A Deep Insight into Different Acidic Additives as Doping Agents for Enhancing Proton Conductivity on Polybenzimidazole Membranes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
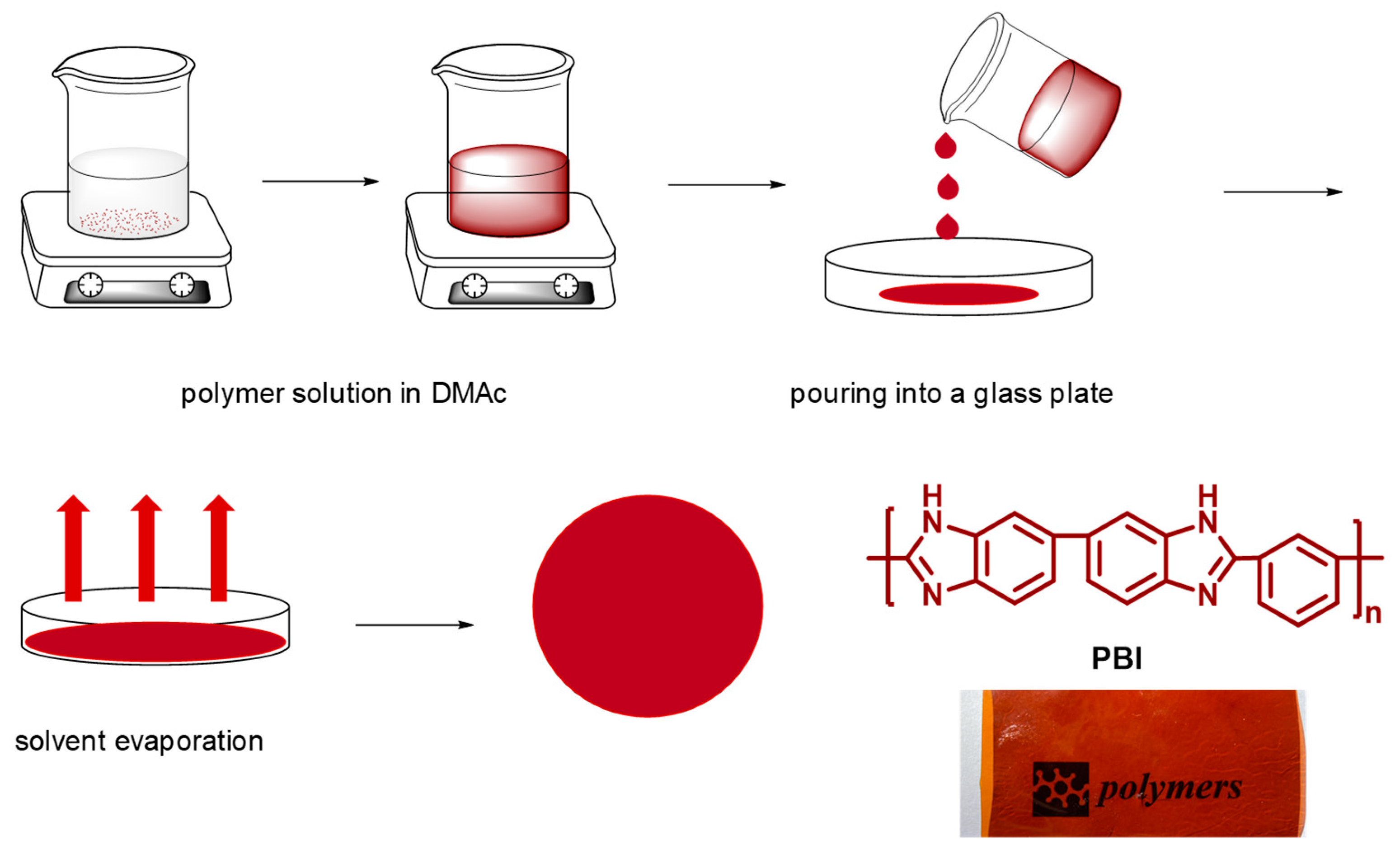
2.2. Membrane Preparation.
2.3. Membrane Characterization
3. Results and Discussion
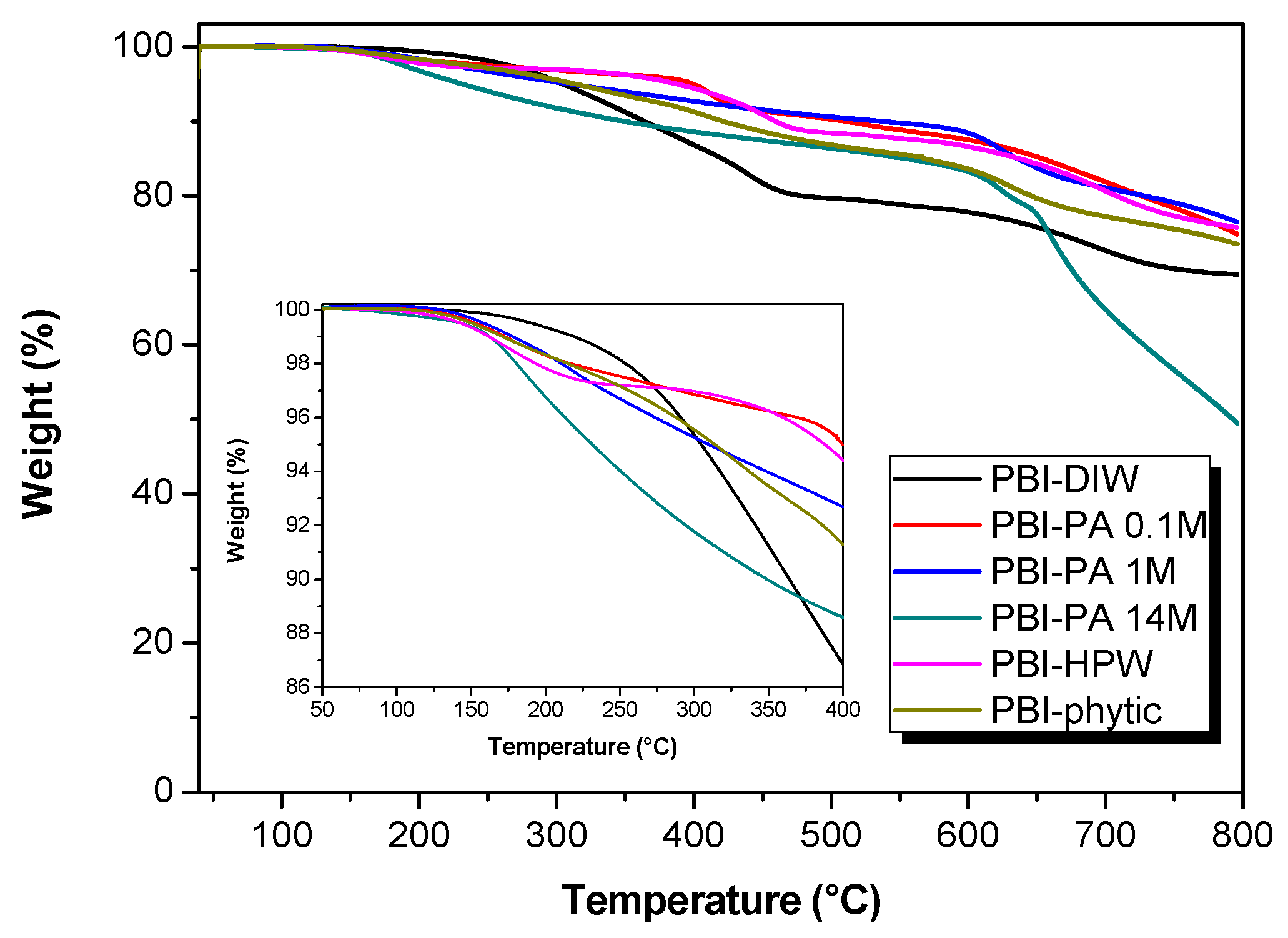
3.1. Membrane Preparation and Characterization
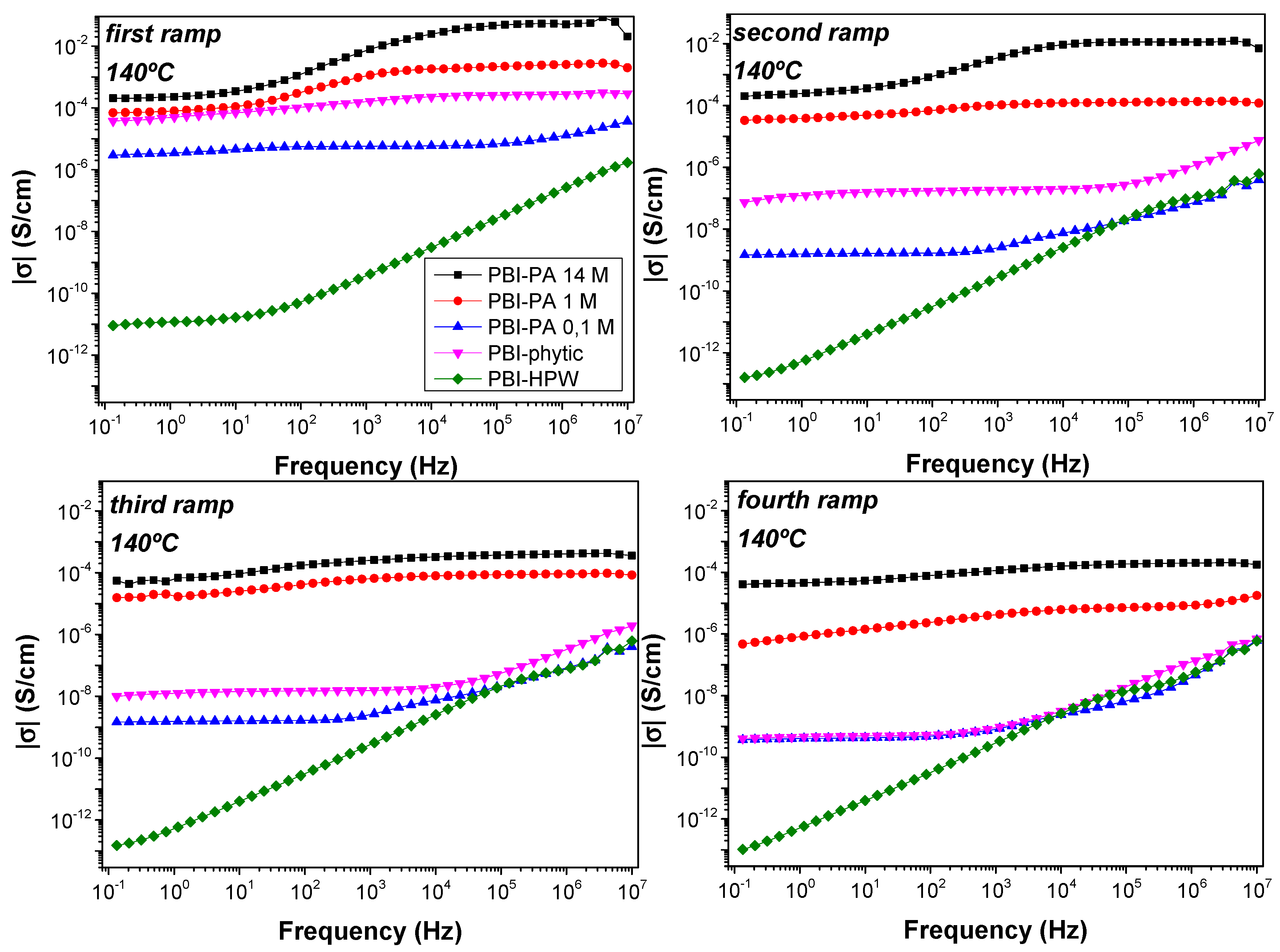
3.2. Proton Conductivity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
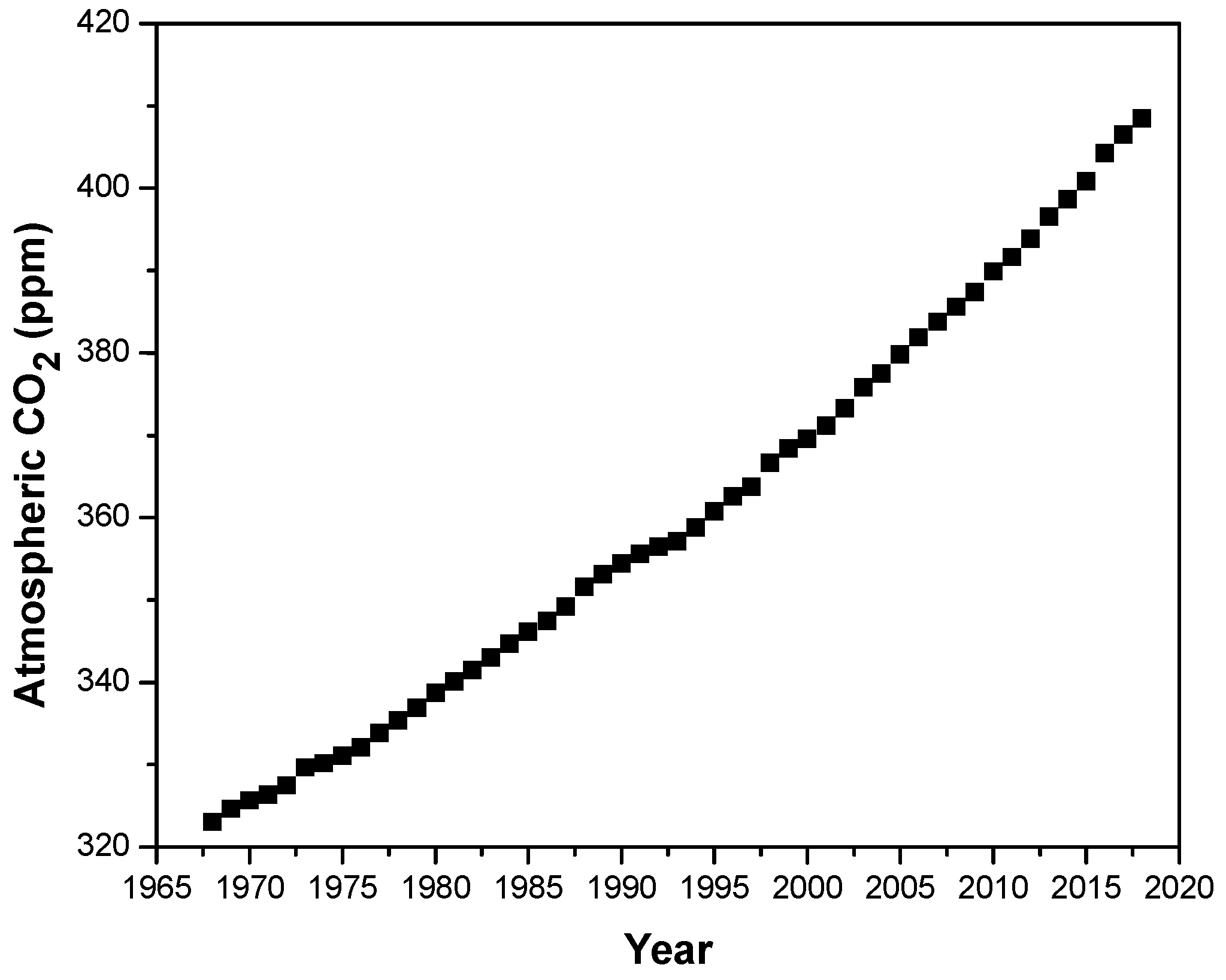
- Earth’s CO2 Home Page. Available online: https://www.co2.earth/ (accessed on 25 March 2020).
- Kreuer, K.-D. Proton conductivity: Materials and applications. Chem. Mater. 1996, 8, 610–641. [Google Scholar] [CrossRef]
- Steele, B.C.H.; Heinzel, A. Materials for fuel-cell technologies. Nature 2001, 414, 345–352. [Google Scholar] [CrossRef]
- Kreuer, K.D.; Portale, G. A critical revision of the nano-morphology of proton conducting ionomers and polyelectrolytes for fuel cell applications. Adv. Funct. Mater. 2013, 23, 5390–5397. [Google Scholar] [CrossRef]
- Bakangura, E.; Wu, L.; Ge, L.; Yang, Z.; Xu, T. Mixed matrix proton exchange membranes for fuel cells: State of the art and perspectives. Prog. Polym. Sci. 2016, 57, 103–152. [Google Scholar] [CrossRef]
- Konstantinia, D.P.; Fotis, P.; Stylianos, G.N.; Joannis, K.K. Cross-linking of side chain unsaturated aromatic polyethers for high temperature polymer electrolyte membrane fuel cell applications. Macromolecules 2011, 44, 4942–4951. [Google Scholar]
- Armand, M.; Chabagno, J.M.; Duclot, M. Polyethers as solid electrolytes. In Fast Ion Transport in Solids: Electrodes and Electrolytes; Vashishta, P., Mundy, J.N., Shenoy, G.K., Eds.; North Holland Publishers: Amsterdan, The Netherlands, 1979. [Google Scholar]
- Di Noto, V.; Lavina, S.; Giffin, G.A.; Negro, E.; Scrosati, B. Polymer electrolytes: Present, past and future. Electrochim. Acta 2011, 57, 4–13. [Google Scholar] [CrossRef]
- Tarascon, J.-M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef]
- Mauritz, K.A.; Moore, R.B. State of understanding of Nafion. Chem. Rev. 2004, 104, 4535–4586. [Google Scholar] [CrossRef]
- Casciola, M.; Alberti, G.; Sganappa, M.; Narducci, R. On the decay of Nafion proton conductivity at high temperature and relative humidity. J. Power Sources 2006, 162, 141–145. [Google Scholar] [CrossRef]
- Li, Q.; He, R.; Gao, J.-A.; Jensen, J.O.; Bjerrum, N. The CO poisoning effect in PEMFCs operational at temperatures up to 200 °C. J. Electrochem. Soc. 2003, 150, A1599–A1605. [Google Scholar] [CrossRef] [Green Version]
- Jannasch, P. Recent developments in high-temperature proton conducting polymer electrolyte membranes. Curr. Opin. Colloid Interface Sci. 2003, 8, 96–102. [Google Scholar] [CrossRef]
