Radiation Grafting of a Polymeric Prodrug onto Silicone Rubber for Potential Medical/Surgical Procedures
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of 2MBA
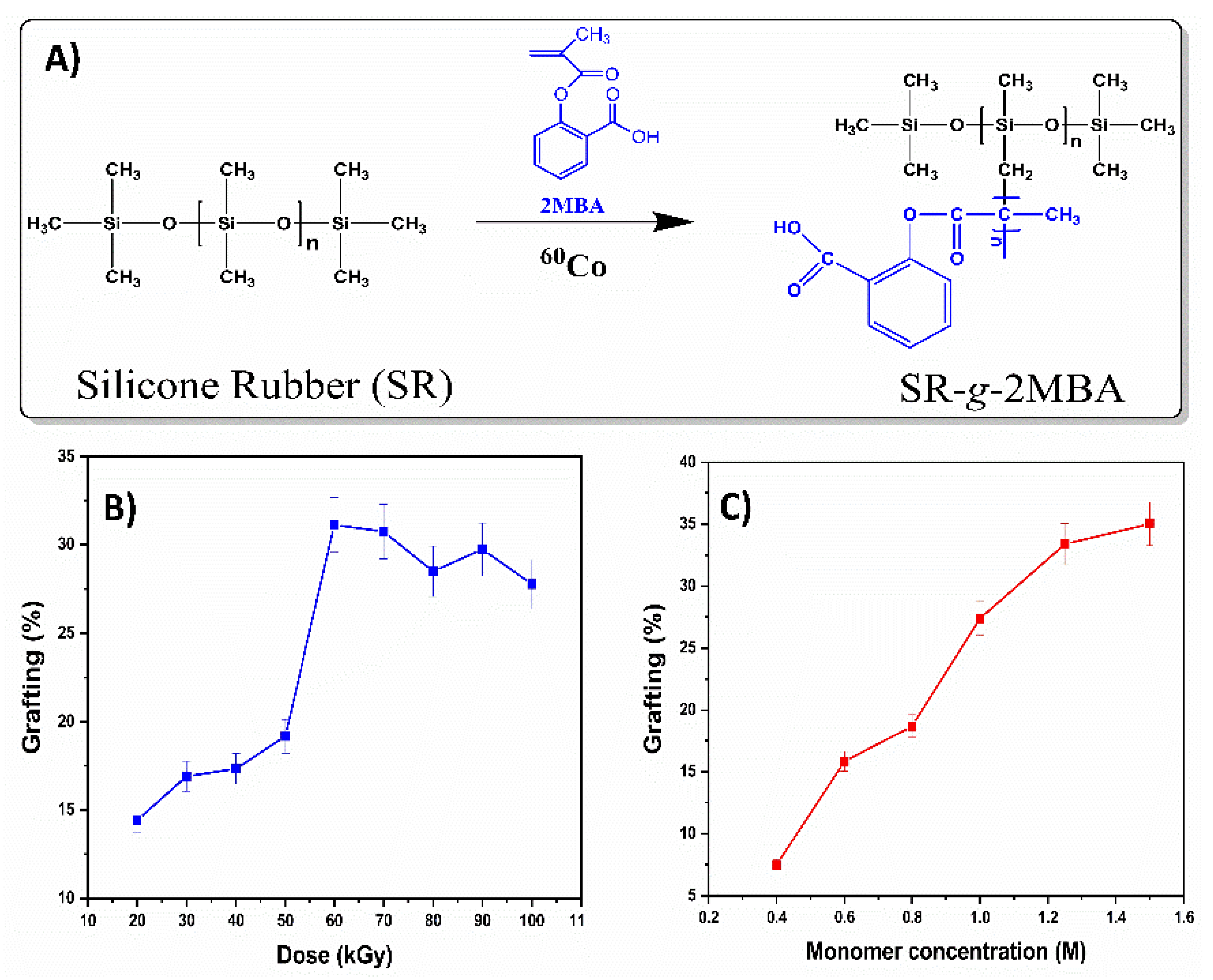
2.3. Grafting Process (SR-g-2MBA)
2.4. Characterization of SR-g-2MBA Films
2.4.1. TGA and FTIR-ATR Analysis
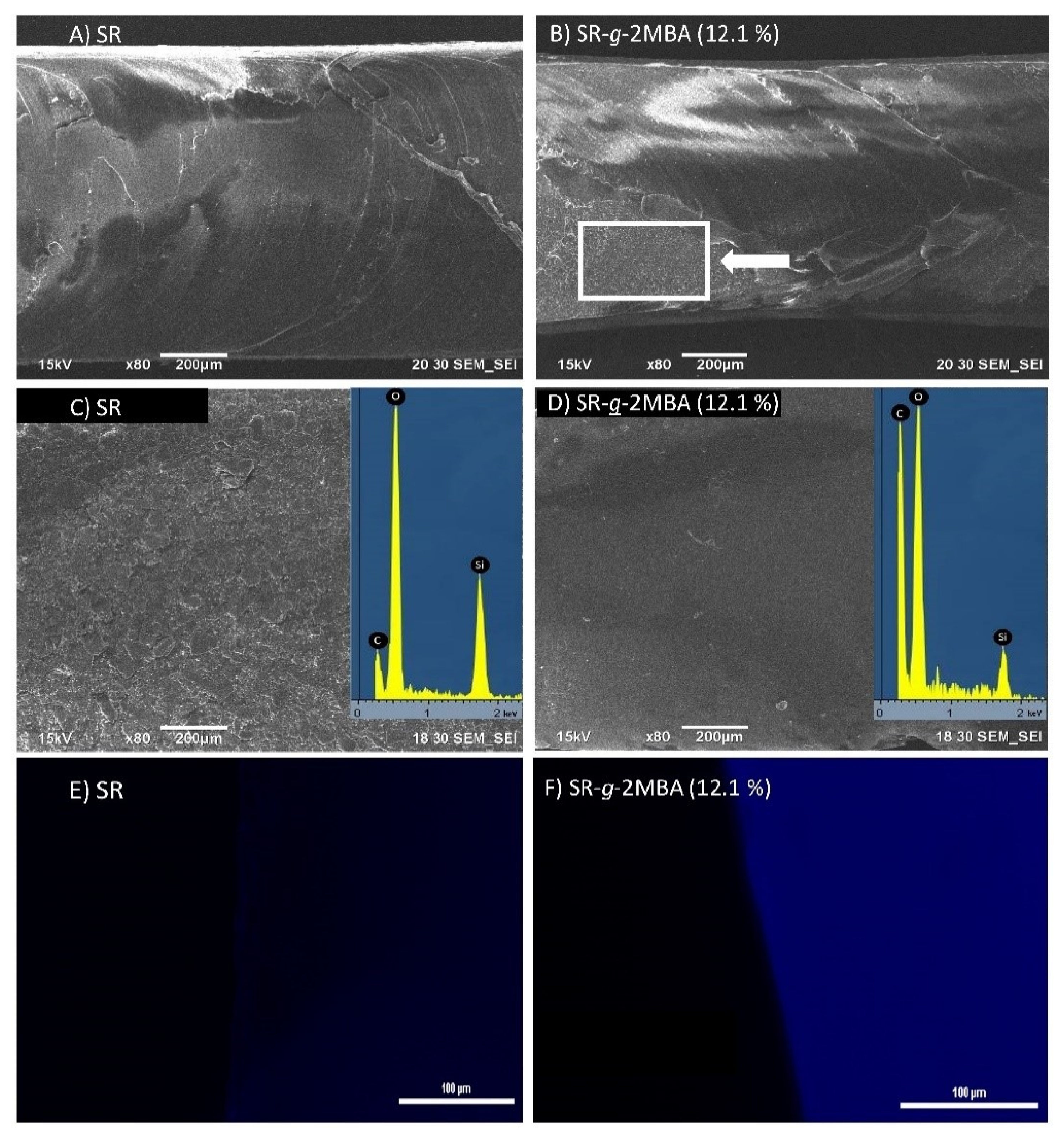
2.4.2. SEM-EDX and Fluorescence Analysis
2.4.3. Contact Angle and Swelling Test
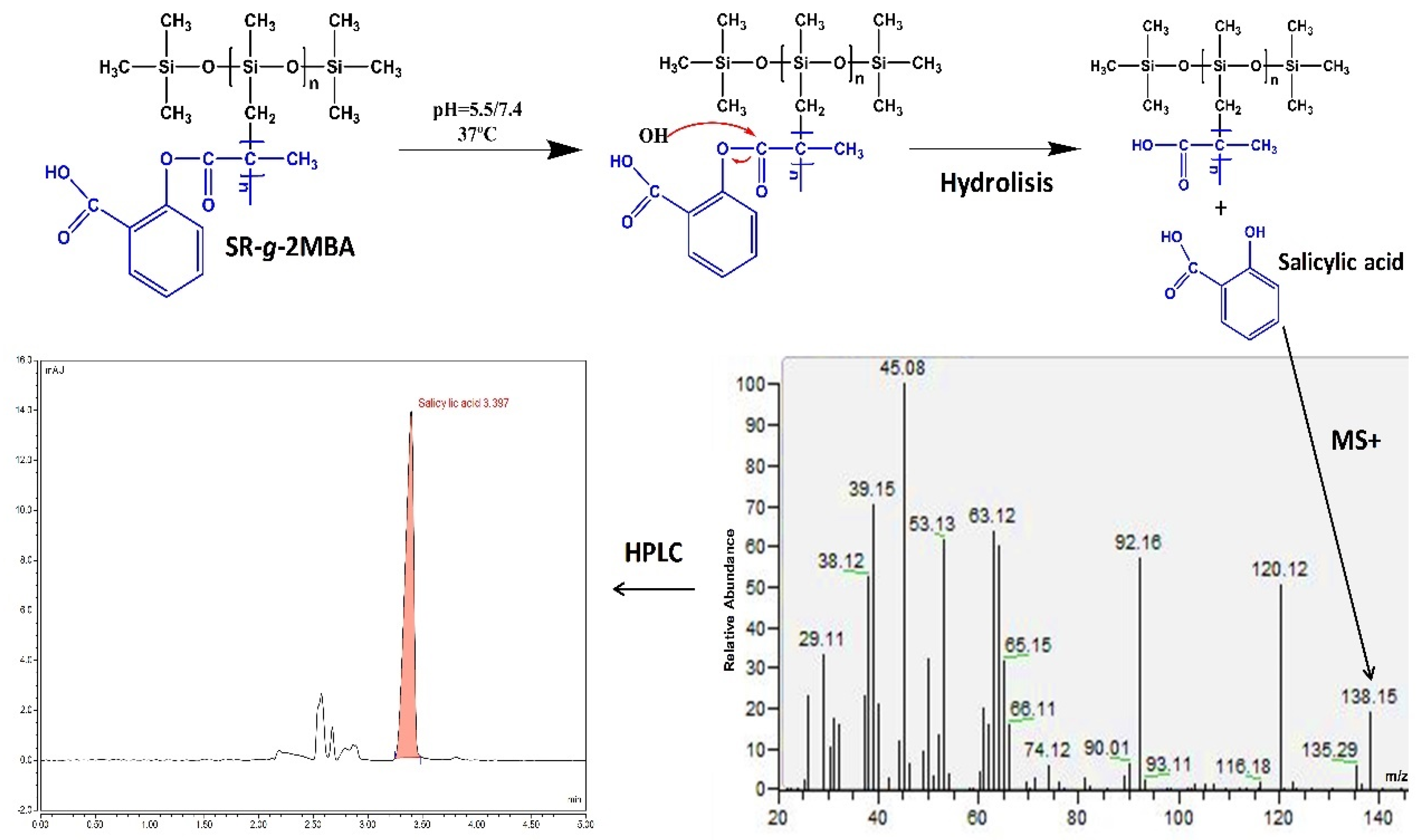
2.5. Salicylic Acid Release and Characterization (HPLC and GC-MS)
2.6. Cytocompatibility Test
3. Results and Discussion
3.1. Synthesis of SR-g-2MBA
3.2. Characterization of SR-g-2MBA
3.2.1. TGA and FTIR-ATR
3.2.2. Contact Angle
3.2.3. SEM-EDX and Fluorescence Analysis
3.2.4. Salicylic Acid Release
3.2.5. Cytocompatibility
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Muskens, I.S.; Gupta, S.; Hulsbergen, A.; Moojen, W.A.; Broekman, M.L.D. Introduction of Novel Medical Devices in Surgery: Ethical Challenges of Current Oversight and Regulation. J. Am. Coll. Surg. 2017, 225, 558–565. [Google Scholar] [CrossRef] [PubMed]
- Zitnik, R.J. Treatment of chronic inflammatory diseases with implantable medical devices. Ann. Rheum. Dis. 2011, 70, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.; Cramer, S. Perspectives on the Inflammatory, Healing, and Foreign Body Responses to Biomaterials and Medical Devices; Elsevier Inc.: Amsterdam, Netherlands, 2015; ISBN 9780128005002. [Google Scholar]
- Almalki, M.A.; Varghese, R. Prevalence of catheter associated biofilm producing bacteria and their antibiotic sensitivity pattern. J. King Saud Univ. Sci. 2020. [Google Scholar] [CrossRef]
- Khatoon, Z.; McTiernan, C.D.; Suuronen, E.J.; Mah, T.F.; Alarcon, E.I. Bacterial biofilm formation on implantable devices and approaches to its treatment and prevention. Heliyon 2018, 4, e01067. [Google Scholar] [CrossRef]
