the Cotton Fabrics Before and After the Treatments
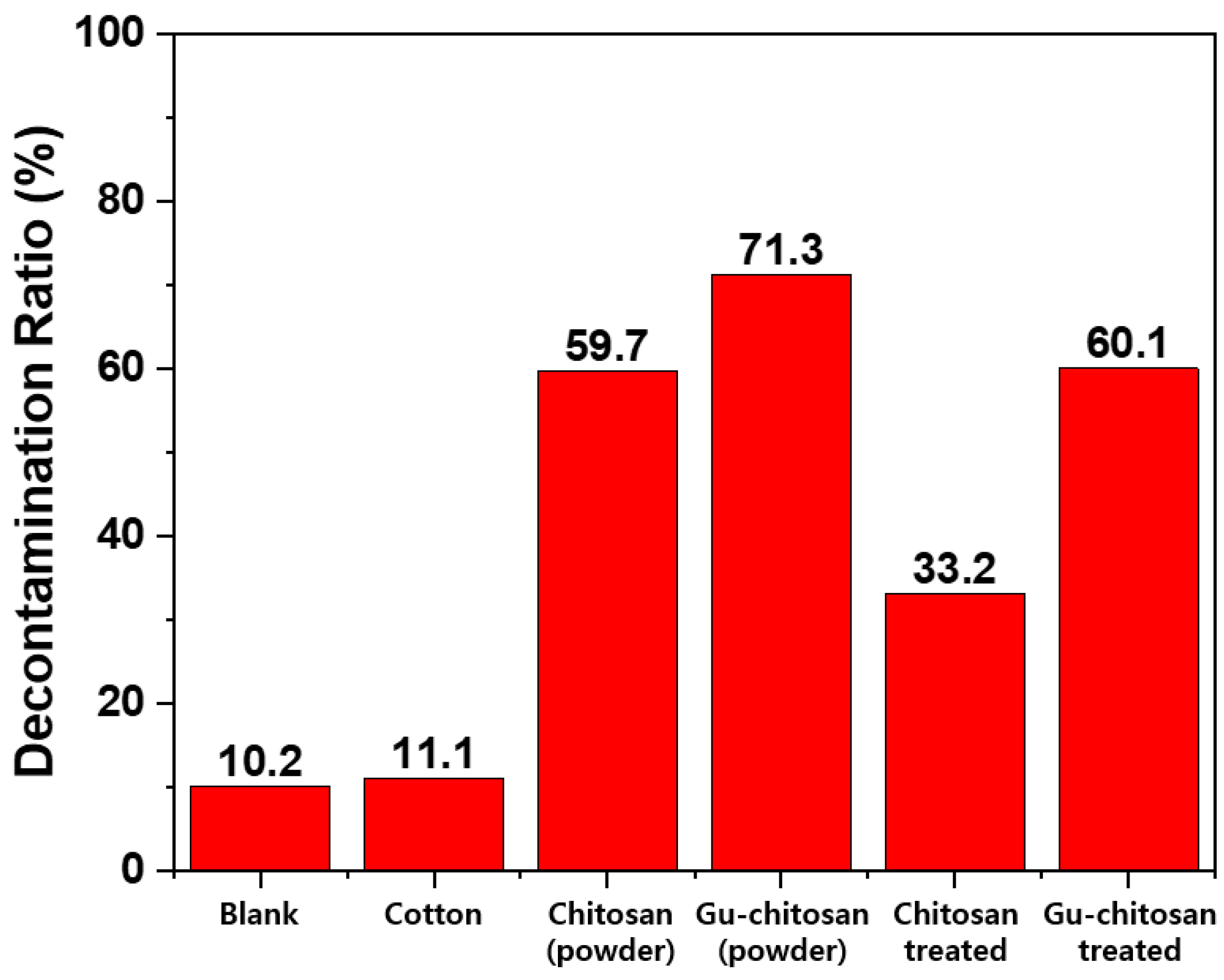
3.3. Detoxification Properties of the Prepared Samples
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Havenith, G.; den Hartog, E.; Martini, S. Heat stress in chemical protective clothing: Porosity and vapour resistance. Ergonomics 2011, 54, 497–507. [Google Scholar] [CrossRef]
- Khalil, E. A technical overview on protective clothing against chemical hazards. AASCIT J. Chem. 2015, 2, 67–76. [Google Scholar]
- Schreuder-Gibson, H.L.; Truong, Q.; Walker, J.E.; Owens, J.R.; Wander, J.D.; Jones, W.E. Chemical and biological protection and detection in fabrics for protective clothing. MRS Bull. 2003, 28, 574–578. [Google Scholar] [CrossRef] [Green Version]
- Boopathi, M.; Singh, B.; Vijayaraghavan, R. A review on NBC body protective clothing. Open Text. J. 2008, 1, 1–8. [Google Scholar] [CrossRef]
- Bhuiyan, M.R.; Wang, L.; Shaid, A.; Shanks, R.A.; Ding, J. Advances and applications of chemical protective clothing system. J. Ind. Text. 2019, 49, 97–138. [Google Scholar] [CrossRef]
- Gil-San-Millan, R.; López-Maya, E.; Hall, M.; Padial, N.M.; Peterson, G.W.; DeCoste, J.B.; Rodríguez-Albelo, L.M.; Oltra, J.E.; Barea, E.; Navarro, J.A. Chemical warfare agents detoxification properties of zirconium metal–organic frameworks by synergistic incorporation of nucleophilic and basic sites. ACS Appl. Mater. Interfaces 2017, 9, 23967–23973. [Google Scholar] [CrossRef]
