Effects of a Reactive Phosphorus–Sulfur Containing Flame-Retardant Monomer on the Flame Retardancy and Thermal and Mechanical Properties of Unsaturated Polyester Resin
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis and Sample Preparation
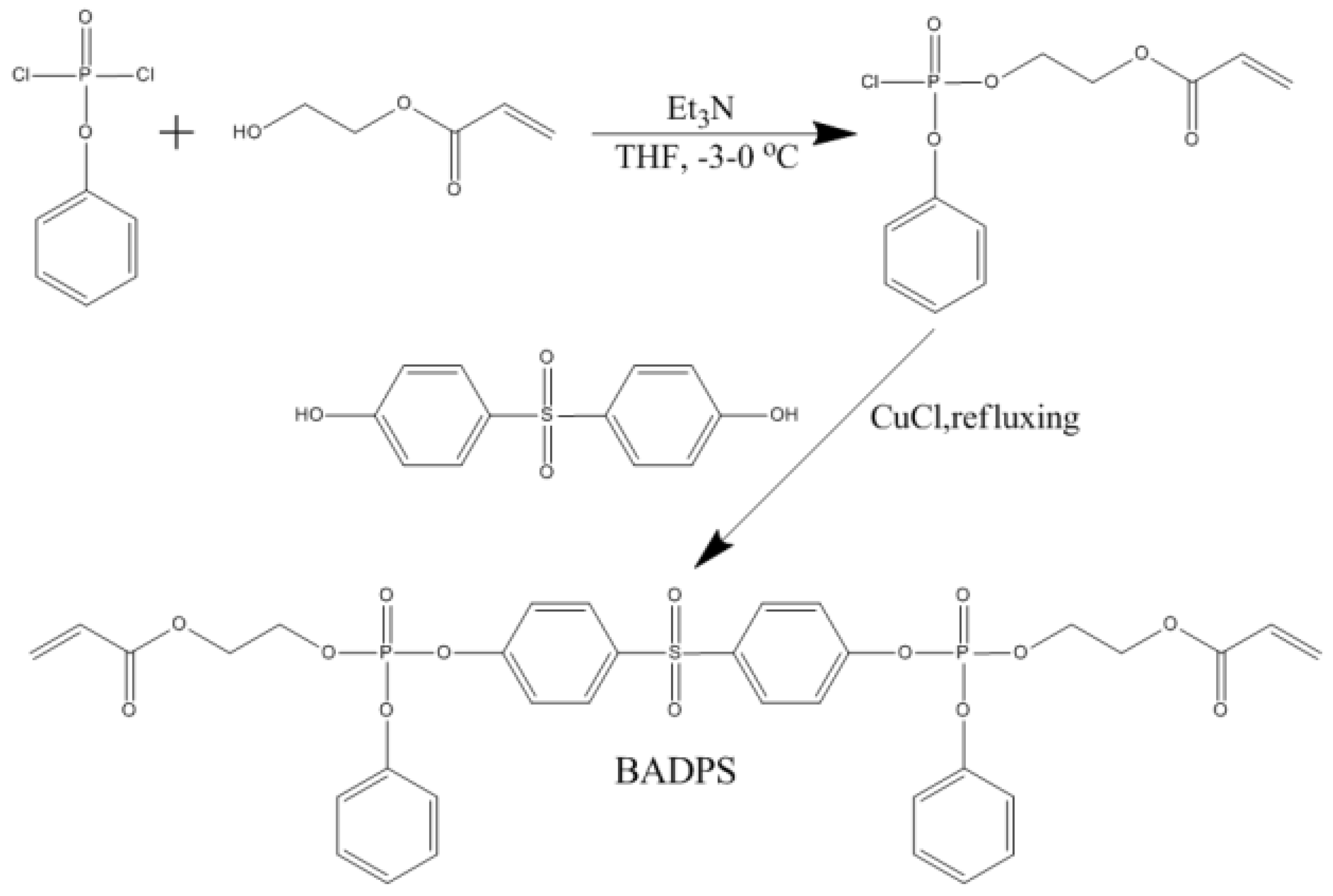
2.2.1. Synthesis of Bis (acryloxyethyl diphenyl phosphate) Sulfone (BADPS)
2.2.2. Sample Preparation
2.3. Characterization
3. Results and Discussion
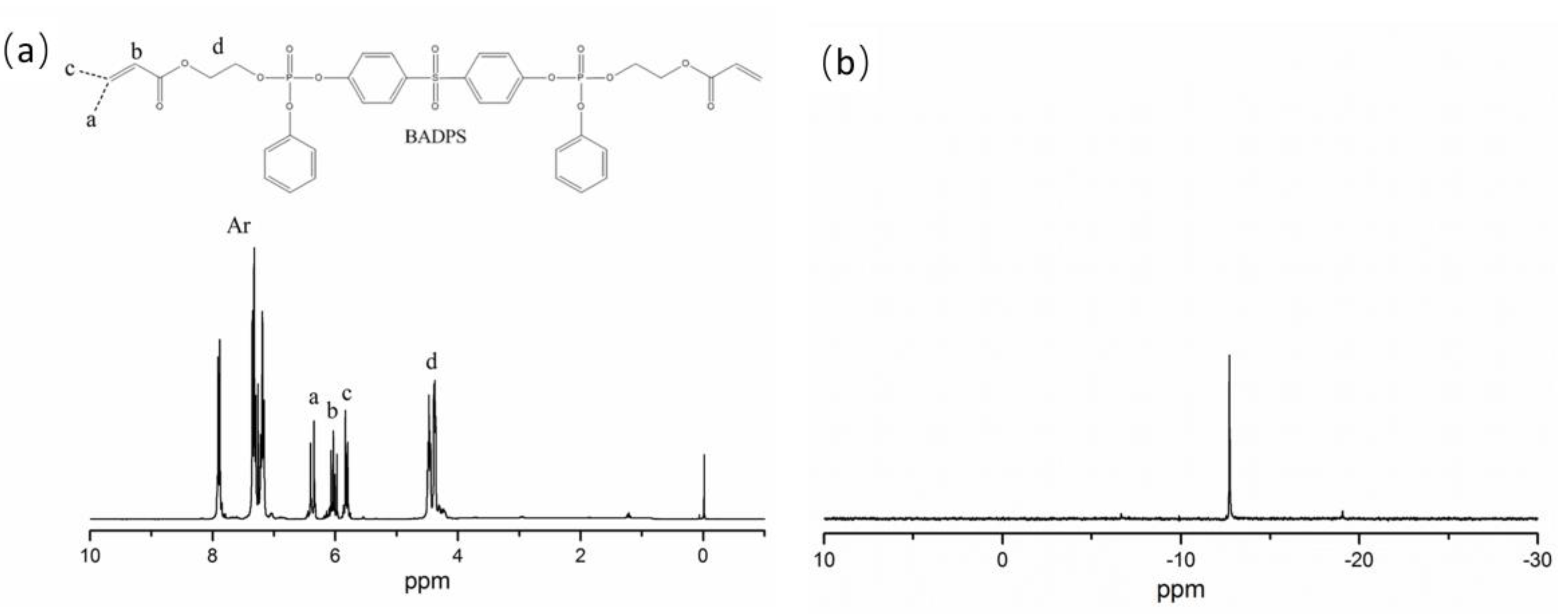
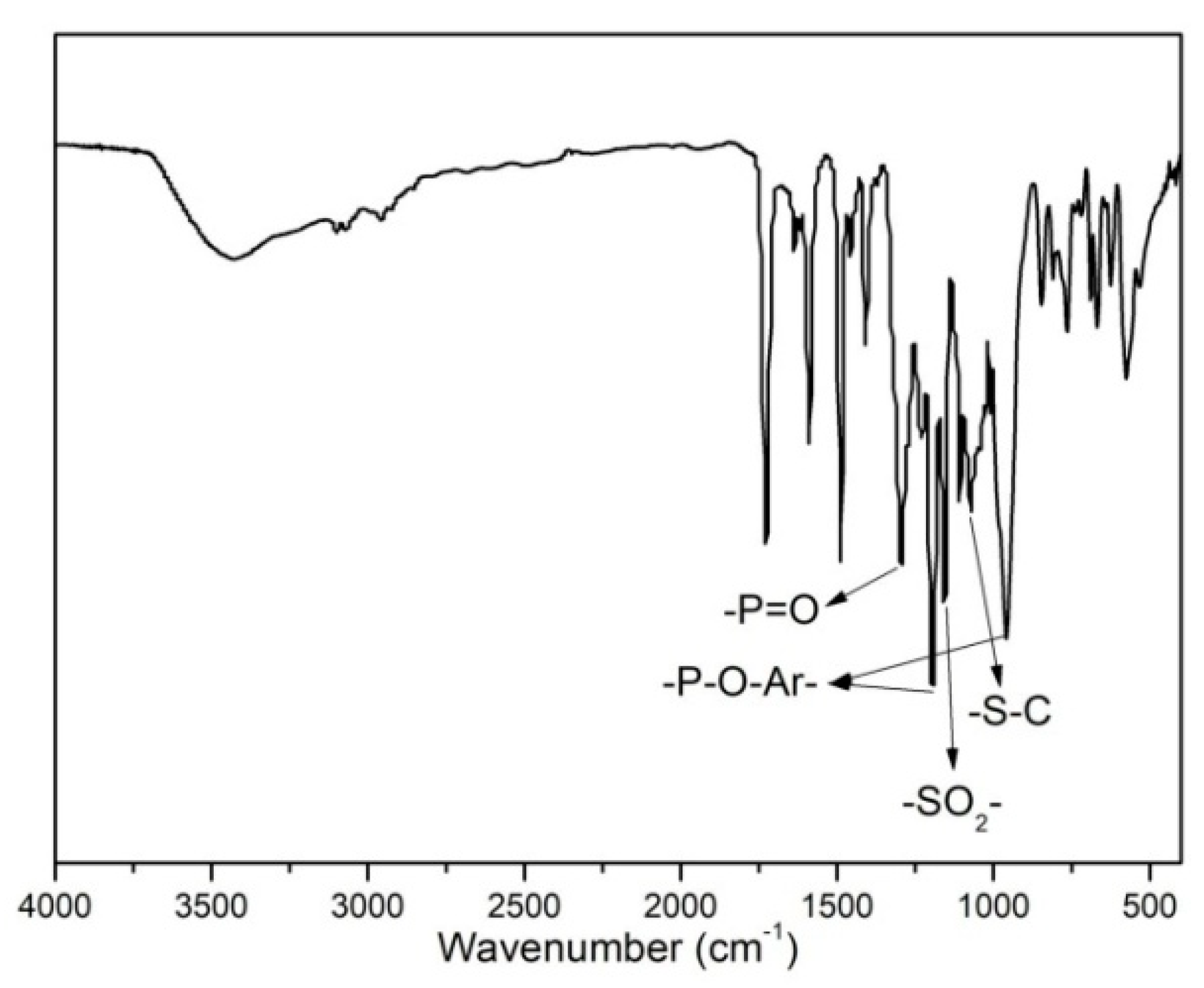
3.1. Structural Characterization of BADPS
3.2. Mechanical Properties
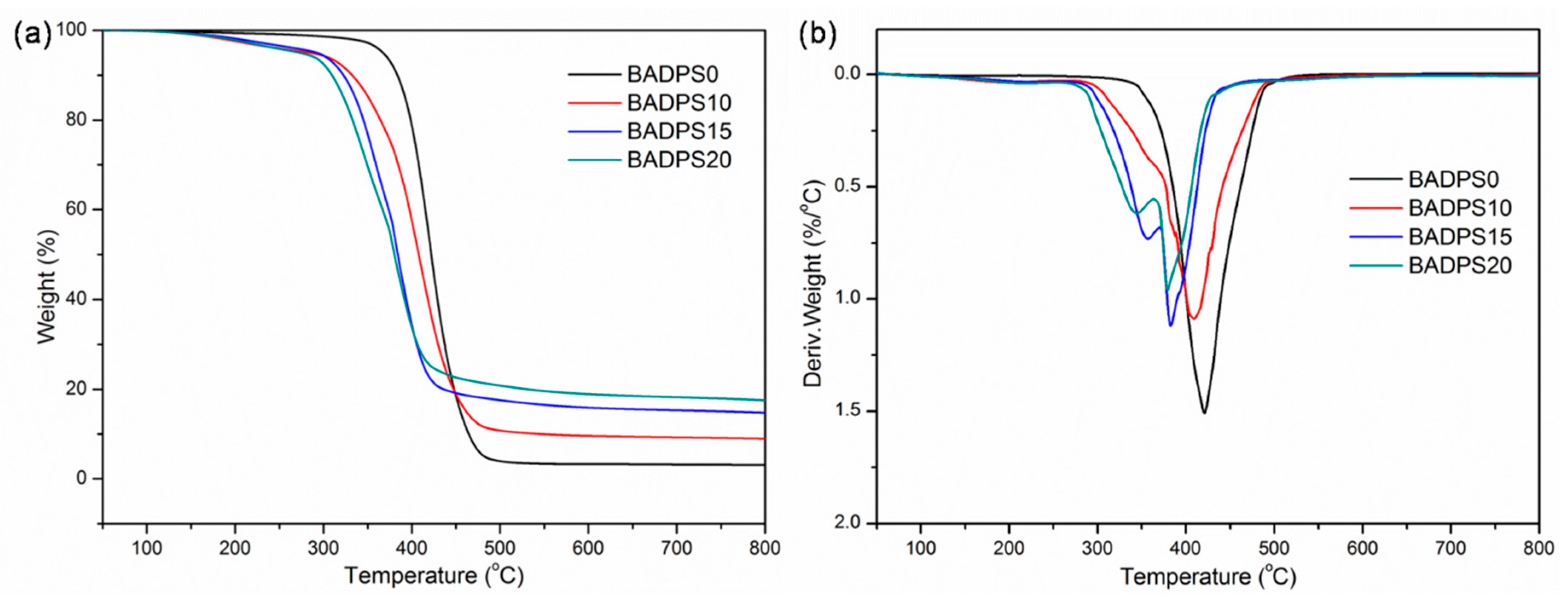
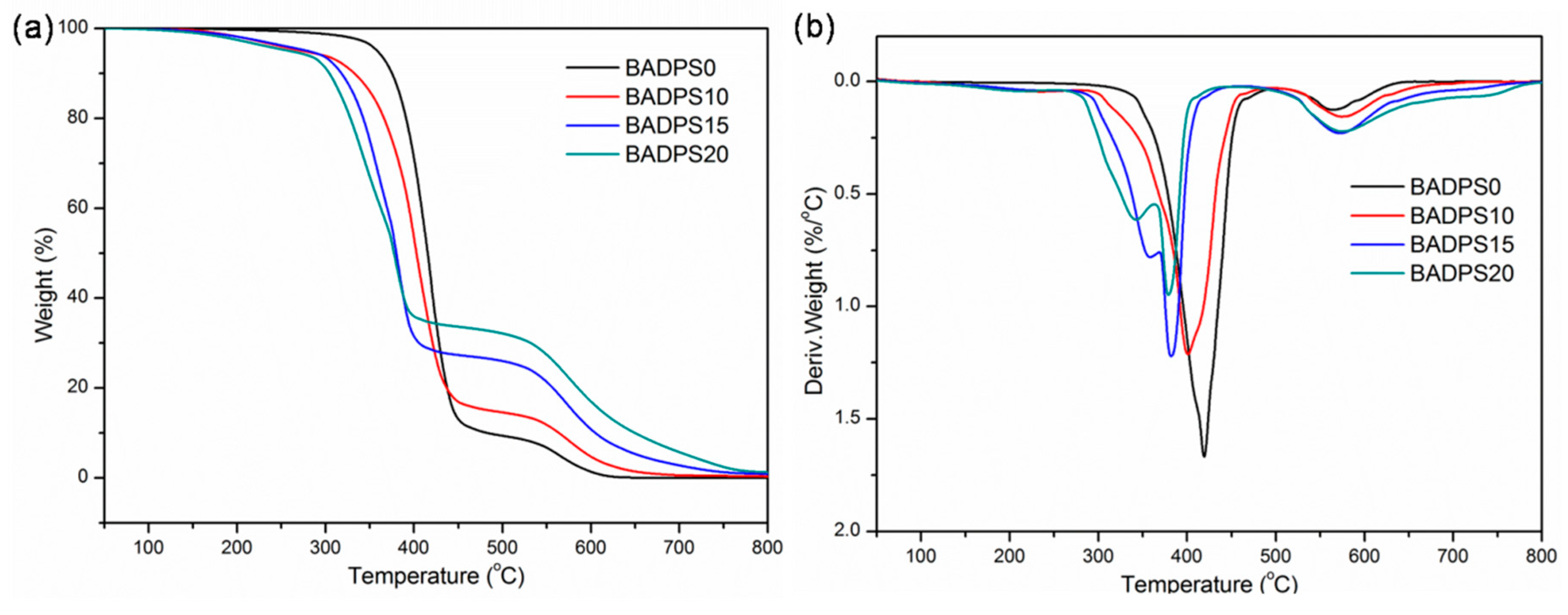
3.3. Thermal Properties
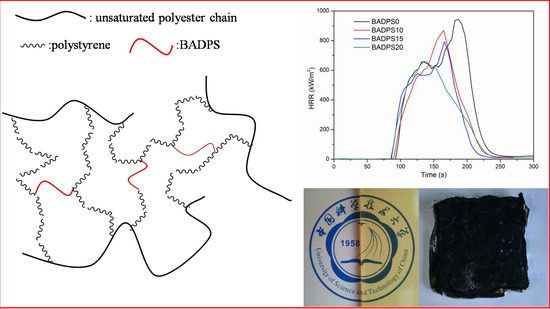
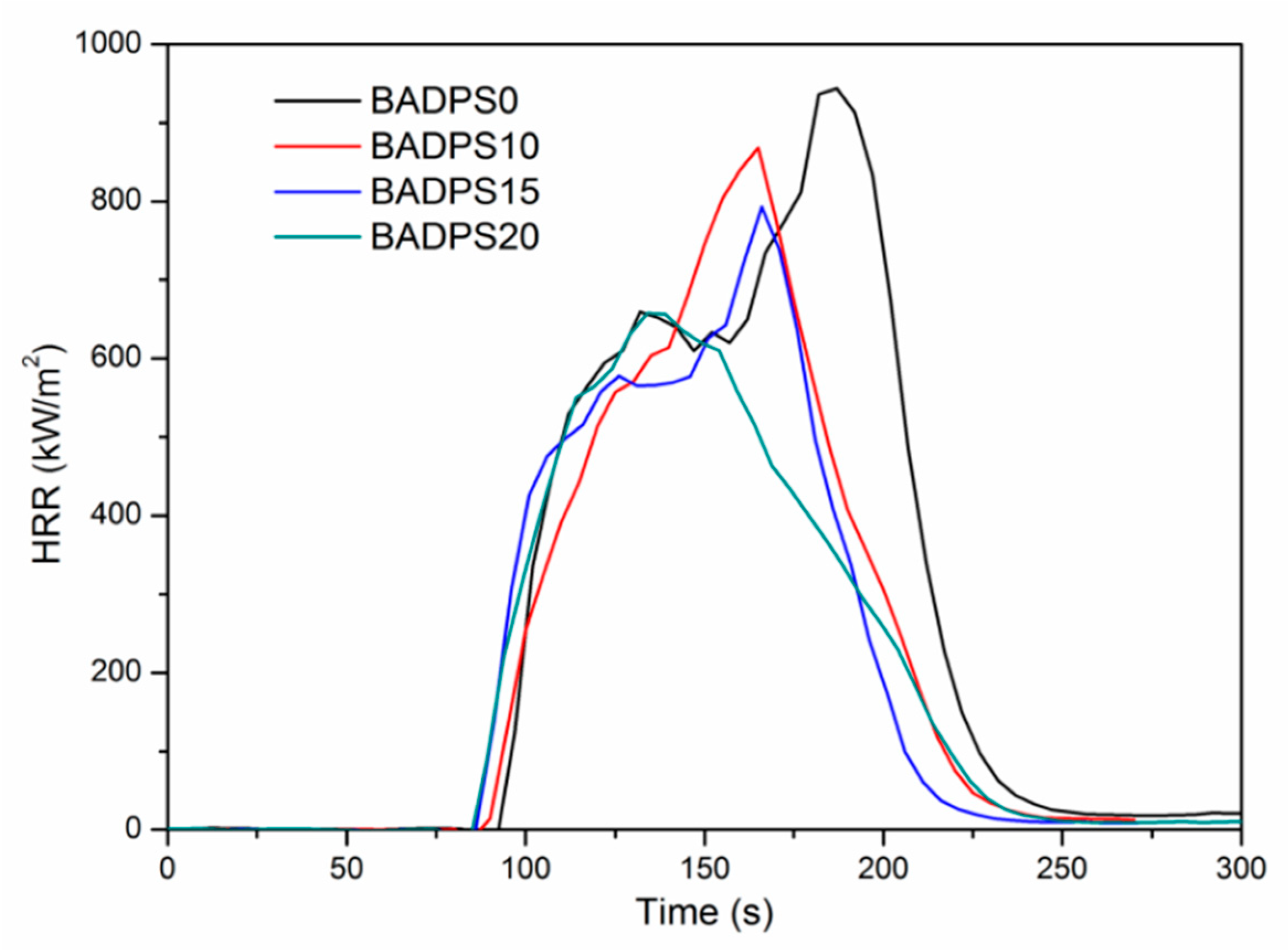
3.4. Flame Retardancy
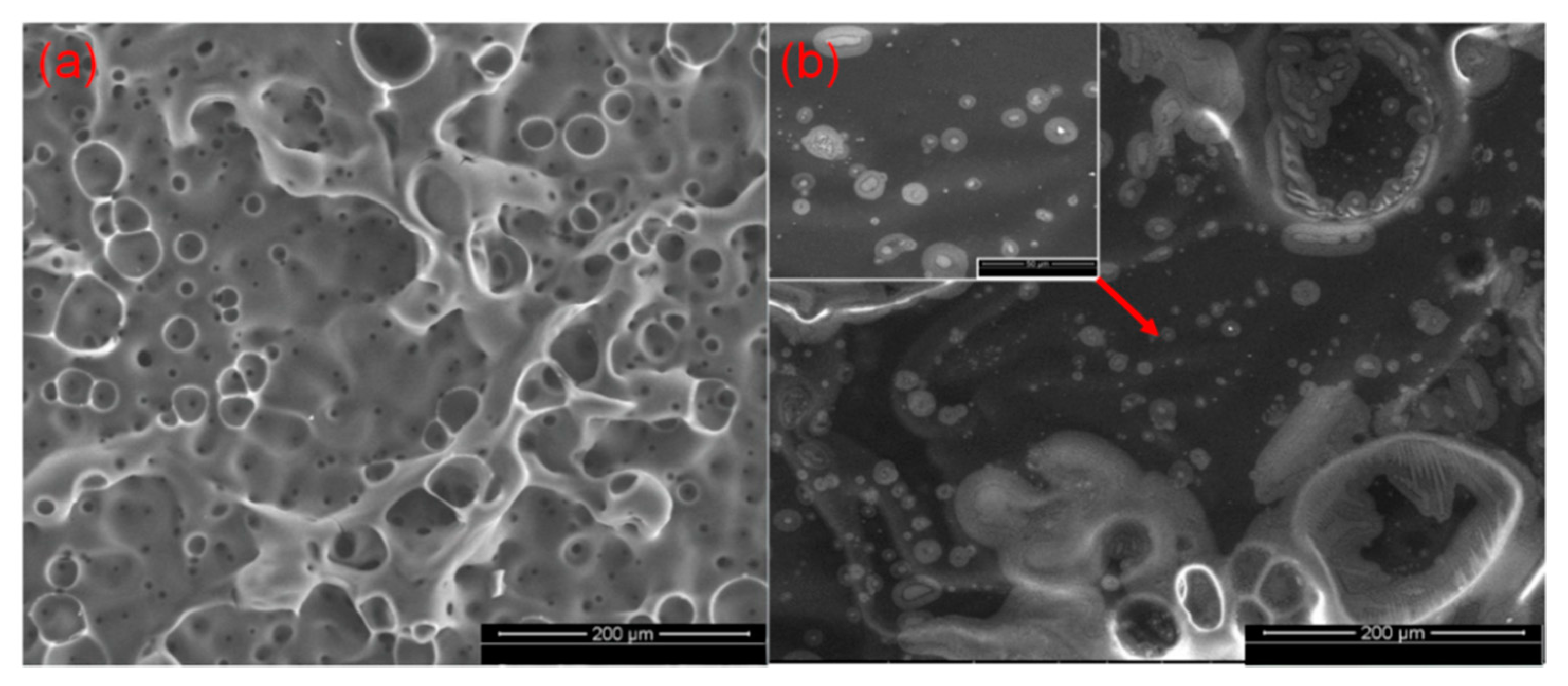
3.5. Char Residue Morphological Study and Spectroscopic and Element Analyses
3.6. TG-FTIR Analysis of the Volatiles
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ruan, T.; Wang, Y.; Wang, C.; Wang, P.; Fu, J.; Yin, Y.; Qu, G.; Wang, T.; Jiang, G. Identification and evaluation of a novel heterocyclic brominated flame retardant tris(2,3-dibromopropyl) isocyanurate in environmental matrices near a manufacturing plant in Southern China. Environ. Sci. Technol. 2009, 43, 3080–3086. [Google Scholar] [CrossRef] [PubMed]
- Birnbaum, L.S.; Staskal, D.F. Brominated flame retardants: Cause for concern? Environ. Health Perspect. 2004, 112, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Kicko-Walczak, E. New generation of fire retardant polyester resins. Macromol.Symp. 2003, 199, 343–350. [Google Scholar] [CrossRef]