- Ceresa, C.; Fracchia, L.; Williams, M.; Banat, I.M.; Díaz De Rienzo, M.A. The effect of sophorolipids against microbial biofilms on medical-grade silicone. J. Biotechnol. 2020, 309, 34–43. [Google Scholar] [CrossRef]
- Boutrand, J.P. Methods for the Characterization and Evaluation of Drug-Device Combination Products, 2nd ed.; Elsevier Ltd.: Amsterdam, Netherlands, 2020; ISBN 9780081026434. [Google Scholar]
- Lewis, A.L. Sensitive Combination Products: Devices, Pharmaceuticals, and Biologics; Elsevier Inc.: Amsterdam, Netherlands, 2020; ISBN 9780128050828. [Google Scholar]
- Sastri, V.R. Material Requirements for Plastics used in Medical Devices. Plast. Med. Devices 2010, 33–54. [Google Scholar] [CrossRef]
- Bizjak, M.; Selmi, C.; Praprotnik, S.; Bruck, O.; Perricone, C.; Ehrenfeld, M.; Shoenfeld, Y. Silicone implants and lymphoma: The role of inflammation. J. Autoimmun. 2015, 65, 64–73. [Google Scholar] [CrossRef]
- von Hanstein, H.; Horch, R.E.; Schubert, D.W. Resistance of silicone breast explants under cyclic compressive load as a function of implantation time and explant mass. Polym. Test. 2020, 84, 106377. [Google Scholar] [CrossRef]
- Costoya, A.; Velázquez Becerra, L.E.; Meléndez-Ortiz, H.I.; Díaz-Gómez, L.; Mayer, C.; Otero, A.; Concheiro, A.; Bucio, E.; Alvarez-Lorenzo, C. Immobilization of antimicrobial and anti-quorum sensing enzymes onto GMA-grafted poly(vinyl chloride) catheters. Int. J. Pharm. 2019, 558, 72–81. [Google Scholar] [CrossRef]
- López-Saucedo, F.; Flores-Rojas, G.G.; López-Saucedo, J.; Magariños, B.; Alvarez-Lorenzo, C.; Concheiro, A.; Bucio, E. Antimicrobial silver-loaded polypropylene sutures modified by radiation-grafting. Eur. Polym. J. 2018, 100, 290–297. [Google Scholar] [CrossRef]
- Melendez-Ortiz, H.I.; Díaz-Rodríguez, P.; Alvarez-Lorenzo, C.; Concheiro, A.; Bucio, E. Binary graft modification of polypropylene for anti-inflammatory drug-device combo products. J. Pharm. Sci. 2014, 103, 1269–1277. [Google Scholar] [CrossRef] [PubMed]