- Purnima, P.; Jayanti, S. Water neutrality and waste heat management in ethanol reformer—HTPEMFC integrated system for on-board hydrogen generation. Appl. Energy 2017, 199, 169–179. [Google Scholar] [CrossRef]
- Hickner, M.A.; Ghassemi, H.; Kim, Y.S.; Einsla, B.R.; McGrath, J.E. Alternative polymer systems for proton exchange membranes (PEMs). Chem. Rev. 2004, 104, 4587–4612. [Google Scholar] [CrossRef] [PubMed]
- Kraytsberg, A.; Ein-Eli, Y. Review of advanced materials for proton exchange membrane fuel cells. Energy Fuels 2014, 28, 7303–7330. [Google Scholar] [CrossRef]
- Reinholdt, M.X.; Kaliaguine, S. Proton exchange membranes for application in fuel cells: Grafted silica/SPEEK nanocomposite elaboration and characterization. Langmuir 2010, 26, 11184–11195. [Google Scholar] [CrossRef] [PubMed]
- Dhanapal, D.; Xiao, M.; Wang, S.; Meng, Y. A review on sulfonated polymer composite/organic-inorganic hybrid membranes to address methanol barrier issue for methanol fuel cells. Nanomaterials 2019, 9, 668. [Google Scholar] [CrossRef] [Green Version]
- Araya, S.S.; Zhou, F.; Liso, V.; Sahlin, S.L.; Vang, J.R.; Thomas, S.; Gao, X.; Jeppesen, C.; Kaer, S.K. A comprehensive review of PBI-based high temperature PEM fuel cells. Int. J. Hydrog. Energy 2016, 41, 21310–21344. [Google Scholar] [CrossRef]
- Asensio, J.A.; Sánchez, E.M.; Gómez-Romero, P. Proton-conducting membranes based on benzimidazole polymers for high-temperature PEM fuel cells. A chemical quest. Chem. Soc. Rev. 2010, 39, 3210–3239. [Google Scholar] [CrossRef]
- Vogel, H.; Marvel, C.S. Polybenzimidazoles: New thermally stable polymers. J. Polym. Sci. 1961, 50, 511–539. [Google Scholar] [CrossRef]
- Wainright, J.S.; Wang, J.-T.; Weng, D.; Savinell, R.F.; Litt, M. Acid-doped polybenzimidazoles: A new polymer electrolyte. J. Electrochem. Soc. 1995, 142, L121–L123. [Google Scholar] [CrossRef]
- Quartarone, E.; Mustarelli, P. Polymer fuel cells based on polybenzimidazole/H3PO4. Energy Environ. Sci. 2012, 5, 6436–6444. [Google Scholar] [CrossRef]
- Wang, L.; Lium, Z.; Ni, J.; Xu, M.; Pan, C.; Wang, D.; Liu, D.; Wang, L. Preparation and investigation of block polybenzimidazole membranes with high battery performance and low phosphoric acid doping for use in high-temperature fuel cells. J. Membr. Sci. 2019, 572, 350–357. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Z.; Liu, Y.; Wang, L. Crosslinked polybenzimidazole containing branching structure with no sacrifice of effective N-H sites: Towards high-performance high-temperature proton exchange membranes for fuel cells. J. Membr. Sci. 2019, 583, 110–117. [Google Scholar] [CrossRef]