- Ku, E.; Cichy, B.; Kicko-Walczak, E.; Rymarz, G.Z. Rheological and fire properties of a composite of unsaturated polyester resin and halogen-free flame retardants. J. Appl. Polym. Sci. 2016, 134. [Google Scholar] [CrossRef]
- Wazarkar, K.; Kathalewar, M.; Sabnis, A. Reactive modification of thermoplastic and thermoset polymers using flame retardants: An overview. Polym.Plast. Technol. Eng. 2016, 55, 71–91. [Google Scholar] [CrossRef]
- Braun, U.; Knoll, U.; Schartel, B.; Hoffmann, T.; Pospiech, D.; Artner, J.; Ciesielski, M.; Doring, M.; Perez-Gratero, R.; Sandler, J.K.W.; et al. Novel phosphorus-containing poly(ether sulfone)s and their blends with an epoxy resin: Thermal decomposition and fire retardancy. Macromol. Chem. Phys. 2006, 207, 1501–1514. [Google Scholar] [CrossRef]
- Alongi, J.; Han, Z.; Bourbigot, S. Intumescence: Tradition versus novelty. A comprehensive review. Prog. Polym. Sci. 2015, 51, 28–73. [Google Scholar] [CrossRef]
- Song, P.A.; Shen, Y.; Du, B.X.; Peng, M.; Shen, L.; Fang, Z.P. Effects of reactive compatibilization on the morphological, thermal, mechanical, and rheological properties of intumescent flame-retardant polypropylene. ACS Appl. Mater. Interfaces 2009, 1, 452–459. [Google Scholar] [CrossRef]
- Wu, J.N.; Qin, Z.H.; Chen, L.; Liu, B.W.; Wang, X.L.; Wang, Y.Z. Tailoring schiff base cross-linking by cyano group toward excellent flame retardancy, anti-dripping and smoke suppression of PET. Polymer 2018, 153, 78–85. [Google Scholar] [CrossRef]
- Sarychev, I.A.; Sirotin, I.S.; Borisov, R.S.; Mu, J.X.; Sokolskaya, I.B.; Bilichenko, J.V.; Filatov, S.N.; Kireev, V.V. Synthesis of resorcinol-based phosphazene-containing epoxy oligomers. Polymers 2019, 11, 614. [Google Scholar] [CrossRef]