- Pino-Ramos, V.H.; Meléndez-Ortiz, H.I.; Ramos-Ballesteros, A.; Bucio, E. Radiation Grafting of Biopolymers and Synthetic Polymers; Elsevier Inc.: Amsterdam, Netherlands, 2018; ISBN 9780128104620. [Google Scholar]
- Zuñiga-Zamorano, I.; Meléndez-Ortiz, H.I.; Costoya, A.; Alvarez-Lorenzo, C.; Concheiro, A.; Bucio, E. Poly(vinyl chloride) catheters modified with pH-responsive poly(methacrylic acid) with affinity for antimicrobial agents. Radiat. Phys. Chem. 2018, 142, 107–114. [Google Scholar] [CrossRef]
- Hiriart-Ramírez, E.; Contreras-García, A.; Garcia-Fernandez, M.J.; Concheiro, A.; Alvarez-Lorenzo, C.; Bucio, E. Radiation grafting of glycidyl methacrylate onto cotton gauzes for functionalization with cyclodextrins and elution of antimicrobial agents. Cellulose 2012, 19, 2165–2177. [Google Scholar] [CrossRef]
- García-Vargas, M.; González-Chomón, C.; Magariños, B.; Concheiro, A.; Alvarez-Lorenzo, C.; Bucio, E. Acrylic polymer-grafted polypropylene sutures for covalent immobilization or reversible adsorption of vancomycin. Int. J. Pharm. 2015, 461, 286–295. [Google Scholar] [CrossRef]
- Agrawal, N.; Agrawal, N. Polymeric Prodrugs: Recent Achievements and General Strategies. J. Antivir. Antiretrovir. 2015, 1, S15-007. [Google Scholar] [CrossRef]
- Madan, R.K.; Levitt, J. A review of toxicity from topical salicylic acid preparations. J. Am. Acad. Dermatol. 2014, 70, 788–792. [Google Scholar] [CrossRef]
- Cao, Y.; Uhrich, K.E. Salicylic Acid-Based Poly (anhydride-esters): Synthesis, Properties, and Applications; American Chemical Society: Washington, DC, USA, 2018. [Google Scholar]
- Nowatzki, P.J.; Koepsel, R.R.; Stoodley, P.; Min, K.; Harper, A.; Murata, H.; Donfack, J.; Hortelano, E.R.; Ehrlich, G.D.; Russell, A.J. Salicylic acid-releasing polyurethane acrylate polymers as anti-biofilm urological catheter coatings. Acta Biomater. 2012, 8, 1869–1880. [Google Scholar] [CrossRef]
