Boosting the Power Factor of Benzodithiophene Based Donor–Acceptor Copolymers/SWCNTs Composites through Doping
Abstract
1. Introduction
2. Experimental Section
2.1. Raw Materials
2.2. Instruments
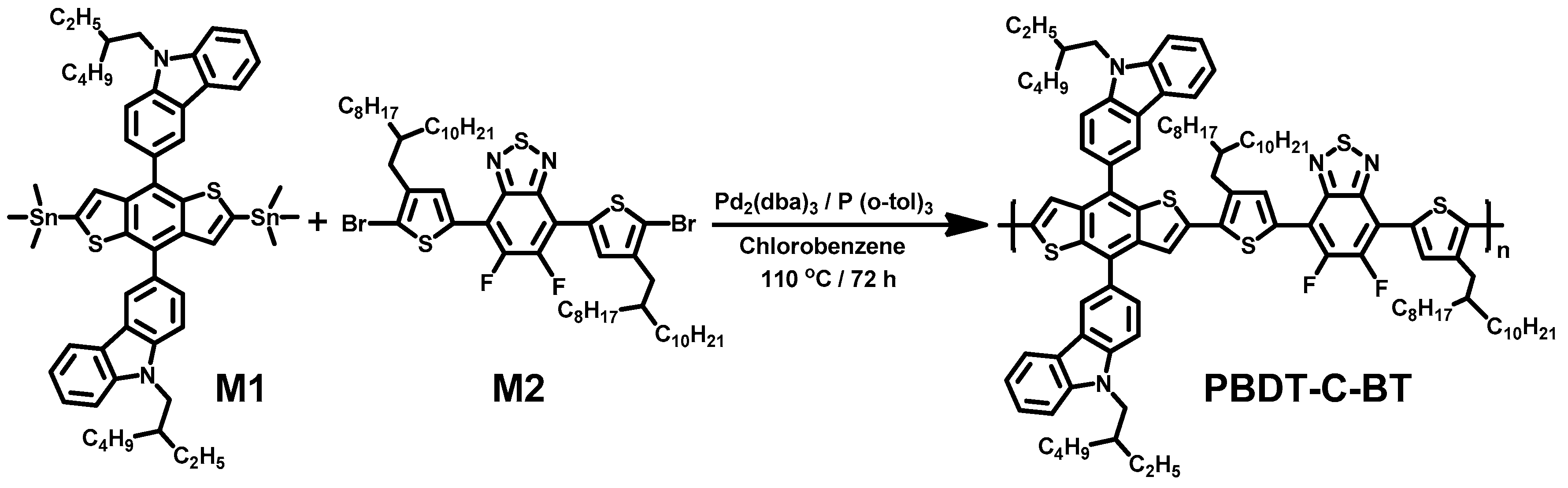
2.3. Synthesis of the Polymer
2.4. Preparation of the PBDT–C–BT/SWCNTs Composites
2.5. Preparation of Doped PBDT–C–BT/SWCNTs Composites
3. Results and Discussion
3.1. Polymer Design, Synthesis and Characterizations
3.2. The UV-Vis-NIR Spectra of the Polymer/SWCNTs Composites
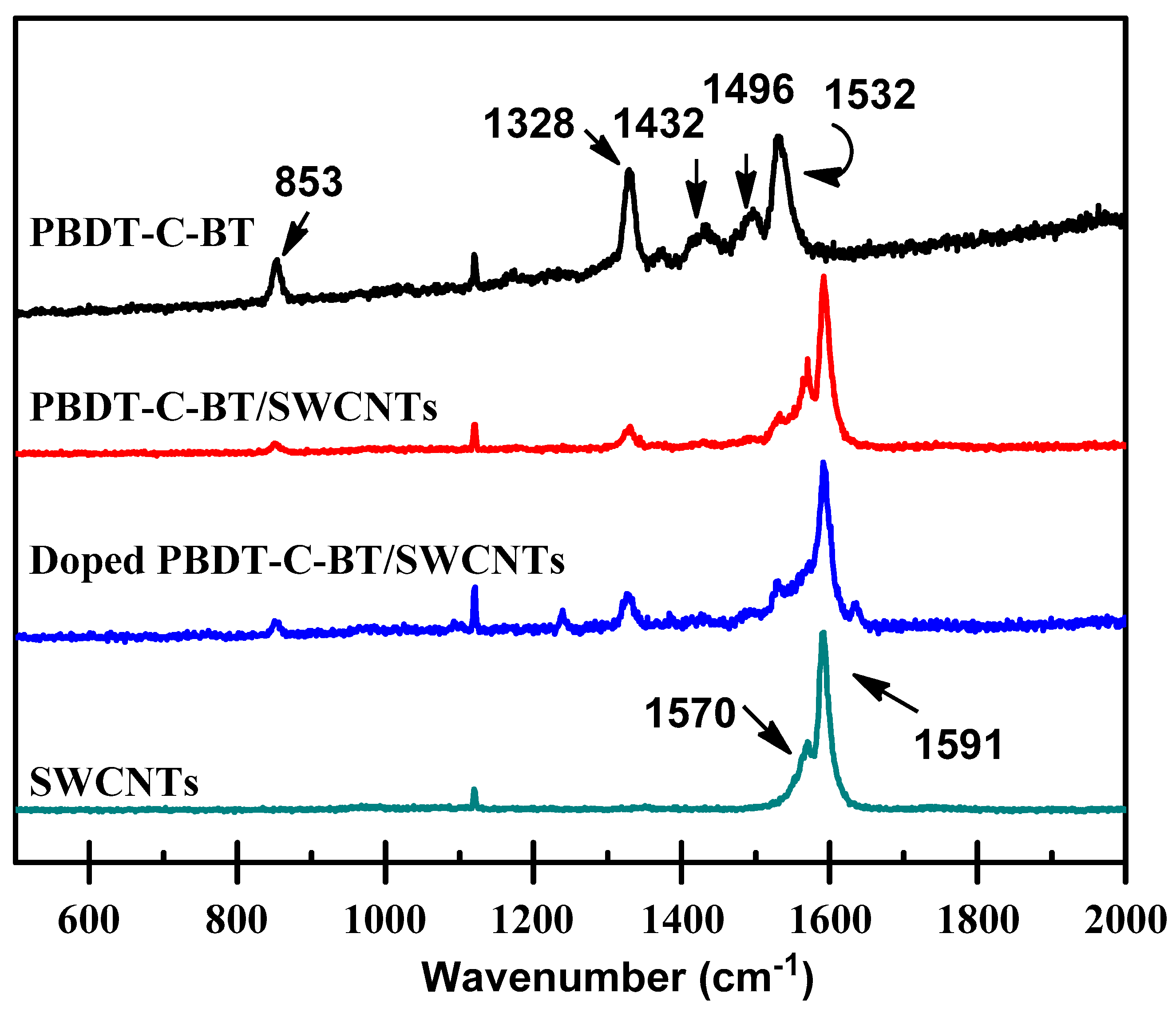
3.3. Raman Spectra of the Polymer/SWCNTs Composites
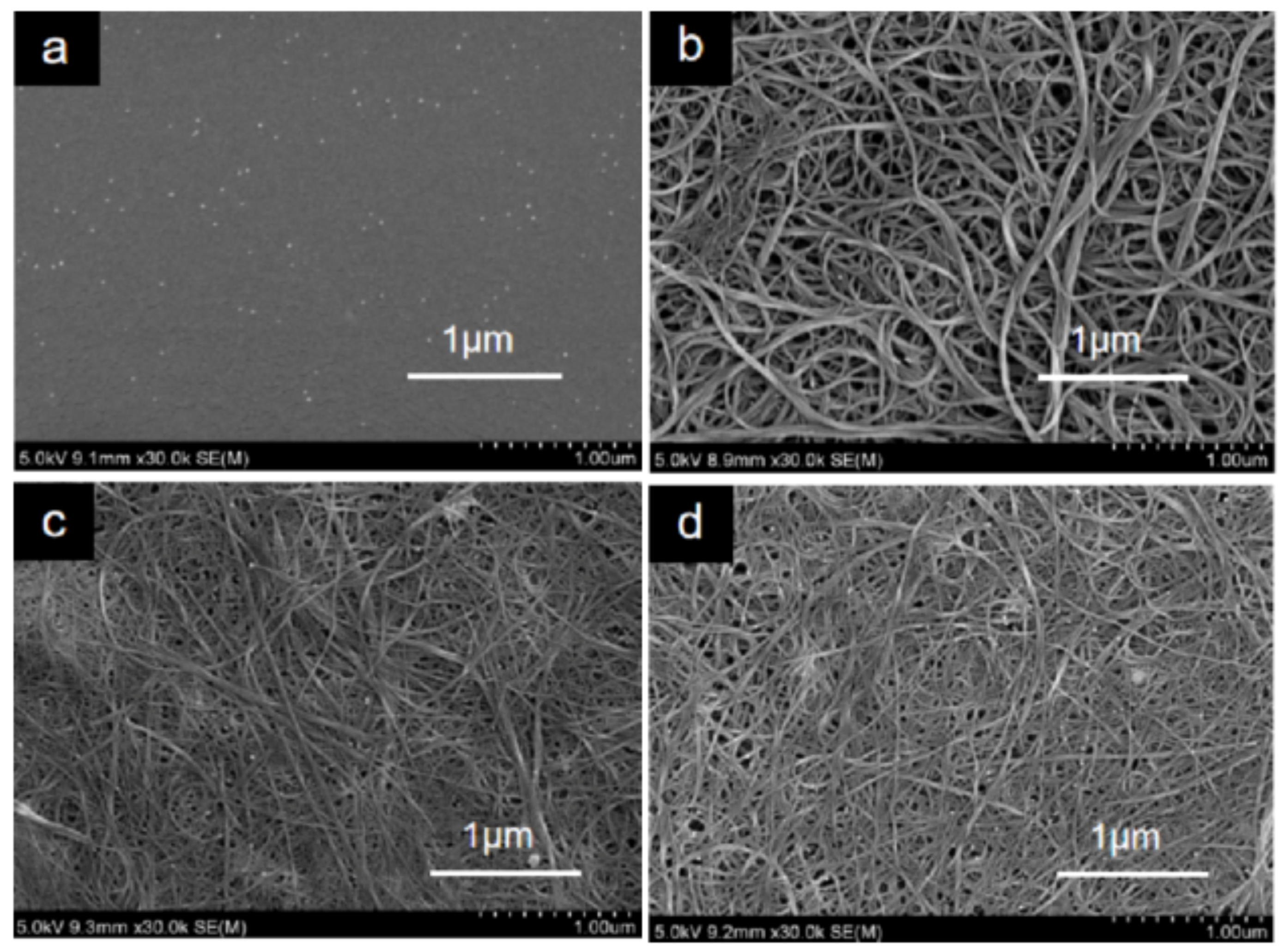
3.4. Surface Morphology of the Composites
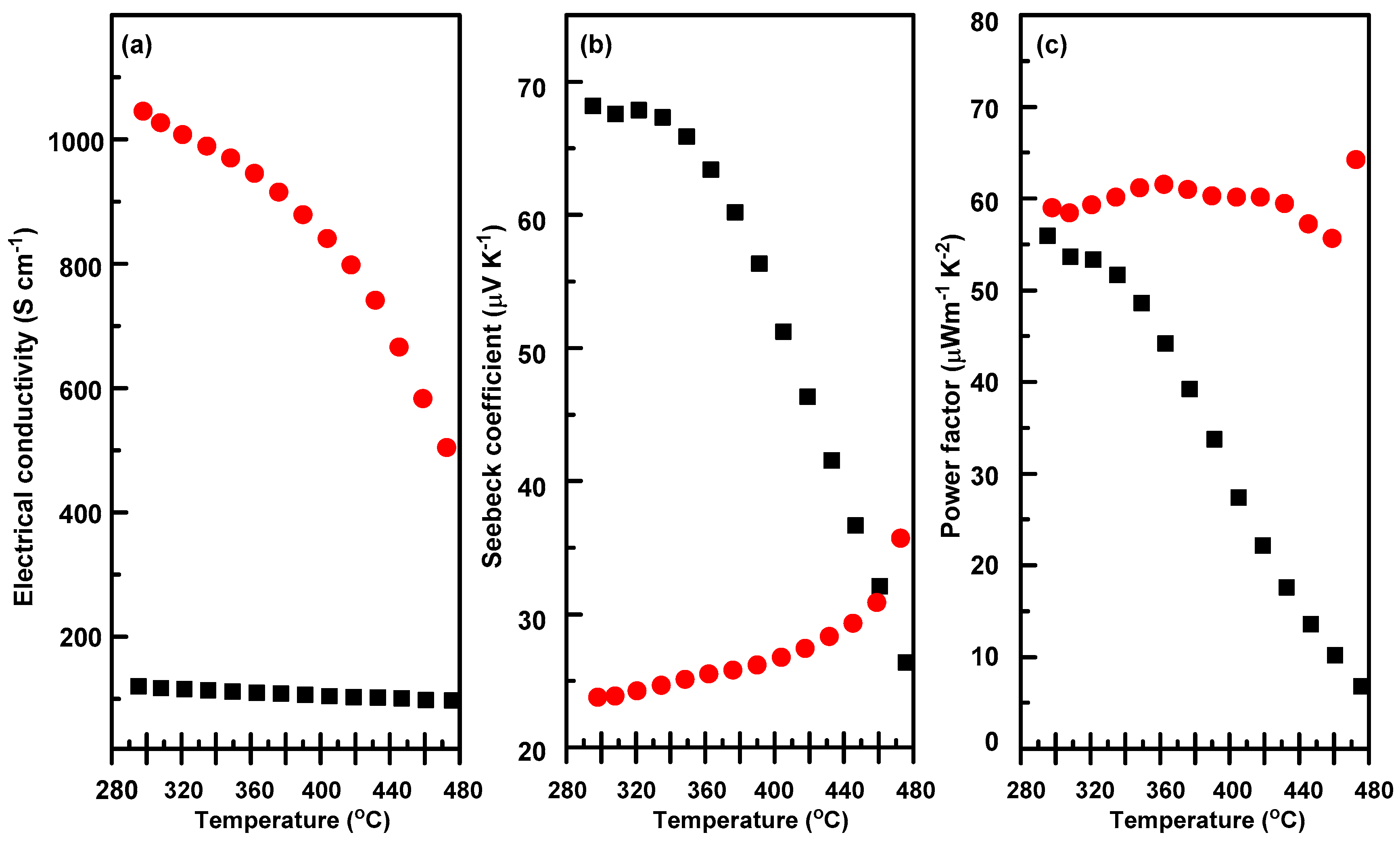
3.5. Thermoelectric Properties of the Composites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Coakley, K.M.; Mcgehee, M.D. Conjugated polymer photovoltaic cells. Chem. Mater. 2004, 16, 4533–4542. [Google Scholar] [CrossRef]
- Deng, Z.; Ai, T.; Li, R.; Yuan, W.; Zhang, K.; Du, H.; Zhang, H. Conjugated polymers containing building blocks 1, 3, 4, 6-tetraarylpyrrolo[3, 2-b]pyrrole-2, 5-dione(iso-DPP), benzodipyrrolidone (BDP) or naphthodipyrrolidone (NDP): A review. Polymers 2019, 11, 1683. [Google Scholar] [CrossRef]
- Gao, C.; Wang, L.; Li, X.; Wang, H. Rational design on D-A conjugated P(BDT-DTBT) polymers for polymer solar cells. Polym. Chem. 2014, 5, 5200–5210. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, S.; Zhang, Y.; Du, H.; Zhao, J. Design and characterization of new D-A type electrochromic conjugated copolymers based on indolo[3,2-b]carbazole, isoindigo and thiophene units. Polymers 2019, 11, 1626. