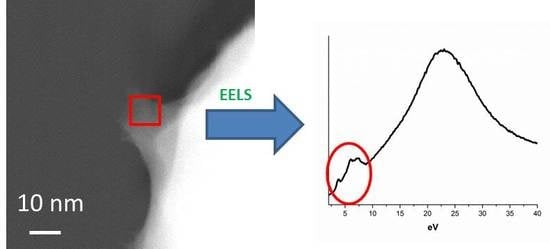
Reliable Characterization of Organic & Pharmaceutical Compounds with High Resolution Monochromated EEL Spectroscopy
Abstract
1. Introduction
2. Materials and Specimen Preparation/Instrumental Configuration—Data Collection
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Censi, R.; Di Martino, P. Polymorph Impact on the Bioavailability and Stability of Poorly Soluble Drugs. Molecules 2015, 20, 18759–18776. [Google Scholar] [CrossRef] [PubMed]
- Savjani, K.T.; Gajjar, A.K.; Savjani, J.K. Drug Solubility: Importance and Enhancement Techniques. ISRN Pharm. 2012, 20, 195727–195736. [Google Scholar] [CrossRef] [PubMed]
- Haleblian, J.; McCrone, W. Pharmaceutical applications of polymorphism. J. Pharm. Sci. 1969, 58, 911–929. [Google Scholar] [CrossRef] [PubMed]
- Bauer, J.; Spanton, S.; Henry, R.; Quick, J.; Dziki, W.; Porter, W.; Morris, J. Ritonavir: An Extraordinary Example of Conformational Polymorphism. Pharm. Res. 2001, 18, 859–866. [Google Scholar] [CrossRef] [PubMed]
- Morissette, S.L.; Soukasene, S.; Levinson, D.; Cima, M.J.; Almarsson, O. Elucidation of Crystal Form Diversity of the HIV Protease Inhibitor Ritonavir By High-Throughput Crystallization. Proc. Natl. Acad. Sci. USA 2003, 100, 2180–2184. [Google Scholar] [CrossRef] [PubMed]
- Aaltonen, J.; Alleso, M.; Mirza, S.; Koradia, V.; Gordon, K.C.; Rantanen, J. Solid form screening—A review. Eur. J. Pharm. Biopharm. 2009, 71, 23–37. [Google Scholar] [CrossRef] [PubMed]
- Newman, A. X-ray Powder Diffraction in Solid Form Screening and Selection. Am. Pharm. Rev. 2011, 14, 44–51. [Google Scholar]
- Rozo, J.I.; Zarow, A.; Zhou, B.; Pinal, R.; Iqbal, Z.; Romanach, R. Complementary Near-Infrared and Raman Chemical Imaging of Pharmaceutical Thin Films. J. Pharm. Sci. 2011, 100, 4888–4895. [Google Scholar] [CrossRef]
- Van Eerdenbrugh, B.; Lo, M.; Kjoller, K.; Marcott, C.; Taylor, L.S. Nanoscale Mid-Infrared Imaging of Phase Separation in A Drug–Polymer Blend. J. Pharm. Sci. 2012, 101, 2066–2073. [Google Scholar] [CrossRef]
- Berendt, R.T.; Sperger, D.M.; Munson, E.J. Solid-State NMR Spectroscopy in Pharmaceutical Research and Analysis. Trends Anal. Chem. 2006, 25, 977–984. [Google Scholar] [CrossRef]
- Byrn, S.R.; Zografi, G.; Chen, X. Solid State Properties of Pharmaceutical Materials, 1st ed.; Wiley: New York, NY, USA, 2017. [Google Scholar] [CrossRef]
- Eddleston, M.D.; Bithell, E.G.; Jones, W. Transmission Electron Microscopy of Pharmaceutical Materials. J. Pharm. Sci. 2010, 99, 4072–4083. [Google Scholar] [CrossRef] [PubMed]
- Eddleston, M.D.; Hejczyk, K.E.; Bithell, E.G.; Day, G.M.; Jones, W. Polymorph Identification and Crystal Structure Determination by a Combined Crystal Structure Prediction and Transmission Electron Microscopy Approach. Chem. Eur. J. 2013, 19, 7874–7882. [Google Scholar] [CrossRef]
- Eddleston, M.D.; Hejczyk, K.E.; Bithell, E.G.; Day, G.M.; Jones, W. Determination of the Crystal Structure of a New Polymorph of Theophylline. Chem. Eur. J. 2013, 19, 7883–7888. [Google Scholar] [CrossRef]