- Marisol Arteaga-Luna, M.; Hugo Pino-Ramos, V.; Magaña, H.; Bucio, E. Polymeric pro-drug sutures for potential local release of salicylic acid. Int. J. Polym. Mater. Polym. Biomater. 2018, 67, 792–799. [Google Scholar] [CrossRef]
- Demirdirek, B.; Uhrich, K.E. Novel salicylic acid-based chemically crosslinked pH-sensitive hydrogels as potential drug delivery systems. Int. J. Pharm. 2017, 528, 406–415. [Google Scholar] [CrossRef]
- Erdmann, L.; Uhrich, K.E. Synthesis and degradation characteristics of salicylic acid-derived poly (anhydride-esters). Biomaterials 2000, 21, 1941–1946. [Google Scholar] [CrossRef]
- Magaña, H.; Palomino, K.; Cornejo-Bravo, J.M.; Díaz-Gómez, L.; Concheiro, A.; Zavala-Lagunes, E.; Alvarez-Lorenzo, C.; Bucio, E. Polymeric prodrug–functionalized polypropylene films for sustained release of salicylic acid. Int. J. Pharm. 2016, 511, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Price, C.T.D.; Lee, I.R.; Gustafson, J.E. The effects of salicylate on bacteria. Int. J. Biochem. Cell Biol. 2000, 32, 1029–1043. [Google Scholar] [CrossRef]
- Licea-Claveríe, A.; Rogel-Hernández, E.; Lopéz-Sanchez, J.A.; Castillo-Arámbula, L.A.; Cornejo-Bravo, J.M.; Arndt, K.F. A facile synthesis route for carboxyaryl-methacrylates: A way to obtain aromatic polyelectrolytes. Des. Monomers Polym. 2003, 6, 67–80. [Google Scholar] [CrossRef]
- Vázquez-González, B.; Meléndez-Ortiz, H.I.; Díaz-Gómez, L.; Alvarez-Lorenzo, C.; Concheiro, A.; Bucio, E. Silicone rubber modified with methacrylic acid to host antiseptic drugs. Macromol. Mater. Eng. 2014, 299, 1240–1250. [Google Scholar] [CrossRef]
- In Jeong, S.; Park, S.C.; Park, S.J.; Kim, E.J.; Heo, H.; Park, J.S.; Gwon, H.J.; Lim, Y.M.; Jang, M.K. One-step synthesis of gene carrier via gamma irradiation and its application in tumor gene therapy. Int. J. Nanomed. 2018, 13, 525–536. [Google Scholar] [CrossRef]
- Segura, T.; Burillo, G. Radiation modification of silicone rubber with glycidylmethacrylate. Radiat. Phys. Chem. 2013, 91, 101–107. [Google Scholar] [CrossRef]