- Hu, M.; Li, T.; Neelakandan, S.; Wang, L.; Chen, Y. Cross-linked polybenzimidazoles containing hyperbranched cross-linkers and quaternary ammoniums as high-temperature proton exchange membranes: Enhanced stability and conductivity. J. Membr. Sci. 2020, 593, 117435. [Google Scholar] [CrossRef]
- Ni, J.; Hu, M.; Liu, D.; Xie, H.; Xiang, X.; Wang, L. Synthesis and properties of highly branched polybenzimidazoles as proton exchange membranes for high-temperature fuel cells. J. Mater. Chem. C 2016, 4, 4814–4821. [Google Scholar] [CrossRef]
- Qingfeng, L.; Hjuler, H.A.; Bjerrum, N.J. Phosphoric acid doped polybenzimidazole membranes: Physiochemical characterization and fuel cell applications. J. Appl. Electrochem. 2001, 31, 773–779. [Google Scholar] [CrossRef]
- Samms, S.R.; Wasmus, S.; Savinell, R.F. Thermal stability of proton conducting acid doped polybenzimidazole in simulated fuel cell environments. J. Electrochem. Soc. 1996, 143, 1225–1232. [Google Scholar] [CrossRef]
- Ghosh, S.; Maity, S.; Jana, T. Polybenzimidazole/silica nanocomposites: Organic-inorganic hybrid membranes for PEM fuel cell. J. Mater. Chem. 2011, 21, 14897–14906. [Google Scholar] [CrossRef]
- Escorihuela, J.; García-Bernabé, A.; Montero, A.; Andrio, A.; Sahuquillo, O.; Giménez, E.; Compañ, V. Proton conductivity through polybenzimidazole composite membranes containing silica nanofiber mats. Polymers 2019, 11, 1182. [Google Scholar] [CrossRef] [Green Version]
- Fuentes, I.; Andrio, A.; Garcia-Bernabé, A.; Escorihuela, J.; Viñas, C.; Teixidor, F.; Compañ, V. Structural and dielectric properties of cobaltacarborane composite polybenzimidazole membranes as solid polymer electrolytes at high temperature. Phys. Chem. Chem. Phys. 2018, 20, 10173–10184. [Google Scholar] [CrossRef]
- Ozdemir, Y.; Uregen, N.; Devrim, Y. Polybenzimidazole based nanocomposite membranes with enhanced proton conductivity for high temperature PEM fuel cells. Int. J. Hydrog. Energy 2017, 42, 2648–2657. [Google Scholar] [CrossRef]
- Uregen, N.; Pehlivanoglu, K.; Ozdemir, Y.; Devrim, Y. Development of polybenzimidazole/graphene oxide composite membranes for high temperature PEM fuel cells. Int. J. Hydrog. Energy 2017, 42, 2636–2647. [Google Scholar] [CrossRef]
- Reyes-Rodriguez, J.L.; Escorihuela, J.; Garcia-Bernabé, A.; Giménez, E.; Solorza-Feria, O.; Compañ, V. Proton conducting electrospun sulfonated polyether ether ketone graphene oxide composite membranes. RSC Adv. 2017, 7, 53481–53491. [Google Scholar] [CrossRef] [Green Version]
- Escorihuela, J.; Sahuquillo, O.; García-Bernabé, A.; Giménez, E.; Compañ, V. Phosphoric acid doped polybenzimidazole (PBI)/zeolitic imidazolate framework composite membranes with significantly enhanced proton conductivity under low humidity conditions. Nanomaterials 2018, 8, 775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barjola, A.; Escorihuela, J.; Andrio, A.; Giménez, E.; Compañ, V. Enhanced conductivity of composite membranes based on sulfonated poly(ether ether ketone) (SPEEK) with zeolitic imidazolate frameworks (ZIFs). Nanomaterials 2018, 8, 1042. [Google Scholar] [CrossRef] [Green Version]