- Ying, W.B.; Kim, S.; Lee, M.W.; Go, N.Y.; Jung, H.; Ryu, S.G.; Lee, B.; Lee, K.J. Toward a detoxification fabric against nerve gas agents: Guanidine-functionalized poly [2-(3-butenyl)-2-oxazoline]/Nylon-6, 6 nanofibers. RSC Adv. 2017, 7, 15246–15254. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Ying, W.B.; Jung, H.; Ryu, S.G.; Lee, B.; Lee, K.J. Zirconium hydroxide-coated nanofiber mats for nerve agent decontamination. Chem. Asian J. 2017, 12, 698–705. [Google Scholar] [CrossRef]
- Lee, J.; Seo, E.; Yoo, M.; Kim, S.; Choi, J.; Jung, H.; Lee, H.W.; Lee, H.M.; Kim, H.Y.; Lee, B. Preparation of non-woven nanofiber webs for detoxification of nerve gases. Polymer 2019, 179, 121664. [Google Scholar] [CrossRef]
- Choi, J.; Moon, D.S.; Ryu, S.G.; Lee, B.; Lee, K.J. Highly functionalized thermoplastic polyurethane from surface click reactions. J. Appl. Polym. Sci. 2018, 135, 46519. [Google Scholar] [CrossRef]
- Chen, L.; Bromberg, L.; Schreuder-Gibson, H.; Walker, J.; Hatton, T.A.; Rutledge, G.C. Chemical protection fabrics via surface oximation of electrospun polyacrylonitrile fiber mats. J. Mater. Chem. 2009, 19, 2432–2438. [Google Scholar] [CrossRef]
- López-Maya, E.; Montoro, C.; Rodríguez-Albelo, L.M.; Aznar Cervantes, S.D.; Lozano-Pérez, A.A.; Cenís, J.L.; Barea, E.; Navarro, J.A. Textile/Metal–Organic-Framework Composites as Self-Detoxifying Filters for Chemical-Warfare Agents. Angew. Chem. Int. Ed. 2015, 54, 6790–6794. [Google Scholar] [CrossRef] [PubMed]