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Park, S.-J. Flexible organic thermoelectric materials and devices for wearable green energy harvesting. Polymers 2019, 11, 909. [Google Scholar] [CrossRef]
- Veysel Tunc, A.; De Sio, A.; Riedel, D.; Deschler, F.; Da Como, E.; Paris, J.; von Hauff, E. Molecular doping of low-bandgap-polymer:fullerene solar cells: Effects on transport and solar cells. Org. Eletron. 2012, 13, 290–296. [Google Scholar] [CrossRef]
- Russ, B.; Glaudell, A.; Urban, J.J.; Chabinyc, M.L.; Segalman, R.A. Organic thermoelectric materials for energy harvesting and temperature control. Nat. Rev. Mater. 2016, 1, 16050. [Google Scholar] [CrossRef]
- Kroon, R.; Mengistie, D.A.; Kiefer, D.; Hynynen, J.; Ryan, J.D.; Yu, L.; Muller, C. Thermoelectric plastics: From design to synthesis, processing and structure-property relationships. Chem. Soc. Rev. 2016, 45, 6147–6164. [Google Scholar] [CrossRef]
- Zhang, Y.; Heo, Y.-J.; Park, M.; Park, S.-J. Recent advances in organic thermoelectric materials: Principle mechanisms and emerging carbon-based green energy materials. Polymers 2019, 11, 167. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.; Wang, L.; Zhou, W.; Cai, L.; Xie, D.; Chen, Z.; Wang, L. Preparation and thermoelectric properties study of bipyridine-containing polyfluorene derivative/SWCNT composites. Polymers 2019, 11, 278. [Google Scholar] [CrossRef] [PubMed]