- Kandare, E.; Kandola, B.K.; Price, D.; Nazare, S.; Horrocks, R.A. Study of the thermal decomposition of flame-retarded unsaturated polyester resins by thermogravimetric analysis and Py-GC/MS. Polym. Degrad. Stab. 2008, 93, 1996–2006. [Google Scholar] [CrossRef]
- Hu, W.Z.; Zhan, J.; Hong, N.N.; Hull, T.R.; Stec, A.A.; Song, L.; Wang, J.; Hu, Y. Flame retardant polystyrene copolymers: Preparation, thermal properties, and fire toxicities. Polym. Adv. Technol. 2014, 25, 631–637. [Google Scholar] [CrossRef]
- Tian, C.; Xu, T.C.; Zhang, L.F.; Cheng, Z.P.; Zhu, X.L. RAFT copolymerization of a phosphorus-containing monomer with alpha-hydroxy phosphonate and methyl methacrylate. RSC Adv. 2016, 6, 34659–34665. [Google Scholar] [CrossRef]
- Chiu, S.H.; Wu, C.L.; Lee, H.T.; Gu, J.H.; Suen, M.C. Synthesis and characterization of novel flame retardant polyurethanes containing designed phosphorus units. J. Polym. Res. 2016, 23, 205. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, X.L.; Zhang, W.Q.; Yin, X.-W.; Shi, Y.Q.; Wang, Y.Z. Bi-DOPO structure flame retardants with or without reactive group: Their effects on thermal stability and flammability of unsaturated polyester. Ind. Eng.Chem.Res. 2017, 56, 5913–5924. [Google Scholar] [CrossRef]
- Lin, Y.; Jiang, S.; Gui, Z.; Li, G.; Shi, X.; Chen, G.; Peng, X. Synthesis of a novel highly effective flame retardant containing multivalent phosphorus and its application in unsaturated polyester resins. RSC Adv. 2016, 6, 86632–86639. [Google Scholar] [CrossRef]
- Zhang, C.; Huang, J.Y.; Liu, S.M.; Zhao, J.Q. The synthesis and properties of a reactive flame-retardant unsaturated polyester resin from a phosphorus-containing diacid. Polym. Adv. Technol. 2011, 22, 1768–1777. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, K.; Xu, J. Synthesis and characterizations of novel phosphorous-nitrogen containing poly(ether sulfone)s. Polym. Degrad. Stab. 2011, 96, 197–203. [Google Scholar] [CrossRef]