- Colovic, M.B.; Krstic, D.Z.; Lazarevic-Pasti, T.D.; Bondzic, A.M.; Vasic, V.M. Acetylcholinesterase inhibitors: Pharmacology and toxicology. Curr. Neuropharmacol. 2013, 11, 315–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delfino, R.T.; Ribeiro, T.S.; Figueroa-Villar, J.D. Organophosphorus compounds as chemical warfare agents: A review. J. Braz. Chem. Soc. 2009, 20, 407–428. [Google Scholar] [CrossRef]
- Talmage, S.S.; Watson, A.P.; Hauschild, V.; Munro, N.B.; King, J. Chemical warfare agent degradation and decontamination. Curr. Org. Chem. 2007, 11, 285–298. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.-C. Chemical detoxification of nerve agent VX. Acc. Chem. Res. 1999, 32, 109–115. [Google Scholar] [CrossRef]
- Gallis, D.F.S.; Harvey, J.A.; Pearce, C.J.; Hall, M.G.; DeCoste, J.B.; Kinnan, M.K.; Greathouse, J.A. Efficient MOF-based degradation of organophosphorus compounds in non-aqueous environments. J. Mater. Chem. A 2018, 6, 3038–3045. [Google Scholar] [CrossRef]
- Giannakoudakis, D.A.; Hu, Y.; Florent, M.; Bandosz, T.J. Smart textiles of MOF/gC 3 N 4 nanospheres for the rapid detection/detoxification of chemical warfare agents. Nanoscale Horiz. 2017, 2, 356–364. [Google Scholar] [CrossRef]