- Escorihuela, J.; Narducci, R.; Compañ, V.; Costantino, F. Proton conductivity of composite polyelectrolyte membranes with metal-organic frameworks for fuel cell applications. Adv. Mater. Interfaces 2019, 6, 1801146. [Google Scholar] [CrossRef]
- Liu, S.; Zhou, L.; Wang, P.; Zhang, F.; Yu, S.; Shao, Z.; Yi, B. Ionic-liquid-based proton conducting membranes for anhydrous H2/Cl2 fuel-cell applications. ACS Appl. Mater. Interfaces 2014, 6, 3195–3200. [Google Scholar] [CrossRef]
- Kallem, P.; Eguizabal, A.; Mallada, R.; Pina, M.P. Constructing straight polyionic liquid microchannels for fast anhydrous proton transport. ACS Appl. Mater. Interfaces 2016, 8, 35377–35389. [Google Scholar] [CrossRef]
- Escorihuela, J.; García-Bernabé, A.; Montero, A.; Sahuquillo, O.; Giménez, E.; Compañ, V. Ionic liquid composite polybenzimidazol membranes for high temperature PEMFC Applications. Polymers 2019, 11, 732. [Google Scholar] [CrossRef] [Green Version]
- Mack, F.; Aniol, K.; Ellwein, C.; Kerres, J.; Zeis, R. Novel phosphoric acid-doped PBI-blends as membranes for high-temperature PEM fuel cells. J. Mater. Chem. A 2015, 3, 10864–10874. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; He, G.; Zhang, B.; Cao, Y.; Wu, H.; Jiang, Z.; Tiantian, Z. Enhanced proton conductivity of Nafion hybrid membrane under different humidities by incorporating metal–organic frameworks with high phytic acid loading. ACS Appl. Mater. Interfaces 2014, 6, 9799–9807. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Takeda, Y.; Wakiya, T.; Wakamoto, Y.; Harigaya, K.; Ito, T.; Tarao, T.; Kawakami, H. Acid-doped polymer nanofiber framework: Three-dimensional proton conductive network for high-performance fuel cells. J. Power Sources 2017, 342, 125–134. [Google Scholar] [CrossRef]
- Zeng, J.; Zhou, Y.; Li, L.; Jiang, S.P. Phosphotungstic acid functionalized silica nanocomposites with tunable bicontinuous mesoporous structure and superior proton conductivity and stability for fuel cells. Phys. Chem. Chem. Phys. 2011, 13, 10249–10257. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, J.; Su, H.; Zeng, J.; Jiang, S.P.; Goddard, W.A. Insight into proton transfer in phosphotungstic acid functionalized mesoporous silica-based proton exchange membrane fuel cells. J. Am. Chem. Soc. 2014, 136, 4954–4964. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Li, Y.; Xue, J.; Zhu, W.; Zhang, J.; Yin, Y.; Qin, Y.; Jiao, K.; Du, Q.; Cheng, B.; et al. Magnetic field alignment of stable proton-conducting channels in an electrolyte membrane. Nat. Commun. 2019, 10, 842. [Google Scholar] [CrossRef] [Green Version]