- Li, Z.G.; Harlow, R.L.; Foris, C.M.; Li, H.; Ma, P.; Vickery, R.D.; Maurin, M.B.; Toby, B.H. New Applications of Electron Diffraction in the Pharmaceutical Industry: Polymorph Determination by Using a Combination of Electron Diffraction and Synchrotron X-ray Powder Diffraction Techniques. Mircosc. Mircroanal. 2002, 8, 134–138. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.G.; Harlow, R.L.; Foris, C.M.; Li, H.; Ma, P.; Vickery, R.D.; Maurin, M.B.; Toby, B.H. Polymorph Determination for the gp Iib/Iiia Antagonist, Roxifiban, using a Combination of Electron Diffraction and Synchrotron X-Ray Powder Diffraction Techniques. J. Pharm. Sci. 1999, 88, 297–301. [Google Scholar] [CrossRef]
- Kolb, U.; Gorelik, T.E.; Kubel, C.; Otten, M.T.; Hubert, D. Towards Automated Diffraction Tomography: Part I-Data Acquisition. Ultramicroscopy 2007, 107, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Kolb, U.; Gorelik, T.E.; Mugnaioli, E.; Stewart, A. Structural Characterization of Organics Using Manual and Automated Electron Diffraction. Polym. Rev. 2010, 50, 385–409. [Google Scholar] [CrossRef]
- Gemmi, M.; Mugnaioli, E.; Gorelik, T.E.; Kolb, U.; Palatinus, L.; Boullay, P.; Hovmöller, S.; Abrahams, J.P. 3D Electron Diffraction: The Nanocrystallography Revolution. ACS Cent. Sci. 2019, 5, 1315–1329. [Google Scholar] [CrossRef]
- Van Genderen, E.; Clabbers, M.T.B.; Das, P.P.; Stewart, A.; Nederlof, I.; Barentsen, K.C.; Portillo, J.; Pannu, N.S.; Nicolopoulos, S.; Gruene, T.; et al. Ab Initio Structure Determination of Nanocrystals of Organic Pharmaceutical Compounds by Electron Diffraction at Room Temperature using a Timepix Quantum Area Direct Electron Detector. Acta Crystallogr. Sect. A. Found. Adv. 2016, 72, 236–242. [Google Scholar] [CrossRef]
- Das, P.P.; Mugnaioli, E.; Nicolopoulos, S.; Tossi, C.; Gemmi, M.; Galanis, A.; Borodi, G.; Pop, M.M. Crystal Structures of two Important Pharmaceuticals Solved by 3D Precession Electron Diffraction Tomography. Org. Process Res. Dev. 2018, 22, 1365–1372. [Google Scholar] [CrossRef]
- Newman, A. Pharmaceutical Amorphous Solid Dispersions; Wiley: New York, NY, USA, 2015; ISBN 978-1-118-45520-3. [Google Scholar]
- Gorelik, T.; Schmidt, M.; Kolb, U.; Billinge, S. Total-Scattering Pair-Distribution Function of Organic Material from Powder Electron Diffraction Data. Microsc. Microanal. 2015, 21, 459–471. [Google Scholar] [CrossRef] [PubMed]
- Ricarte, R.; Lodge, T.; Hillmyer, M. Nanoscale Concentration Quantification of Pharmaceutical Actives in Amorphous Polymer Matrices by Electron Energy-Loss Spectroscopy. Langmuir 2016, 32, 7411–7419. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Adams, W.W. Electron Beam Damage ιn High Temperature Polymers. Polymer 1990, 31, 15–19. [Google Scholar] [CrossRef]
- Williams, D.B.; Carter, C.B. Transmission Electron Microscopy: A Textbook for Materials Science; Springer: New York, NY, USA, 2004; ISBN 978-0-387-76501-3. [Google Scholar]