- Kalaj, M.; Denny, M.S., Jr.; Bentz, K.C.; Palomba, J.M.; Cohen, S.M. Nylon–MOF composites through postsynthetic polymerization. Angew. Chem. 2019, 131, 2358–2362. [Google Scholar] [CrossRef]
- Lee, D.T.; Zhao, J.; Oldham, C.J.; Peterson, G.W.; Parsons, G.N. UiO-66-NH2 Metal–Organic Framework (MOF) Nucleation on TiO2, ZnO, and Al2O3 Atomic Layer Deposition-Treated Polymer Fibers: Role of Metal Oxide on MOF Growth and Catalytic Hydrolysis of Chemical Warfare Agent Simulants. ACS Appl. Mater. Interfaces 2017, 9, 44847–44855. [Google Scholar] [CrossRef] [Green Version]
- Han, J.; Xu, Y.; Su, Y.; She, X.; Pan, X. Guanidine-catalyzed Henry reaction and Knoevenagel condensation. Catal. Commun. 2008, 9, 2077–2079. [Google Scholar] [CrossRef]
- Lohmeijer, B.G.; Pratt, R.C.; Leibfarth, F.; Logan, J.W.; Long, D.A.; Dove, A.P.; Nederberg, F.; Choi, J.; Wade, C.; Waymouth, R.M. Guanidine and amidine organocatalysts for ring-opening polymerization of cyclic esters. Macromolecules 2006, 39, 8574–8583. [Google Scholar] [CrossRef]
- Sun, S.; An, Q.; Li, X.; Qian, L.; He, B.; Xiao, H. Synergistic effects of chitosan–guanidine complexes on enhancing antimicrobial activity and wet-strength of paper. Bioresour. Technol. 2010, 101, 5693–5700. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jiang, J.; Chen, Y. Synthesis and antimicrobial activity of polymeric guanidine and biguanidine salts. Polymer 1999, 40, 6189–6198. [Google Scholar] [CrossRef]