- Zhai, L.; Li, H. Polyoxometalate–Polymer Hybrid Materials as Proton Exchange Membranes for Fuel Cell Applications. Molecules 2019, 24, 3425. [Google Scholar] [CrossRef] [Green Version]
- Yuan, J.; Zhou, G.; Pu, H. Preparation and properties of Nafion®/hollow silica spheres composite membranes. J. Membr. Sci. 2008, 325, 742–748. [Google Scholar] [CrossRef]
- Zhang, X.; Fu, X.; Yang, S.; Zhang, Y.; Zhang, R.; Hu, S.; Bao, X.; Zhao, F.; Li, X.; Liu, Q. Design of sepiolite-supported ionogel-embedded composite membranes without proton carrier wastage for wide-temperature-range operation of proton exchange membrane fuel cells. J. Mater. Chem. A 2019, 7, 15288–15301. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, C.J.; Ma, W.J.; Zhang, G.; Liu, Z.G.; Ni, J.; Li, M.Y.; Zhang, N.; Na, H. Preparation and properties of epoxy-cross-linked porous polybenzimidazole for high temperature proton exchange membrane fuel cells. J. Membr. Sci. 2012, 411–412, 54–63. [Google Scholar] [CrossRef]
- Bose, S.; Kuila, T.; Nguyen, T.X.H.; Kim, N.H.; Lau, K.; Lee, J.H.P. Polymer membranes for high temperature proton exchange membrane fuel cell: Recent advances and challenges. Prog. Polym. Sci. 2011, 36, 813–843. [Google Scholar] [CrossRef]
- Choi, S.-W.; Park, J.O.; Pak, C.; Choi, K.H.; Lee, J.-C.; Chang, H. Design and synthesis of cross-linked copolymer membranes based on poly(benzoxazine) and polybenzimidazole and their application to an electrolyte membrane for a high-temperature PEM fuel cell. Polymers 2013, 5, 77–111. [Google Scholar] [CrossRef] [Green Version]
- Lua, Z.; Lugo, M.; Santare, M.H.; Karlsson, A.M.; Busby, F.C.; Walsh, P. An experimental investigation of strain rate, temperature and humidity effects on the mechanical behavior of a perfluorosulfonic acid membrane. J. Power Sources 2012, 214, 130–136. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.S.; Li, Q.F.; Cleemann, L.N.; Xu, C.X.; Jensen, J.O.; Pan, C.; Bjerrumb, N.J.; He, R.H. Synthesis and properties of poly(aryl sulfone benzimidazole) and its copolymers for high temperature membrane electrolytes for fuel cells. J. Mater. Chem. 2012, 22, 11185–11195. [Google Scholar] [CrossRef]
- Sana, B.; Unnikrishnan, G.; Jana, T.; KS, S.K. Polybenzimidazole co-polymers: Their synthesis, morphology and high temperature fuel cell membrane properties. Polym. Chem. 2020, 11, 1043–1054. [Google Scholar]
- Li, Q.; Pan, C.; Jensen, J.O.; Noyé, P.; Bjerrum, N.J. Cross-Linked Polybenzimidazole Membranes for Fuel Cells. Chem. Mater. 2007, 19, 350–352. [Google Scholar] [CrossRef]
- Mader, J.A.; Benicewicz, B.C. Synthesis and properties of segmented block copolymers of functionalised polybenzimidazoles for high-temperature PEM fuel cells. Fuel Cells 2011, 11, 222–237. [Google Scholar] [CrossRef]