- Lin, C.H.; Chang, S.L.; Wei, T.P. High-Tg Transparent poly(ether sulfone)s based on phosphinated bisphenols. Macromol. Chem. Phys. 2011, 212, 455–464. [Google Scholar] [CrossRef]
- Hou, S.; Zhang, Y.J.; Jiang, P. Phosphonium sulfonates as flame retardants for polycarbonate. Polym. Degrad. Stab. 2016, 130, 165–172. [Google Scholar] [CrossRef]
- Zhao, W.; Liu, J.P.; Peng, H.; Liao, J.Y.; Wang, X.J. Synthesis of a novel PEPA-substituted polyphosphoramide with high char residues and its performance as an intumescent flame retardant for epoxy resins. Polym. Degrad. Stab. 2015, 118, 120–129. [Google Scholar] [CrossRef]
- Tai, Q.; Chen, L.; Song, L.; Nie, S.; Hu, Y.; Yuen, R.K.K. Preparation and thermal properties of a novel flame retardant copolymer. Polym. Degrad. Stab. 2010, 95, 830–836. [Google Scholar] [CrossRef]
- Price, D.; Bullett, K.J.; Cunliffe, L.K.; Hull, T.R.; Milnes, G.J.; Ebdon, J.R.; Hunt, B.J.; Joseph, P. Cone calorimetry studies of polymer systems flame retarded by chemically bonded phosphorus. Polym. Degrad. Stab. 2005, 88, 74–79. [Google Scholar] [CrossRef]
- Dai, K.; Song, L.; Yuen, R.K.K.; Jiang, S.; Pan, H.; Hu, Y. Enhanced properties of the incorporation of a novel reactive phosphorus- and sulfur-containing flame-retardant monomer into unsaturated polyester resin. Ind. Eng. Chem. Res. 2012, 51, 15918–15926. [Google Scholar] [CrossRef]
- Wang, H.; Xu, S.; Shi, W. Photopolymerization behaviors of hyperbranched polyphosphonate acrylate and properties of the UV cured film. Prog. Org. Coat. 2009, 65, 417–424. [Google Scholar] [CrossRef]