- Qu, S.; Yao, Q.; Yu, B.; Zeng, K.; Shi, W.; Chen, Y.; Chen, L. Optimizing the thermoelectric performance of poly(3-hexylthiophene) through molecular-weight engineering. Chem. Asian J. 2018, 13, 3246–3253. [Google Scholar] [CrossRef]
- Wu, S.; Wu, X.; Xing, W.; Sun, Y.; Zou, Y.; Xu, W.; Zhu, D. Backbone structure effect on the thermoelectric properties of IDT-based p-type conjugated polymers. Macromol. Rapid Commun. 2020, 41, 1900322. [Google Scholar] [CrossRef]
- Pan, C.; Wang, L.; Liu, T.; Zhou, X.; Wan, T.; Wang, S.; Chen, Z.; Gao, C.; Wang, L. Polar side chain effects on the thermoelectric properties of benzo[1,2-b:4,5-b’]dithiophene-based conjugated polymers. Macromol. Rapid Commun. 2019, 40, e1900082. [Google Scholar] [CrossRef]
- Liu, J.; Qiu, L.; Portale, G.; Torabi, S.; Stuart, M.C.A.; Qiu, X.; Koopmans, M.; Chiechi, R.C.; Hummelen, J.C.; Koster, L.J.A. Side-chain effects on N-type organic thermoelectrics: A case study of fullerene derivatives. Nano Energy 2018, 52, 183–191. [Google Scholar] [CrossRef]
- Wei, C.; Wang, L.; Pan, C.; Chen, Z.; Zhao, H.; Wang, L. Effect of backbone structure on the thermoelectric performance of indacenodithiophene-based conjugated polymers. React. Funct. Polym. 2019, 142, 1–6. [Google Scholar] [CrossRef]
- Glaudell, A.M.; Cochran, J.E.; Patel, S.N.; Chabinyc, M.L. Impact of the doping method on conductivity and thermopower in semiconducting polythiophenes. Adv. Energy Mater. 2015, 5, 1401072. [Google Scholar] [CrossRef]