- Gao, C.; Hu, M.; Wang, L.; Wang, L. Synthesis and properties of phosphoric-acid-doped polybenzimidazole with hyperbranched cross-linkers decorated with imidazolium groups as high-temperature proton exchange membranes. Polymers 2020, 12, 515. [Google Scholar] [CrossRef] [Green Version]
- Xiao, L.; Apple, T.; Zhang, H.; Scanlon, E.; Ramanathan, L.S.; Choe, E.-W.; Rogers, D.; Benicewicz, B.C. High-temperature polybenzimidazole fuel cell membranes via a sol-gel process. Chem. Mater. 2005, 17, 5328–5333. [Google Scholar] [CrossRef]
- Sacco, A. Electrochemical impedance spectroscopy: Fundamentals and application in dye-sensitized solar cells. Renew. Sust. Energy Rev. 2017, 79, 814–829. [Google Scholar] [CrossRef]
- Randviir, E.P.; Banks, C.E. Electrochemical impedance spectroscopy: An overview of bioanalytical applications. Anal. Methods 2013, 5, 1098–1115. [Google Scholar] [CrossRef]
- Gomadam, P.M.; Weidner, J.W. Analysis of electrochemical impedance spectroscopy in proton exchange membrane fuel cells. Int. J. Energy Res. 2005, 29, 1133–1151. [Google Scholar] [CrossRef]
- Donne, S.W. General principles of electrochemistry. In Supercapacitors: Materials, Systems, and Applications; Béguin, F., Frąckowiak, E., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2013; pp. 1–68. [Google Scholar]
- Nawn, G.; Pace, G.; Lavina, S.; Vezzù, K.; Negro, E.; Bertasi, F.; Polizzi, S.; Di Noto, V. Interplay between composition, structure, and properties of new H3PO4-doped PBI4N−HfO2 nanocomposite membranes for high-temperature proton exchange membrane fuel cells. Macromolecules 2015, 48, 15–27. [Google Scholar] [CrossRef]
- Liu, F.; Wang, S.; Chen, H.; Li, J.; Tian, X.; Wang, X.; Mao, T.; Xu, J.; Wang, Z. Cross-linkable polymeric ionic liquid improve phosphoric acid retention and long-term conductivity stability in polybenzimidazole based PEMs. ACS Sustain. Chem. Eng. 2018, 6, 16352–16362. [Google Scholar] [CrossRef]
- Vilčiauskas, L.; Tuckerman, M.E.; Bester, G.; Paddison, S.J.; Kreuer, K.-D. The Mechanism of Proton Conduction in Phosphoric Acid. Nat. Chem. 2012, 4, 461–466. [Google Scholar] [CrossRef]
- Bose, A.B.; Gopu, S.; Li, W. Enhancement of proton exchange membrane fuel cells performance at elevated temperatures and lower humidities by incorporating immobilized phosphotungstic acid in electrodes. J. Power Sources 2014, 263, 217–222. [Google Scholar] [CrossRef]
- Crea, F.; De Stefano, C.; Milea, D.; Sammartano, S. Formation and stability of phytate complexes in solution. Coord. Chem. Rev. 2008, 252, 1108–1120. [Google Scholar] [CrossRef]