- Liaw, D.J. Synthesis of sulfone-containing polyphosphates: Low temperature solution polycondensation of bisphenol S analogues and aryl phosphorodichlorides. Polym. Degrad. Stab. 1997, 55, 301–308. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, H.; Wang, M.; Liao, C.; Zhang, J. Thermal degradation behavior and mechanism of polybenzoxazine based on bisphenol-S and methylamine. J. Therm. Anal. Calorim. 2013, 112, 1213–1219. [Google Scholar] [CrossRef]
- Shah, D.; Maiti, P.; Gunn, E.; Schmidt, D.F.; Jiang, D.D.; Batt, C.A.; Giannelis, E.R. Dramatic enhancements in toughness of polyvinylidene fluoride nanocomposites via nanoclay-directed crystal structure and morphology. Adv. Mater. 2004, 16, 1173–1177. [Google Scholar] [CrossRef]
- Zulfiqar, S.; Ahmad, Z.; Sarwar, M.I. Soluble aromatic polyamide bearing ether linkages: Synthesis and characterization. Colloid Poly. Sci. 2007, 285, 1749–1754. [Google Scholar] [CrossRef]
- Carlier, V.; Devaux, J.; Legras, R.; Mcgrail, P.T. Percentage of rigid chain-length, a new concept for predicting glass-transition temperatures and melting-points of poly(aryl ether ketone)s and poly(aryl ether sulfone)s. Macromolecules 1992, 25, 6646–6650. [Google Scholar] [CrossRef]
- Tenbrinke, G.; Karasz, F.E.; Ellis, T.S. Depression of glass-transition temperatures of polymer networks by diluents. Macromolecules 1983, 16, 244–249. [Google Scholar] [CrossRef][Green Version]
- Benin, V.; Durganala, S.; Morgan, A.B. Synthesis and flame retardant testing of new boronated and phosphonated aromatic compounds. J. Mater. Chem. 2012, 22, 1180–1190. [Google Scholar] [CrossRef]
- Kandola, B.K.; Horrocks, A.R.; Myler, P.; Blair, D. New developments in flame retardancy of glass-reinforced epoxy composites. J. Appl. Polym. Sci. 2003, 88, 2511–2521. [Google Scholar] [CrossRef]