- Egerton, R.F. Scattering delocalization and radiation damage in STEM EELS. Ultramicroscopy 2017, 180, 115–124. [Google Scholar] [CrossRef] [PubMed]
- De la Peña, F.; Prestat, E.; Fauske, V.T.; Burdet, P.; Jokubauskas, P.; Nord, M.; Garmannslund, A. hyperspy/hyperspy: HyperSpy v1.5.2. Zndo. 2019. [Google Scholar] [CrossRef]
- Barlos, K.; Gatos, D.; Kallitsis, J.; Papaphotiou, G.; Sotiriou, P.; Wenqing, Y.; Schiller, W. Darstellung geschützter peptid-fragmente unter einsatz substituierter triphenylmethyl-harze. Tetrahedron Lett. 1989, 30, 3943. [Google Scholar] [CrossRef]
- Sheth, A.R.; Bates, S.; Muller, F.X.; Grant, D.J.W. Polymorphism in Piroxicam. Cryst. Growth Des. 2004, 4, 1091–1098. [Google Scholar] [CrossRef]
- Zeidan, T.A.; Trotta, J.T.; Tilak, P.A.; Olivera, M.A.; Chiarella, R.A.; Foxman, B.M.; Almarsson, O.; Hickey, M.B. An Unprecedented Case of Dodecamorphism: The Twelfth Polymorph of Aripiprazole Formed by Seeding with its Active Metabolite. CrystEngComm 2016, 18, 1486–1488. [Google Scholar] [CrossRef]
- Egerton, R.F. Electron Energy-Loss Spectroscopy in the Electron Microscope, 3rd ed.; Springer: New York, NY, USA, 2011; ISBN 978-1-4419-9582-7. [Google Scholar]
- Malis, T.; Cheng, S.C.; Egerton, R.F. EELS log ratio technique for specimen-thickness measurement in the TEM. J. Electron Microsc. Tech. 1988, 8, 193–200. [Google Scholar] [CrossRef]
- Blaha, P.; Schwarz, K.; Tran, F.; Laskowski, R.; Madsen, G.K.H.; Marks, L.D. WIEN2k: An APW+lo Program for Calculating the Properties of Solids. J. Chem. Phys. 2020, 152, 074101. [Google Scholar] [CrossRef] [PubMed]
- Hébert, C. Practical Aspects of Running the WIEN2k Code for Electron Spectroscopy. Micron 2007, 38, 12–28. [Google Scholar] [CrossRef] [PubMed]
- Hébert, C.; Luitz, J.; Schattschneider, P. Improvement of Energy Loss Near Edge Structure Calculation Using Wien2k. Micron 2003, 34, 219–225. [Google Scholar] [CrossRef]
- Blaha, P.; Schwarz, K.; Madsen, G.K.; Kvasnicka, D.; Luitz, J.; Laskowsk, R.; Tran, F.; Marks, L. An Augmented Plane Wave+Local Orbitals Program for Calculating Crystal Properties; Techn. Universitat Wien: Vienna, Austria, 2019; Volume 2. [Google Scholar]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Keast, V.J. Ab Initio Calculations of Plasmons and Interband Transitions in The Low-Loss Electron Energy-Loss Spectrum. J. Electron Spectrosc. Relat. Phenom. 2005, 143, 97–104. [Google Scholar] [CrossRef]
- Eljarrat, A.; Sastre, X.; Peiró, F.; Estradé, S. Density Functional Theory Modeling of Low-Loss Electron Energy-Loss Spectroscopy in Wurtzite III-Nitride Ternary Alloys. Microsc. Microanal 2016, 22, 706–716. [Google Scholar] [CrossRef]
- Štukelj, J.; Svanbäck, S.; Agopov, M.; Löbmann, K.; Strachan, C.J.; Rades, T.; Yliruusi, J. Direct Measurement of Amorphous Solubility. Anal. Chem. 2019, 91, 7411–7417. [Google Scholar] [CrossRef]
- Sun, Y.; Zhu, L.; Wu, T.; Cai, T.; Gunn, E.M.; Yu, L. Stability of Amorphous Pharmaceutical Solids: Crystal Growth Mechanisms and Effect of Polymer Additives. AAPS J. 2012, 14, 380–388. [Google Scholar] [CrossRef]