- Lua, J.L.; Fang, Q.H.; Li, S.L.; Jiang, S.P. A novel phosphotungstic acid impregnated meso-Nafion multilayer membrane for proton exchange membrane fuel cells. J. Membr. Sci. 2013, 427, 101–107. [Google Scholar] [CrossRef]
- Hasani-Sadrabadi, M.M.; Dashtimoghadam, E.; Majedi, F.S.; Moaddel, H.; Bertsch, A.; Renaudand, P. Superacid-doped polybenzimidazole-decorated carbon nanotubes: A novel high-performance proton exchange nanocomposite membrane. Nanoscale 2013, 5, 11710–11717. [Google Scholar] [CrossRef]
- Wang, S.; Sun, P.; Li, Z.; Liu, G.; Yin, X. Comprehensive performance enhancement of polybenzimidazole based high temperature proton exchange membranes by doping with a novel intercalated proton conductor. Int. J. Hydrog. Energy 2018, 43, 9994–10003. [Google Scholar] [CrossRef]
- Kim, A.R.; Vinothkannan, M.; Kim, J.S.; Yoo, D.J. Proton-conducting phosphotungstic acid/sulfonated fluorinated block copolymer composite membrane for polymer electrolyte fuel cells with reduced hydrogen permeability. Polym. Bull. 2018, 75, 2779–2804. [Google Scholar] [CrossRef]
- Kim, A.R.; Park, C.J.; Vinothkannan, M.; Yoo, D.J. Sulfonated poly ether sulfone/heteropoly acid composite membranes as electrolytes for the improved power generation of proton exchange membrane fuel cells. Compos. Part B Eng. 2018, 155, 272–281. [Google Scholar] [CrossRef]
- Agmon, N. The Grotthuss mechanism. Chem. Phys. Lett. 1995, 244, 456–462. [Google Scholar] [CrossRef]
- Kreuer, K.D.; Rabenau, A.; Weppner, W. Vehicle mechanism, a new model for the interpretation of the conductivity of fast proton conductors. Angew. Chem. Int. Ed. Engl. 1982, 21, 208–209. [Google Scholar] [CrossRef]
- Xu, C.; Cao, Y.; Kumar, R.; Wu, X.; Wang, X.; Scott, K. A polybenzimidazole/sulfonated graphite oxide composite membrane for high temperature polymer electrolyte membrane fuel cells. J. Mater. Chem. 2011, 21, 11359–11364. [Google Scholar] [CrossRef]
- Rewar, A.S.; Chaudhari, H.D.; Illathvalappil, R.; Sreekumar, K.; Kharul, U.K. New approach of blending polymeric ionic liquid with polybenzimidazole (PBI) for enhancing physical and electrochemical properties. J. Mater. Chem. A 2014, 2, 14449–14458. [Google Scholar] [CrossRef]
- He, R.; Li, Q.; Xiao, G.; Bjerrum, N.J. Proton conductivity of phosphoric acid doped polybenzimidazole and its composites with inorganic proton conductors. J. Membr. Sci. 2003, 226, 169–184. [Google Scholar] [CrossRef]
- Monroe, C.W. Ionic Mobility and Diffusivity. In Encyclopedia of Applied Electrochemistry; Kreysa, G., Ota, K., Savinell, R.F., Eds.; Springer: New York, NY, USA, 2014. [Google Scholar]
- Bandara, T.M.W.J.; Dissanayake, M.A.K.L.; Alboinsson, I.; Mellander, B.-E. Mobile charge carrier concentration and mobility of a polymer electrolyte containing PEO and Pr4N+I- using electrical and dielectric measurements. Solid State Ion. 2011, 189, 63–68. [Google Scholar] [CrossRef]