- Braun, U.; Balabanovich, A.I.; Schartel, B.; Knoll, U.; Artner, J.; Ciesielski, M.; Doring, M.; Perez, R.; Sandler, J.K.W.; Altstadt, V.; et al. Influence of the oxidation state of phosphorus on the decomposition and fire behaviour of flame-retarded epoxy resin composites. Polymer 2006, 47, 8495–8508. [Google Scholar] [CrossRef]
- Levchik, S.V.; Weil, E.D. Overview of recent developments in the flame retardancy of polycarbonates. Polym. Int. 2005, 54, 981–998. [Google Scholar] [CrossRef]
- Wang, B.B.; Tang, Q.B.; Hong, N.N.; Song, L.; Wang, L.; Shi, Y.Q.; Hu, Y. Effect of cellulose acetate butyrate microencapsulated ammonium polyphosphate on the flame retardancy, mechanical, electrical, and thermal properties of intumescent flame-retardant ethylene-vinyl acetate copolymer/microencapsulated ammonium polyphosphate/polyamide-6 blends. ACS Appl. Mater. Inter. 2011, 3, 3754–3761. [Google Scholar]
- Schartel, B.; Hull, T.R. Development of fire-retarded materials—Interpretation of cone calorimeter data. Fire Mater. 2007, 31, 327–354. [Google Scholar] [CrossRef]
- Gilman, J.W.; Bourbigot, S.; Shields, J.R.; Nyden, M.; Kashiwagi, T.; Davis, R.D.; Vanderhart, D.L.; Demory, W.; Wilkie, C.A.; Morgan, A.B.; et al. High throughput methods for polymer nanocomposites research: Extrusion, NMR characterization and flammability property screening. J. Mater. Sci. 2003, 38, 4451–4460. [Google Scholar] [CrossRef]
- Ma, H.Y.; Tong, L.F.; Xu, Z.B.; Fang, Z.P. Functionalizing carbon nanotubes by grafting on intumescent flame retardant: Nanocomposite synthesis, morphology, rheology, and flammability. Adv. Funct. Mater. 2008, 18, 414–421. [Google Scholar] [CrossRef]
- Sadezky, A.; Muckenhuber, H.; Grothe, H.; Niessner, R.; Poschl, U. Raman micro spectroscopy of soot and related carbonaceous materials: Spectral analysis and structural information. Carbon 2005, 43, 1731–1742. [Google Scholar] [CrossRef]