- Hussain, M.R.; Iman, M.; Maji, T.K. Determination of degree of deacetylation of chitosan and their effect on the release behavior of essential oil from chitosan and chitosan-gelatin complex microcapsules. Int. J. Adv. Eng. Appl. 2013, 6, 4–12. [Google Scholar]
- Dimzon, I.K.D.; Knepper, T.P. Degree of deacetylation of chitosan by infrared spectroscopy and partial least squares. Int. J. Biol. Macromol. 2015, 72, 939–945. [Google Scholar] [CrossRef]
- Hu, Y.; Du, Y.; Yang, J.; Kennedy, J.F.; Wang, X.; Wang, L. Synthesis, characterization and antibacterial activity of guanidinylated chitosan. Carbohydr. Polym. 2007, 67, 66–72. [Google Scholar] [CrossRef]
- Yadav, M.; Ahmad, S. Montmorillonite/graphene oxide/chitosan composite: Synthesis, characterization and properties. Int. J. Biol. Macromol. 2015, 79, 923–933. [Google Scholar] [CrossRef]
- Yadav, M.; Behera, K.; Chang, Y.H.; Chiu, F.C. Cellulose Nanocrystal Reinforced Chitosan Based UV Barrier Composite Films for Sustainable Packaging. Polymers 2020, 12, 202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salama, A.; Hesemann, P. Synthesis and characterization of N-guanidinium chitosan/silica ionic hybrids as templates for calcium phosphate mineralization. Int. J. Biol. Macromol. 2020, 147, 276–283. [Google Scholar] [CrossRef]
- Chung, C.; Lee, M.; Choe, E.K. Characterization of cotton fabric scouring by FT-IR ATR spectroscopy. Carbohydr. Polym. 2004, 58, 417–420. [Google Scholar] [CrossRef]