- Duong, D.T.; Wang, C.; Antono, E.; Toney, M.F.; Salleo, A. The chemical and structural origin of efficient p-type doping of P3HT. Org. Electron. 2013, 14, 1330–1336. [Google Scholar] [CrossRef]
- Salzmann, I.; Heimel, G.; Oehzelt, M.; Winkler, S.; Koch, N. Molecular eletrical doping of organic semiconductors: Fundamental mechanisms and emerging dopant design rules. Acc. Chem. Res. 2016, 49, 370–378. [Google Scholar] [CrossRef]
- Yim, K.-H.; Whiting, G.L.; Murphy, C.E.; Halls, J.J.M.; Burroughes, J.H.; Friend, R.H.; Kim, J.-S. Controlling electrical properties of conjugated polymers via a solution-based p-type doping. Adv. Mater. 2008, 20, 3319–3324. [Google Scholar] [CrossRef]
- Li, J.; Zhang, G.; Holm, D.M.; Jacobs, I.E.; Yin, B.; Stroeve, P.; Mascal, M.; Moulé, A.J. Introducing solubility control for improved organic p-type dopants. Chem. Mater. 2015, 27, 5765–5774. [Google Scholar] [CrossRef]
- Cochran, J.E.; Junk, M.J.N.; Glaudell, A.M.; Miller, P.L.; Cowart, J.S.; Toney, M.F.; Hawker, C.J.; Chmelka, B.F.; Chabinyc, M.L. Molecular interactions and ordering in electrically doped polymers: Blends of PBTTT and F4TCNQ. Macromolecules 2014, 47, 6836. [Google Scholar] [CrossRef]
- Patel, S.N.; Glaudell, A.M.; Peterson, K.A.; Thomas, E.M.; O’Hara, K.A.; Lim, E.; Chabinyc, M.L. Morphology controls the thermoelectric power factor of a doped semiconducting polymer. Sci. Adv. 2017, 3, el700434. [Google Scholar] [CrossRef] [PubMed]