- Bourbigot, S.; LeBras, M.; Delobel, R.; Tremillon, J.M. Synergistic effect of zeolite in an intumescence process-study of the interactions between the polymer and the additives. J. Chem. Soc. Faraday Trans. 1996, 92, 3435–3444. [Google Scholar] [CrossRef]
- Orhan, T.; Ates, S.; Hacaloglu, J.; Yagci, Y. Thermal degradation characteristics of polysulfones with benzoxazine end groups. J. Anal. Appl. Pyrol. 2012, 94, 146–152. [Google Scholar] [CrossRef]
- Lakshmi, R.T.S.M.; Kumari, R.; Varma, I.K. Structure and thermal characterisation of poly(arylene ether sulphone)s. J. Therm. Anal. Calorim. 2004, 78, 809–819. [Google Scholar] [CrossRef]



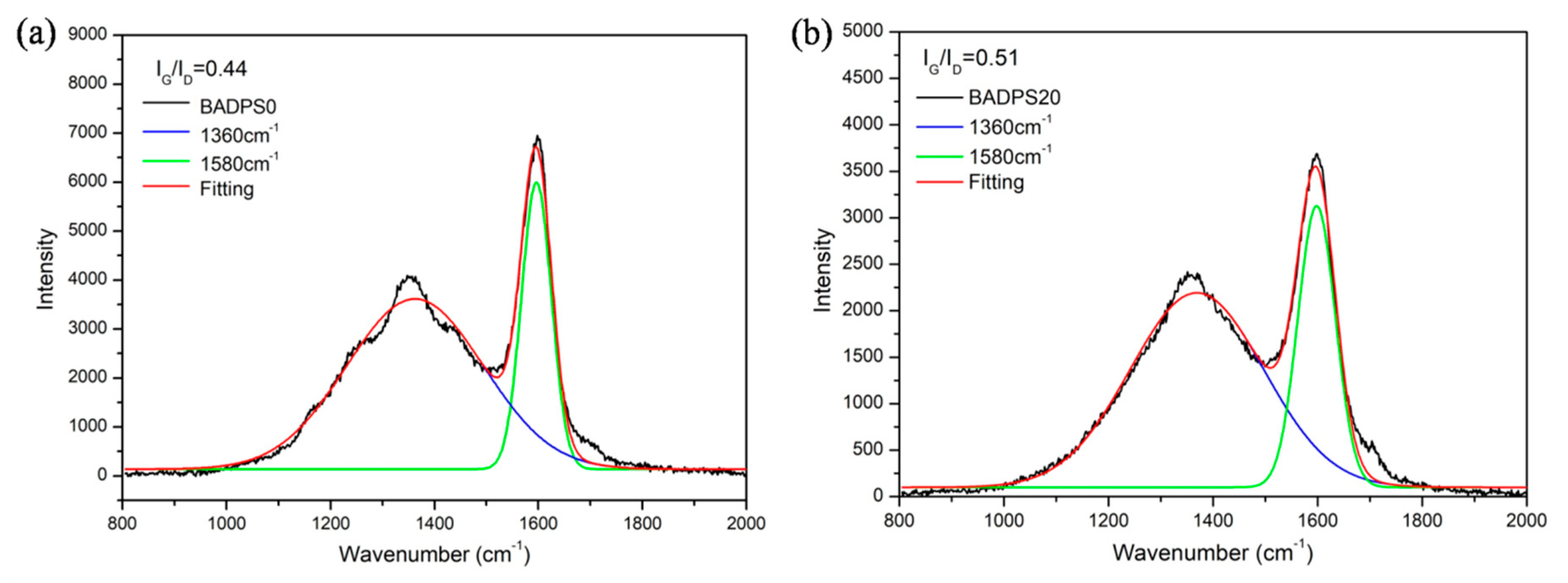
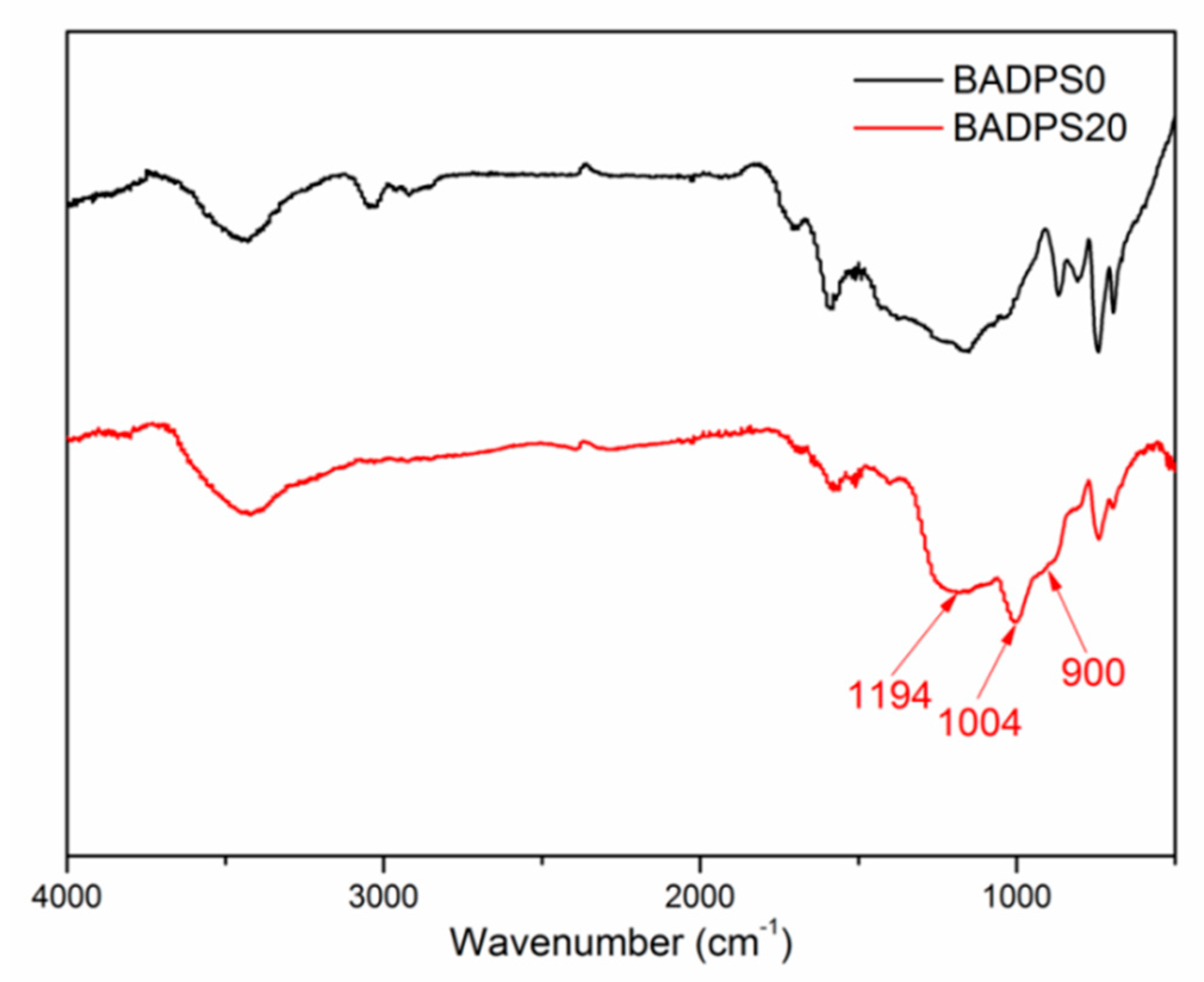
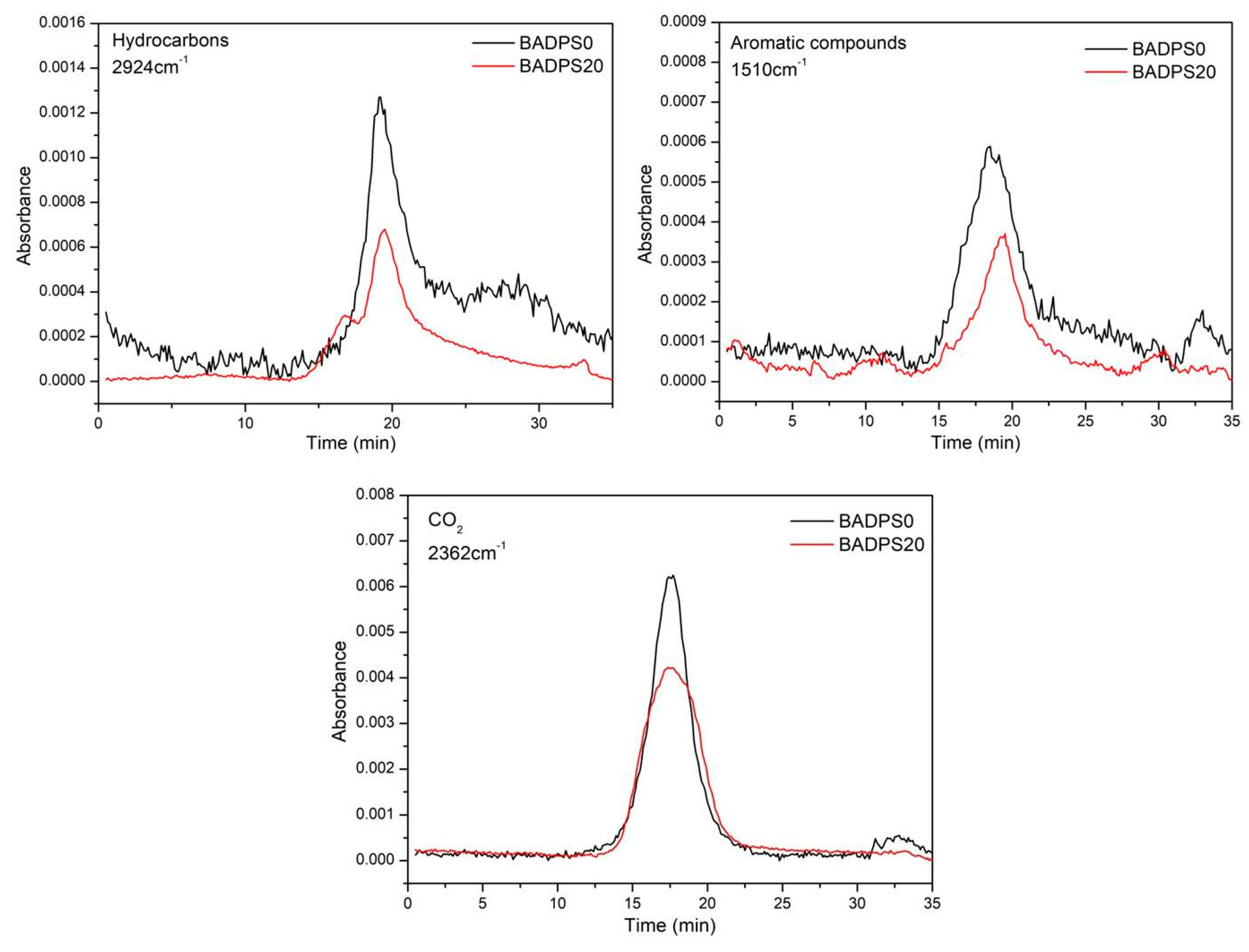
| Sample | Tensile Strength (MPa) | Young’s Modulus (MPa) | Elongation at Break (%) | Toughness |
|---|---|---|---|---|
| BADPS0 | 33.62 ± 3.13 | 3062 ± 97 | 1.35 ± 0.17 | 24.6 ± 2.9 |
| BADPS10 | 47.49 ± 2.58 | 2737 ± 89 | 3.50 ± 0.33 | 109.7 ± 2.7 |
| BADPS15 | 45.42 ± 3.10 | 2657 ± 116 | 3.57 ± 0.27 | 106.3 ± 3.2 |
| BADPS20 | 37.11 ± 2.11 | 2371 ± 78 | 3.85 ± 0.21 | 101.1 ± 2.4 |
| Sample | Tg (°C) | T0.1 (°C) | Tmax (°C) | MMLR (%/°C) | Char (500 °C, wt%) | Char (700 °C, wt%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Air | N2 | Air | N2 | Air | N2 | Air | N2 | Air | N2 | ||
| BADPS0 | 150.6 | 375 | 383 | 419/564 | 421 | 1.67 | 1.51 | 9.4 | 4.0 | 0 | 3.3 |
| BADPS10 | 152.7 | 331 | 333 | 401/574 | 409 | 1.21 | 1.09 | 14.6 | 10.8 | 0.6 | 9.3 |
| BADPS15 | 154.6 | 318 | 322 | 358/382/573 | 357/383 | 1.22 | 1.12 | 26.0 | 17.6 | 2.8 | 15.3 |