- Chen, Y.; Zou, C.; Mastalerz, M.; Hu, S.; Gasaway, C.; Tao, X. Applications of micro-fourier transform infrared spectroscopy (FTIR) in the geological sciences—A review. Int. J. Mol. Sci. 2015, 16, 30223–30250. [Google Scholar] [CrossRef] [PubMed]
- Javid, A.; Raza, Z.A.; Hussain, T.; Rehman, A. Chitosan microencapsulation of various essential oils to enhance the functional properties of cotton fabric. J. Microencapsul. 2014, 31, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Thakur, V.K.; Thakur, M.K. Fibers from natural resources. In Handbook of Composites from Renewable Materials, Functionalization; Kessler, M.R., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2017; Volume 4, pp. 303–304. [Google Scholar]
- Kwon, W.; Han, M.W.; Jeong, E.G. Chemical warfare agent simulant decontamination of chitosan treated cotton fabric. Text. Color. Finish. 2020, 32, 51–56. [Google Scholar]
- Heiss, D.R.; Zehnder, D.W.; Jett, D.A.; Platoff, G.E.; Yeung, D.T.; Brewer, B.N. Synthesis and storage stability of diisopropylfluorophosphate. J. Chem. 2016, 2016, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
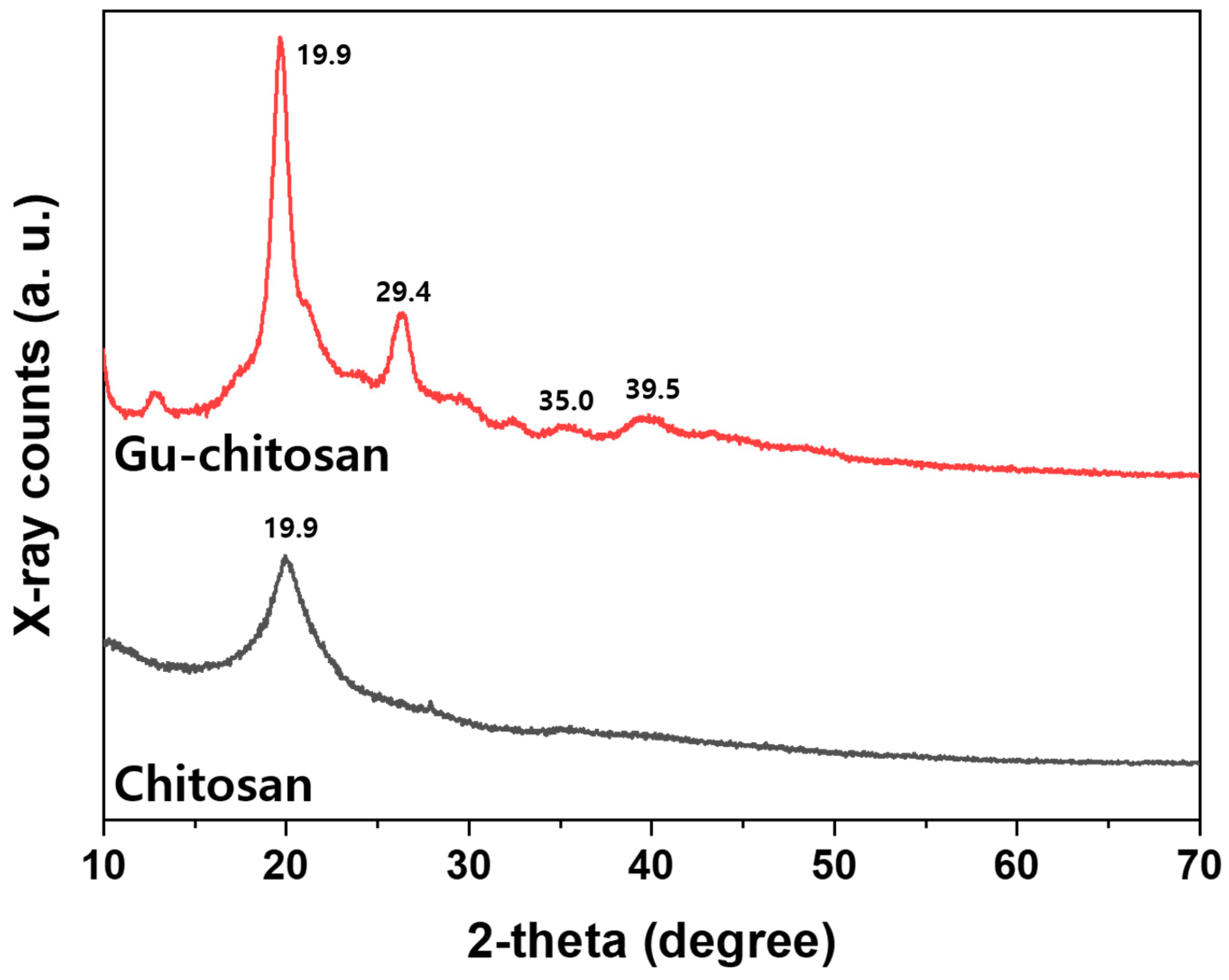
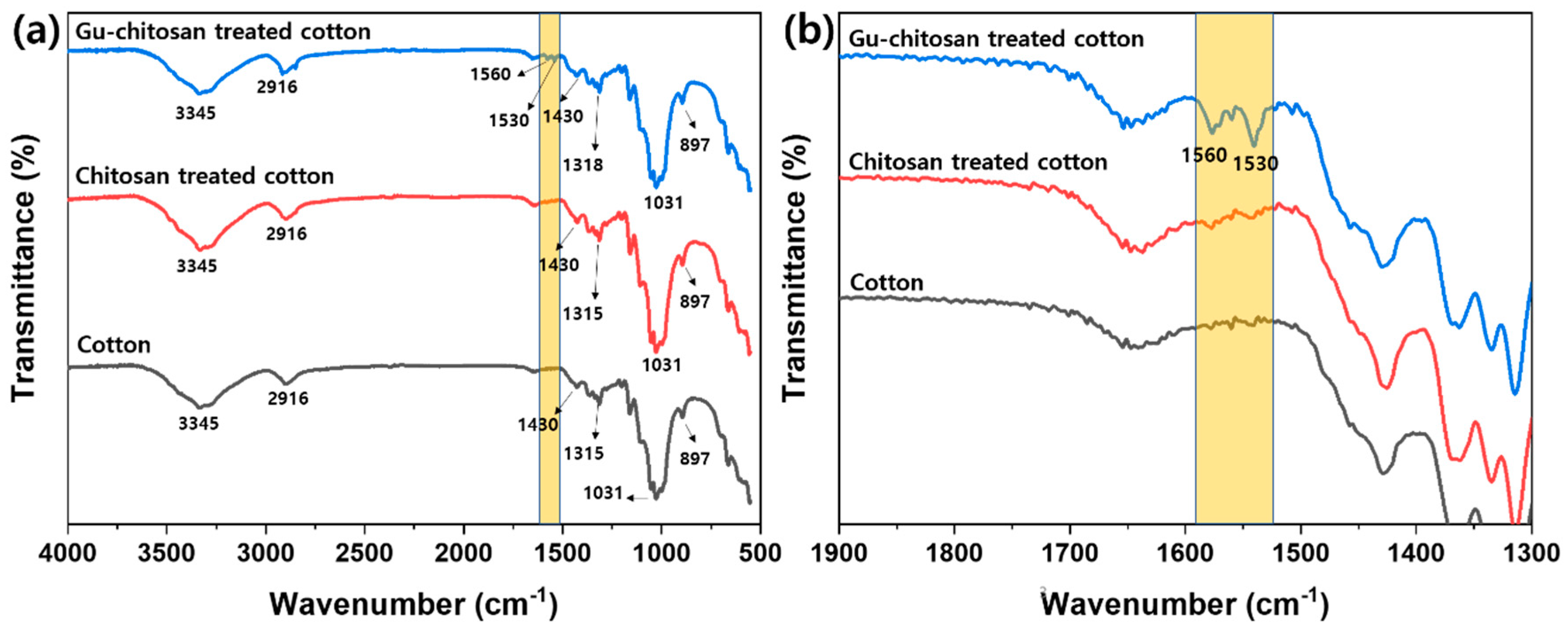
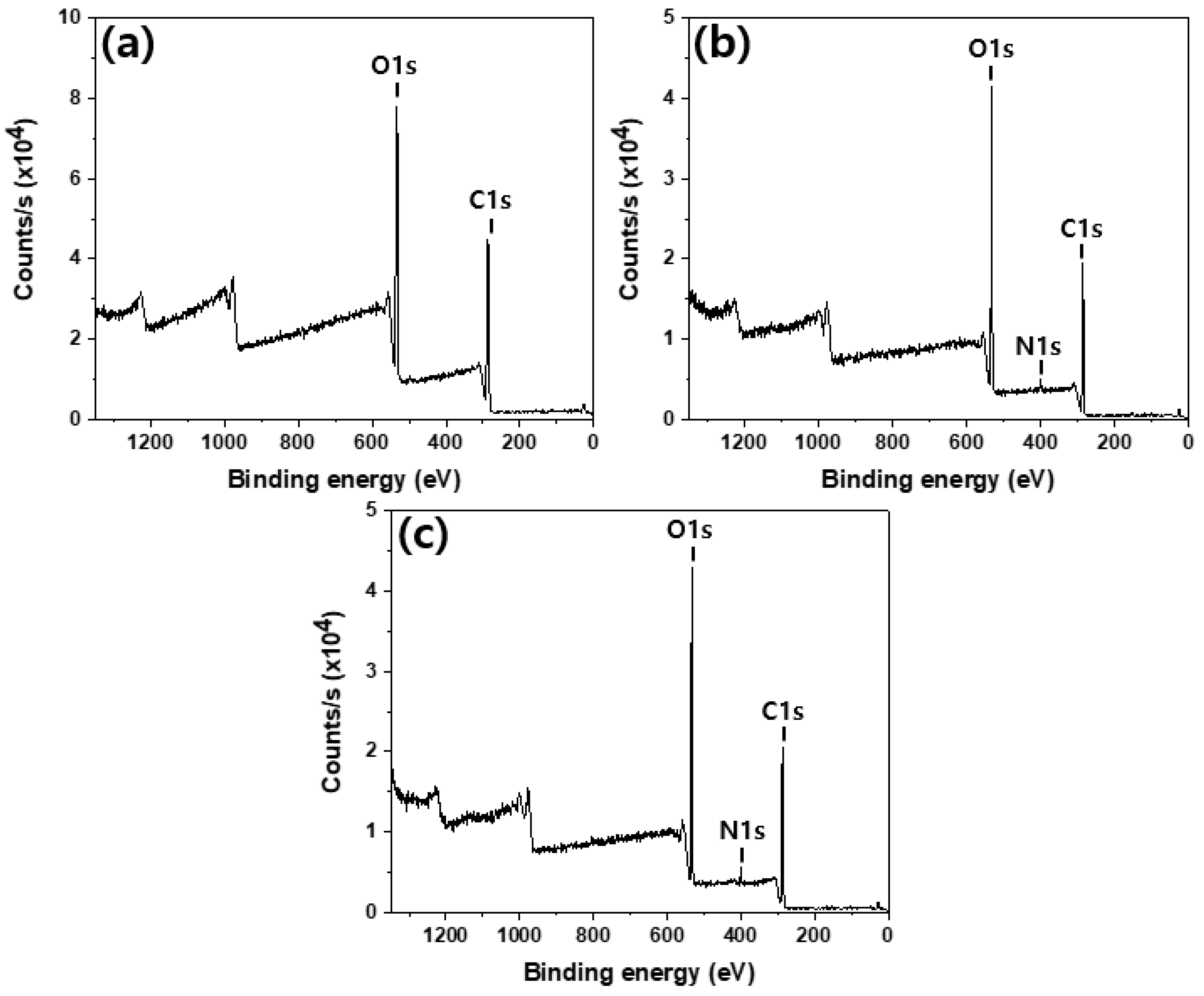

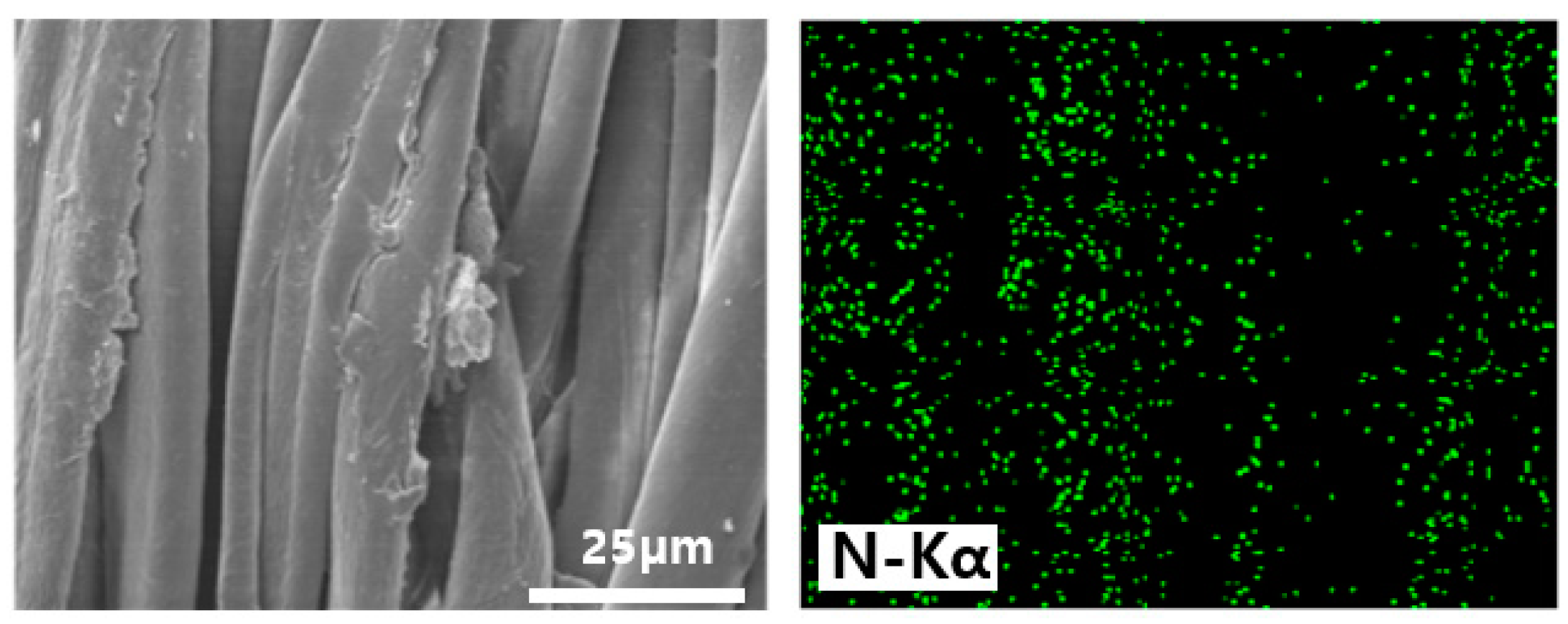

| Sample | Element Content (wt.%) | C/N | DS (Degree of Substitution) | ||