- Sun, S.Q.; Shi, S.-L.; Hunt, J.A.; Leapman, R.D. Quantitative Water Mapping of Cryosectioned Cells by Electron Energy-Loss Spectroscopy. J. Microsc. 1995, 177, 18–30. [Google Scholar] [CrossRef]
- Sousa, A.; Aitouchen, A.; Libera, M. Water Mapping in Hydrated Soft Materials. Ultramicroscopy 2006, 106, 130–145. [Google Scholar] [CrossRef]
- Yakovlev, S.; Misra, M.; Shi, S.; Firlar, E.; Libera, M. Quantitative Nanoscale Water Mapping in Frozen Hydrated Skin by Low-Loss Electron Energy-Loss Spectroscopy. Ultramicroscopy 2010, 110, 866–876. [Google Scholar] [CrossRef]
- Pennycook, S.J. High resolution electron microscopy and microanalysis. Contemp. Phys. 1982, 23, 371–400. [Google Scholar] [CrossRef]
- Qi, S.; Belton, P.; Nollenberger, K.; Gryczke, A.; Craig, D.Q. Compositional analysis of low quantities of phase separation in hot-melt-extruded solid dispersions: A combined atomic force microscopy, photothermal fourier-transform infrared microspectroscopy, and localised thermal analysis approach. Pharm. Res. 2011, 28, 2311–2326. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Taylor, L.S. Nanoscale Infrared, Thermal, and Mechanical Characterization of Telaprevir-Polymer Miscibility in Amorphous Solid Dispersions Prepared by Solvent Evaporation. Mol. Pharm. 2016, 13, 1123–1136. [Google Scholar] [CrossRef] [PubMed]
- Stöger-Pollach, M.; Franco, H.; Schattschneider, P.; Lazar, S.; Schaffer, B.; Grogger, W.; Zandbergen, H.W. Cerenkov losses: A limit for bandgap determination and Kramers-Kronig analysis. Micron 2006, 37, 396–402. [Google Scholar] [CrossRef] [PubMed]






© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Das, P.P.; Guzzinati, G.; Coll, C.; Gomez Perez, A.; Nicolopoulos, S.; Estrade, S.; Peiro, F.; Verbeeck, J.; Zompra, A.A.; Galanis, A.S. Reliable Characterization of Organic & Pharmaceutical Compounds with High Resolution Monochromated EEL Spectroscopy. Polymers 2020, 12, 1434. https://doi.org/10.3390/polym12071434
Das PP, Guzzinati G, Coll C, Gomez Perez A, Nicolopoulos S, Estrade S, Peiro F, Verbeeck J, Zompra AA, Galanis AS. Reliable Characterization of Organic & Pharmaceutical Compounds with High Resolution Monochromated EEL Spectroscopy. Polymers. 2020; 12(7):1434. https://doi.org/10.3390/polym12071434
Chicago/Turabian StyleDas, Partha Pratim, Giulio Guzzinati, Catalina Coll, Alejandro Gomez Perez, Stavros Nicolopoulos, Sonia Estrade, Francesca Peiro, Johan Verbeeck, Aikaterini A. Zompra, and Athanassios S. Galanis. 2020. "Reliable Characterization of Organic & Pharmaceutical Compounds with High Resolution Monochromated EEL Spectroscopy" Polymers 12, no. 7: 1434. https://doi.org/10.3390/polym12071434
APA StyleDas, P. P., Guzzinati, G., Coll, C., Gomez Perez, A., Nicolopoulos, S., Estrade, S., Peiro, F., Verbeeck, J., Zompra, A. A., & Galanis, A. S. (2020). Reliable Characterization of Organic & Pharmaceutical Compounds with High Resolution Monochromated EEL Spectroscopy. Polymers, 12(7), 1434. https://doi.org/10.3390/polym12071434