- Guo, T.; Pei, Y.; Tang, K.; He, X.; Huang, J.; Wang, F. Mechanical and drug release properties of alginate beads reinforced with cellulose. J. Appl. Polym. Sci. 2017, 134, 1–9. [Google Scholar] [CrossRef]
- Magaña, H.; Palomino, K.; Cornejo-Bravo, J.M.; Alvarez- Lorenzo, C.; Concheiro, A.; Bucio, E. Radiation-grafting of acrylamide onto silicone rubber films for diclofenac delivery. Radiat. Phys. Chem. 2015, 107, 164–170. [Google Scholar] [CrossRef]
- Bennett, A.J. The absorption of globular proteins. Biopolymers 1973, 12, 1671–1676. [Google Scholar] [CrossRef]
- Hameed, N.; Guo, Q.; Hanley, T.; Mai, Y.W. Hydrogen bonding interactions, crystallization, and surface hydrophobicity in nanostructured epoxy/block copolymer blends. J. Polym. Sci. Part B Polym. Phys. 2010, 48, 790–800. [Google Scholar] [CrossRef]
- Karim, M.M.; Lee, H.S.; Kim, Y.S.; Bae, H.S.; Lee, S.H. Analysis of salicylic acid based on the fluorescence enhancement of the As(III)–salicylic acid system. Anal. Chim. Acta 2006, 576, 136–139. [Google Scholar] [CrossRef] [PubMed]
- Marcus, F.; Colaizzi, J.L.; Barry, H. pH effects on salicylate absorption from hydrophilic ointment. J. Pharm. Sci. 1970, 59, 1616–1620. [Google Scholar] [CrossRef] [PubMed]
- Chandorkar, Y.; Bhagat, R.K.; Madras, G.; Basu, B. Cross-linked, biodegradable, cytocompatible salicylic acid based polyesters for localized, sustained delivery of salicylic acid: An in vitro study. Biomacromolecules 2014, 15, 863–875. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, Q.; Chatterjee, K.; Madras, G. Controlled Release of Salicylic Acid from Biodegradable Cross-Linked Polyesters. Mol. Pharm. 2015, 12, 3479–3489. [Google Scholar] [CrossRef] [PubMed]
- Chandorkar, Y.; Bhaskar, N.; Madras, G.; Basu, B. Long-term sustained release of salicylic acid from cross-linked biodegradable polyester induces a reduced foreign body response in mice. Biomacromolecules 2015, 16, 636–649. [Google Scholar] [CrossRef]