- Hynynen, J.; Kiefer, D.; Yu, L.; Kroon, R.; Munir, R.; Amassian, A.; Kemerink, M.; Muller, C. Enhanced electrical conductivity of molecularly p-doped poly(3-hexylthiophene) through understanding the correlation with solid-state order. Macromolecules 2017, 50, 8140–8148. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Gao, C.; Liang, L.; Wang, X.; Chen, G. Nanostructure controlled construction of high-performance thermoelectric materials of polymers and their composites. J. Mater. Chem. A 2018, 6, 22381–22390. [Google Scholar] [CrossRef]
- Yin, S.; Lu, W.; Wu, R.; Fan, W.; Guo, C.-Y.; Chen, G. Poly(3,4-ethylenedioxythiophene)/Te/single-walled carbon nanotube composites with high thermoelectric performance promoted by electropolymerization. ACS Appl. Mater. Interfaces 2020, 12, 3547–3553. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Liang, A.; Pan, C.; Wang, L. Effects of oxidative doping on the thermoelectric performance of polyfluorene derivatives/carbon nanotube composite films. Org. Electron. 2018, 52, 281–287. [Google Scholar] [CrossRef]
- Wang, L.; Pan, C.; Chen, Z.; Zhou, W.; Gao, C.; Wang, L. Enhanced thermoelectric performance of conjugated polymer/single-walled carbon nanotube composites with strong stacking. ACS Appl. Energy Mater. 2019, 2, 6664–6671. [Google Scholar] [CrossRef]
- Zeng, Y.-J.; Wu, D.; Cao, X.-H.; Zhou, W.-X.; Tang, L.-M.; Chen, K.-Q. Nanoscale organic thermoelectric materials: Measurement, theoretical models, and optimization strategies. Adv. Funct. Mater. 2019, 30, 1903873. [Google Scholar] [CrossRef]
- Xu, K.; Chen, G.; Qiu, D. Convenient construction of poly(3, 4-ethylenedioxythiophene)/graphene pie-like structure with enhanced thermoelectric performance. J. Mater. Chem. A 2013, 1, 12395–123999. [Google Scholar] [CrossRef]