|---|---|---|---|---|---|
| C | N | H | |||
| Chitosan | 41.7 | 7.57 | 6.65 | 5.51 | |
| Gu-chitosan | 39.3 | 10.8 | 6.21 | 3.64 | 0.295 |
| Sample | Atomic Percent | Atomic Ratios | ||
|---|---|---|---|---|
| C | O | N | N/C | |
| Cotton | 62.8 | 37.2 | ||
| Chitosan-treated cotton | 57.5 | 40.5 | 2.54 | 0.044 |
| Gu-chitosan-treated cotton | 57.5 | 37.4 | 3.74 | 0.065 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, W.; Jeong, E. Detoxification Properties of Guanidinylated Chitosan Against Chemical Warfare Agents and Its Application to Military Protective Clothing. Polymers 2020, 12, 1461. https://doi.org/10.3390/polym12071461
Kwon W, Jeong E. Detoxification Properties of Guanidinylated Chitosan Against Chemical Warfare Agents and Its Application to Military Protective Clothing. Polymers. 2020; 12(7):1461. https://doi.org/10.3390/polym12071461
Chicago/Turabian StyleKwon, Woong, and Euigyung Jeong. 2020. "Detoxification Properties of Guanidinylated Chitosan Against Chemical Warfare Agents and Its Application to Military Protective Clothing" Polymers 12, no. 7: 1461. https://doi.org/10.3390/polym12071461
APA StyleKwon, W., & Jeong, E. (2020). Detoxification Properties of Guanidinylated Chitosan Against Chemical Warfare Agents and Its Application to Military Protective Clothing. Polymers, 12(7), 1461. https://doi.org/10.3390/polym12071461