| BADPS20 | 155.8 | 304 | 309 | 342/379/575 | 344/380 | 0.95 | 0.96 | 32.1 | 20.8 | 5.7 | 18.2 |
| Sample | BADPS Content (wt %) | LOI | TTI a (s) | PHRR (kW/m2) | THR (MJ/m2) | Mass Residue (%) | Mean EHC b (MJ/kg) |
|---|---|---|---|---|---|---|---|
| BADPS0 | 0 | 20.5 | 97 | 944 | 80 | 2.1 | 19.5 |
| BADPS10 | 10 | 24.5 | 95 | 869 | 66 | 3.7 | 16.6 |
| BADPS15 | 15 | 25.5 | 91 | 793 | 61 | 8.7 | 16.6 |
| BADPS20 | 20 | 26.5 | 89 | 657 | 58 | 9.5 | 16.5 |
| Element Content | BADPS0 | BADPS20 |
|---|---|---|
| P (%) | 0 | 11.6 |
| S (%) | 0 | 0.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, K.; Deng, Z.; Liu, G.; Wu, Y.; Xu, W.; Hu, Y. Effects of a Reactive Phosphorus–Sulfur Containing Flame-Retardant Monomer on the Flame Retardancy and Thermal and Mechanical Properties of Unsaturated Polyester Resin. Polymers 2020, 12, 1441. https://doi.org/10.3390/polym12071441
Dai K, Deng Z, Liu G, Wu Y, Xu W, Hu Y. Effects of a Reactive Phosphorus–Sulfur Containing Flame-Retardant Monomer on the Flame Retardancy and Thermal and Mechanical Properties of Unsaturated Polyester Resin. Polymers. 2020; 12(7):1441. https://doi.org/10.3390/polym12071441
Chicago/Turabian StyleDai, Kang, Zhenzhen Deng, Guyue Liu, Yutong Wu, Wenbin Xu, and Yuan Hu. 2020. "Effects of a Reactive Phosphorus–Sulfur Containing Flame-Retardant Monomer on the Flame Retardancy and Thermal and Mechanical Properties of Unsaturated Polyester Resin" Polymers 12, no. 7: 1441. https://doi.org/10.3390/polym12071441
APA StyleDai, K., Deng, Z., Liu, G., Wu, Y., Xu, W., & Hu, Y. (2020). Effects of a Reactive Phosphorus–Sulfur Containing Flame-Retardant Monomer on the Flame Retardancy and Thermal and Mechanical Properties of Unsaturated Polyester Resin. Polymers, 12(7), 1441. https://doi.org/10.3390/polym12071441