- Yao, Q.; Wang, Q.; Wang, L.; Chen, L. Abnormally enhanced thermoelectric transport properties of SWNT/PANI hybrid films by the strengthened PANI molecular ordering. Energ. Environ. Sci. 2014, 7, 3801. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.; Wang, N.; Liang, Z.; Wang, X.; Peng, Y.; Tong, J.; Yang, C.; Xia, Y. Synthesis and photovoltaic effect of electron-withdrawing units for low band gap conjugated polymers bearing bi(thienylenevinylene) side chains. Polymers 2019, 11, 1461. [Google Scholar] [CrossRef]
- Wang, X.; Cheng, C.; Li, Y.; Wang, F. Synthesis and photovoltaic properties of 2D-conjugated polymers based on alkylthiothienyl-substituted benzodithiophene and different accepting units. Polymer 2018, 10, 331. [Google Scholar] [CrossRef]
- Jiang, J.M.; Raghunath, P.; Lin, Y.C.; Lin, H.K.; Ko, C.L.; Su, Y.W.; Lin, M.C.; Wei, K.H. Linear solubilizing side chain substituents enhance the photovoltaic properties of two-dimensional conjugated benzodithiophene-based polymers. Polymer 2015, 79, 262–270. [Google Scholar] [CrossRef]
- Su, Q.; Tong, J.F.; Li, J.F.; Zhang, P.; Yang, C.Y.; Zhang, C.Z.; Wang, F.; Chen, D.J.; Xia, Y.J. Synthesis and characterization of alternating and random conjugated polymers derived from dithieno[2,3-d:20,30-d0]benzo[1,2-b:4,5-b’]dithiophene and 2,1,3-benzothiadiazole derivatives. Polym. J. 2015, 47, 803–809. [Google Scholar] [CrossRef]