- Compañ, V.; Sorensen, T.S.; Diaz-Calleja, R.; Riande, E. Diffusion coefficients of conductive ions in a copolymer of vinylidene cyanide and vinyl acetate obtained from dielectric measurements using the model of Trukhan. J. Appl. Phys. 1996, 79, 403–411. [Google Scholar] [CrossRef]



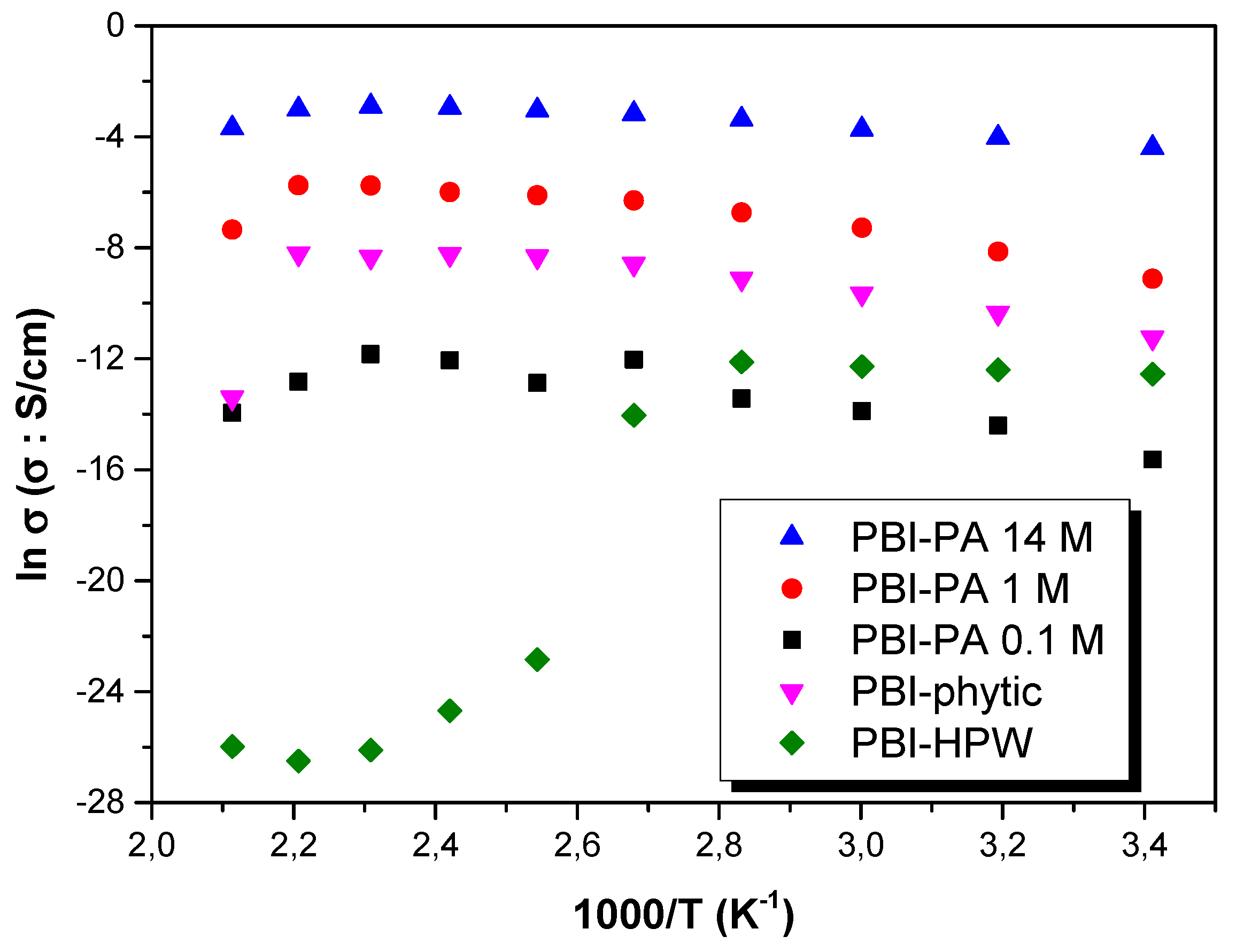

| Membrane | AU (%) | Swelling (%) | TU (%) | Acid Doping Level (%) |
|---|---|---|---|---|
| PBI–DIW | 4 ± 1 | 5 ± 1 | 4 ± 1 | ---- |
| PBI–PA 0.1 M | 19 ± 1 | 9 ± 1 | 8 ± 1 | 0.60 |
| PBI–PA 1 M | 63 ± 3 | 17 ± 1 | 22 ± 2 | 1.98 |
| PBI–PA 14 M | 278 ± 5 | 63 ± 4 | 80 ± 3 | 8.74 |
| PBI–phytic acid | 21 ± 1 | 12 ± 1 | 10 ± 1 | 0.10 |
| PBI–HPW | 25 ± 1 | 15 ± 1 | 9 ± 1 | 0.03 |
| Membrane | Young’s Modulus (GPa) | Tensile Stress (MPa) | Elongation at Break (%) |
|---|---|---|---|
| PBI–dry | 2.52 ± 0.17 | 174 ± 4 | 18 ± 2 |
| PBI–DIW | 2.13 ± 0.21 | 98 ± 6 | 66 ± 3 |
| PBI–PA 0.1 M | 1.58 ± 0.19 | 92 ± 5 | 89 ± 5 |
| PBI–PA 1 M | 1.22 ± 0.12 | 75 ± 4 | 108 ± 7 |
| PBI–PA 14 M | 0.11 ± 0.02 | 19 ± 2 | 189 ± 9 |
| PBI–phytic acid | 1.11 ± 0.15 | 71 ± 2 | 119 ± 5 |
| PBI–HPW | 1.67 ± 0.13 | 90 ± 4 | 125 ± 6 |
| Membrane | σ 20 °C (S·cm−1) | σ 80 °C (S·cm−1) | σ 140 °C (S·cm−1) | Eact (kJ·mol−1) |
|---|---|---|---|---|
| PBI–DIW | 1.1 × 10−14 | 1.4 × 10−13 | 2.5 × 10−12 | 52.6 ± 2.1 |
| PBI–PA 0.1 M | 1.6 × 10−6 | 2.7 × 10−6 | 5.8 × 10−6 | 27.5 ± 3.8 |
| PBI–PA 1 M | 5.8 × 10−4 | 1.2 × 10−3 | 2.5 × 10−3 | 24.7 ± 3.2 |
| PBI–PA 14 M | 2.5 × 10−2 | 3.4 × 10−2 | 5.3 × 10−2 | 11.6 ± 0.7 |
| PBI–phytic acid | 1.3 × 10−5 | 1.1 × 10−4 | 2.6 × 10−4 | 25.1 ± 2.3 |
| PBI–HPW | 4.8 × 10−6 | 2.9 × 10−6 | 1.9 × 10−11 | 30.8 ± 2.8 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Escorihuela, J.; García-Bernabé, A.; Compañ, V. A Deep Insight into Different Acidic Additives as Doping Agents for Enhancing Proton Conductivity on Polybenzimidazole Membranes. Polymers 2020, 12, 1374. https://doi.org/10.3390/polym12061374
Escorihuela J, García-Bernabé A, Compañ V. A Deep Insight into Different Acidic Additives as Doping Agents for Enhancing Proton Conductivity on Polybenzimidazole Membranes. Polymers. 2020; 12(6):1374. https://doi.org/10.3390/polym12061374
Chicago/Turabian StyleEscorihuela, Jorge, Abel García-Bernabé, and Vicente Compañ. 2020. "A Deep Insight into Different Acidic Additives as Doping Agents for Enhancing Proton Conductivity on Polybenzimidazole Membranes" Polymers 12, no. 6: 1374. https://doi.org/10.3390/polym12061374
APA StyleEscorihuela, J., García-Bernabé, A., & Compañ, V. (2020). A Deep Insight into Different Acidic Additives as Doping Agents for Enhancing Proton Conductivity on Polybenzimidazole Membranes. Polymers, 12(6), 1374. https://doi.org/10.3390/polym12061374