- Rochlin, D.H.; Davis, C.R.; Nguyen, D.H. Breast augmentation and breast reconstruction demonstrate equivalent aesthetic outcomes. Plast. Reconstr. Surg. Glob. Open 2016, 4, 1–8. [Google Scholar] [CrossRef]


| Graft (%) | Release Medium pH | Kinetic Model | Rate Constants | R2 |
|---|---|---|---|---|
| 7.1 | 5.5 | Zero order | 0.0419 mg/g h | 0.9853 |
| 31.1 | 5.5 | First order | 0.0166 h−1 | 0.9885 |
| 7.1 | 7.4 | First order | 0.0513 h−1 | 0.9289 |
| 31.1 | 7.4 | First order | 0.0246 h−1 | 0.9986 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magaña, H.; Becerra, C.D.; Serrano-Medina, A.; Palomino, K.; Palomino-Vizcaíno, G.; Olivas-Sarabia, A.; Bucio, E.; Cornejo-Bravo, J.M. Radiation Grafting of a Polymeric Prodrug onto Silicone Rubber for Potential Medical/Surgical Procedures. Polymers 2020, 12, 1297. https://doi.org/10.3390/polym12061297
Magaña H, Becerra CD, Serrano-Medina A, Palomino K, Palomino-Vizcaíno G, Olivas-Sarabia A, Bucio E, Cornejo-Bravo JM. Radiation Grafting of a Polymeric Prodrug onto Silicone Rubber for Potential Medical/Surgical Procedures. Polymers. 2020; 12(6):1297. https://doi.org/10.3390/polym12061297
Chicago/Turabian StyleMagaña, Hector, Claudia D. Becerra, Aracely Serrano-Medina, Kenia Palomino, Giovanni Palomino-Vizcaíno, Amelia Olivas-Sarabia, Emilio Bucio, and José M. Cornejo-Bravo. 2020. "Radiation Grafting of a Polymeric Prodrug onto Silicone Rubber for Potential Medical/Surgical Procedures" Polymers 12, no. 6: 1297. https://doi.org/10.3390/polym12061297
APA StyleMagaña, H., Becerra, C. D., Serrano-Medina, A., Palomino, K., Palomino-Vizcaíno, G., Olivas-Sarabia, A., Bucio, E., & Cornejo-Bravo, J. M. (2020). Radiation Grafting of a Polymeric Prodrug onto Silicone Rubber for Potential Medical/Surgical Procedures. Polymers, 12(6), 1297. https://doi.org/10.3390/polym12061297