- Ye, L.; Zhang, S.Q.; Huo, L.J.; Zhang, M.J.; Hou, J.H. Molecular design toward highly effificient photovoltaic polymers based on two-dimensional conjugated benzodithiophene. Acc. Chem. Res. 2014, 47, 1595–1603. [Google Scholar] [CrossRef]
- Zhou, X.; Pan, C.; Gao, C.; Shinohara, A.; Yin, X.; Wang, L.; Li, Y.; Jiang, Q.; Yang, C.; Wang, L. Thermoelectrics of two-dimensional conjugated benzodithiophene-based polymers: Density-of-states enhancement and semi-metallic behavior. J. Mater. Chem. A 2019, 7, 10422. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.; Lai, M.; Cai, L.; Zhou, W.; Xie, D.; Pan, C.; Qiu, Y. Boosting the Power Factor of Benzodithiophene Based Donor–Acceptor Copolymers/SWCNTs Composites through Doping. Polymers 2020, 12, 1447. https://doi.org/10.3390/polym12071447
Chen Z, Lai M, Cai L, Zhou W, Xie D, Pan C, Qiu Y. Boosting the Power Factor of Benzodithiophene Based Donor–Acceptor Copolymers/SWCNTs Composites through Doping. Polymers. 2020; 12(7):1447. https://doi.org/10.3390/polym12071447
Chicago/Turabian StyleChen, Zhongming, Mengfei Lai, Lirong Cai, Wenqiao Zhou, Dexun Xie, Chengjun Pan, and Yongfu Qiu. 2020. "Boosting the Power Factor of Benzodithiophene Based Donor–Acceptor Copolymers/SWCNTs Composites through Doping" Polymers 12, no. 7: 1447. https://doi.org/10.3390/polym12071447
APA StyleChen, Z., Lai, M., Cai, L., Zhou, W., Xie, D., Pan, C., & Qiu, Y. (2020). Boosting the Power Factor of Benzodithiophene Based Donor–Acceptor Copolymers/SWCNTs Composites through Doping. Polymers, 12(7), 1447. https://doi.org/10.3390/polym12071447





