Experimental Investigation of the Melt Shear Viscosity, Specific Volume and Thermal Conductivity of Low-Density Polyethylene/Multi-Walled Carbon Nanotube Composites Using Capillary Flow
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization
2.2.1. Differential Scanning Calorimetry
2.2.2. Melt Shear Viscosity
2.2.3. Specific Volume
2.2.4. Thermal Conductivity
2.2.5. Solid Density
3. Results and Discussion
3.1. Thermal Properties
3.2. Thermo-Stability of LDPE/MWCNT Composites
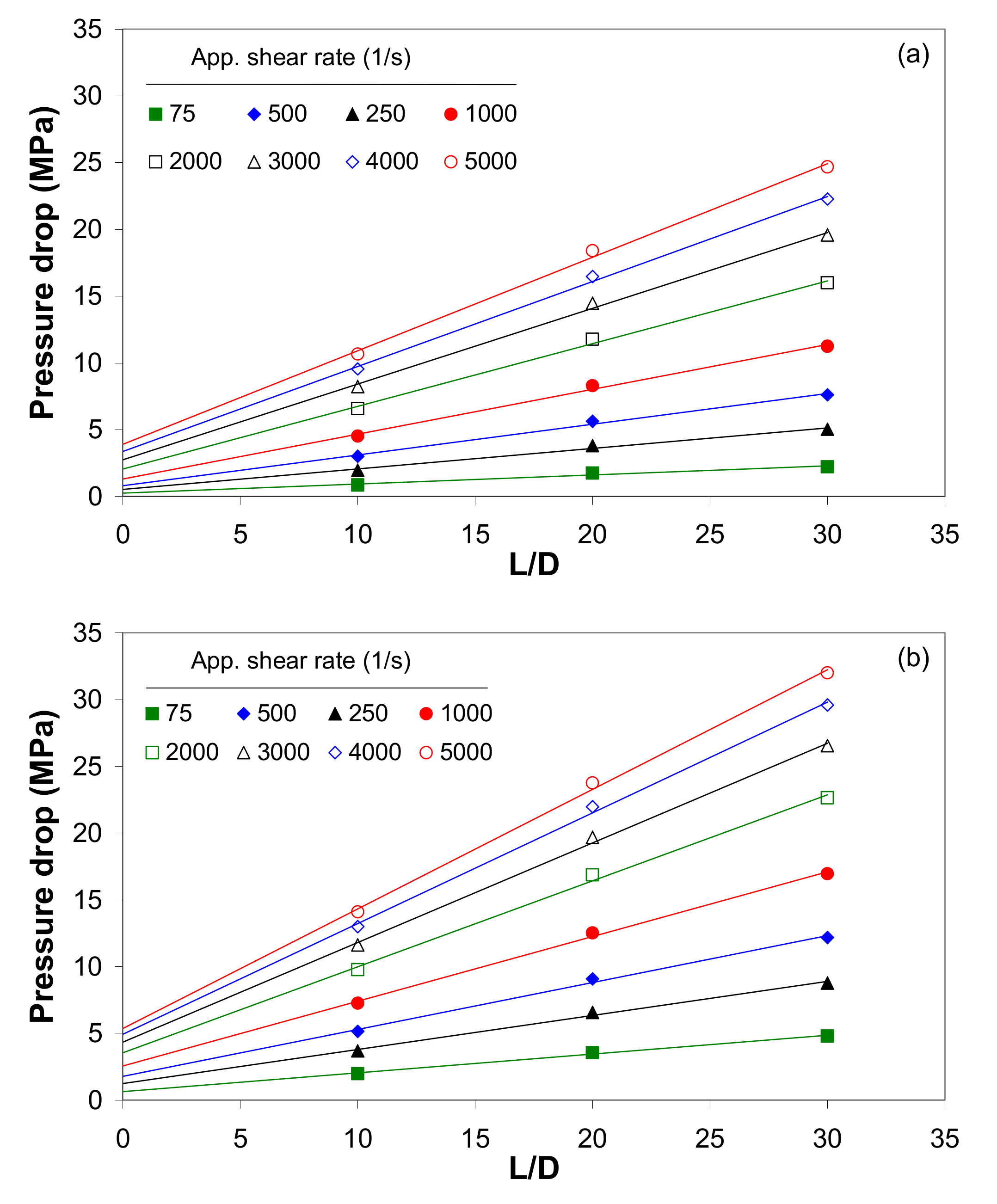
3.3. Effect of Pressure on Capillary Flow
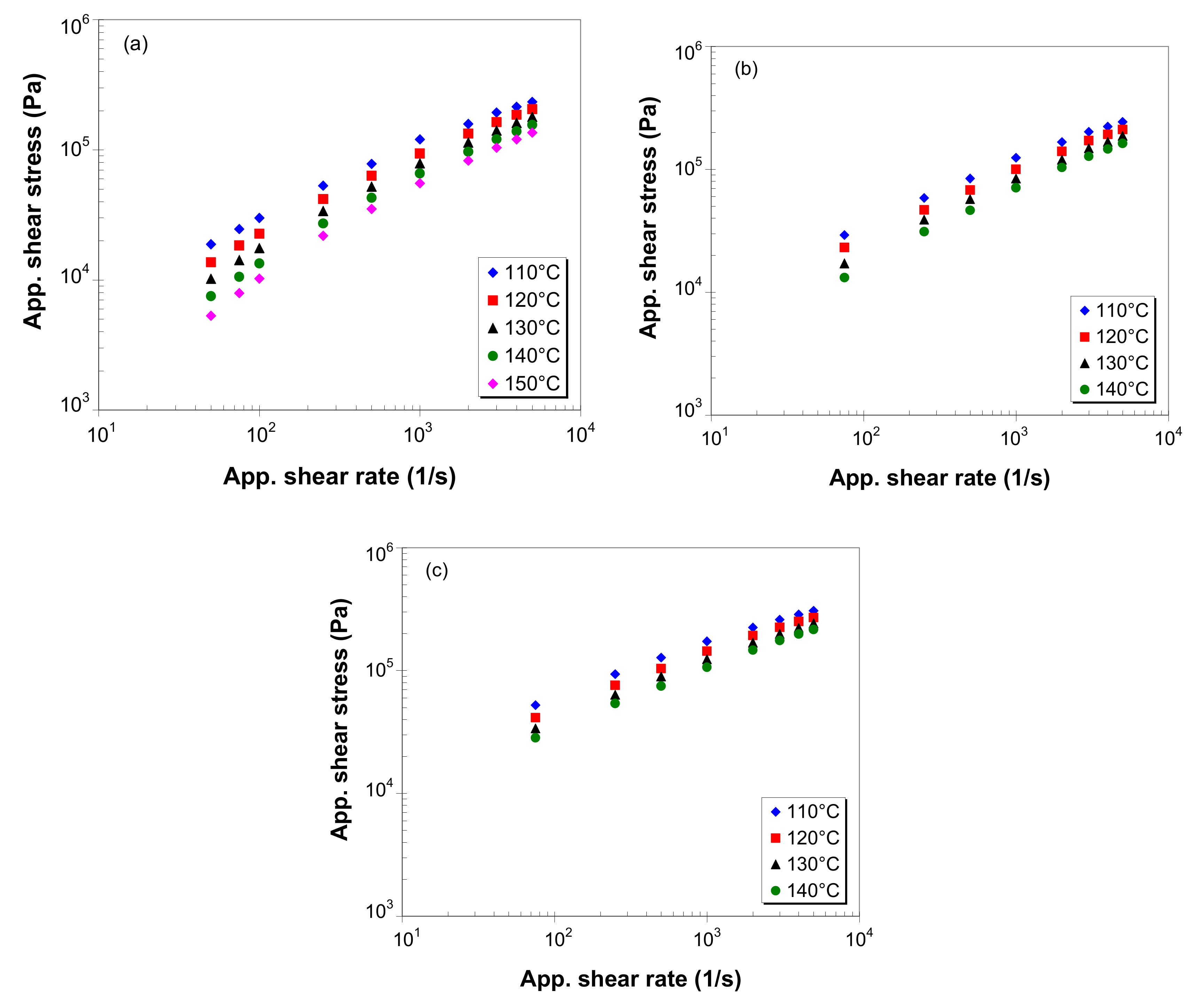
3.4. Apparent Shear Stress
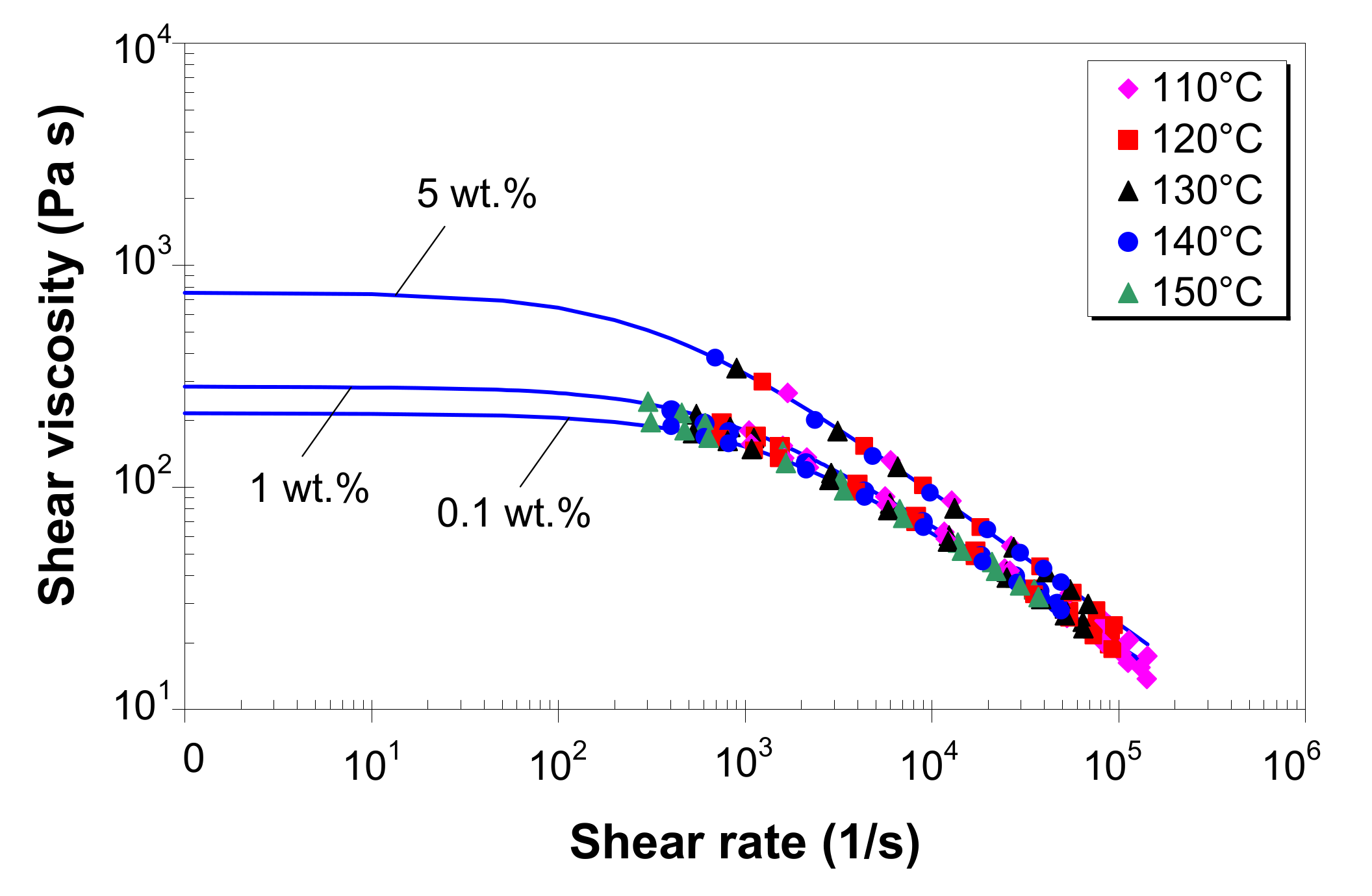
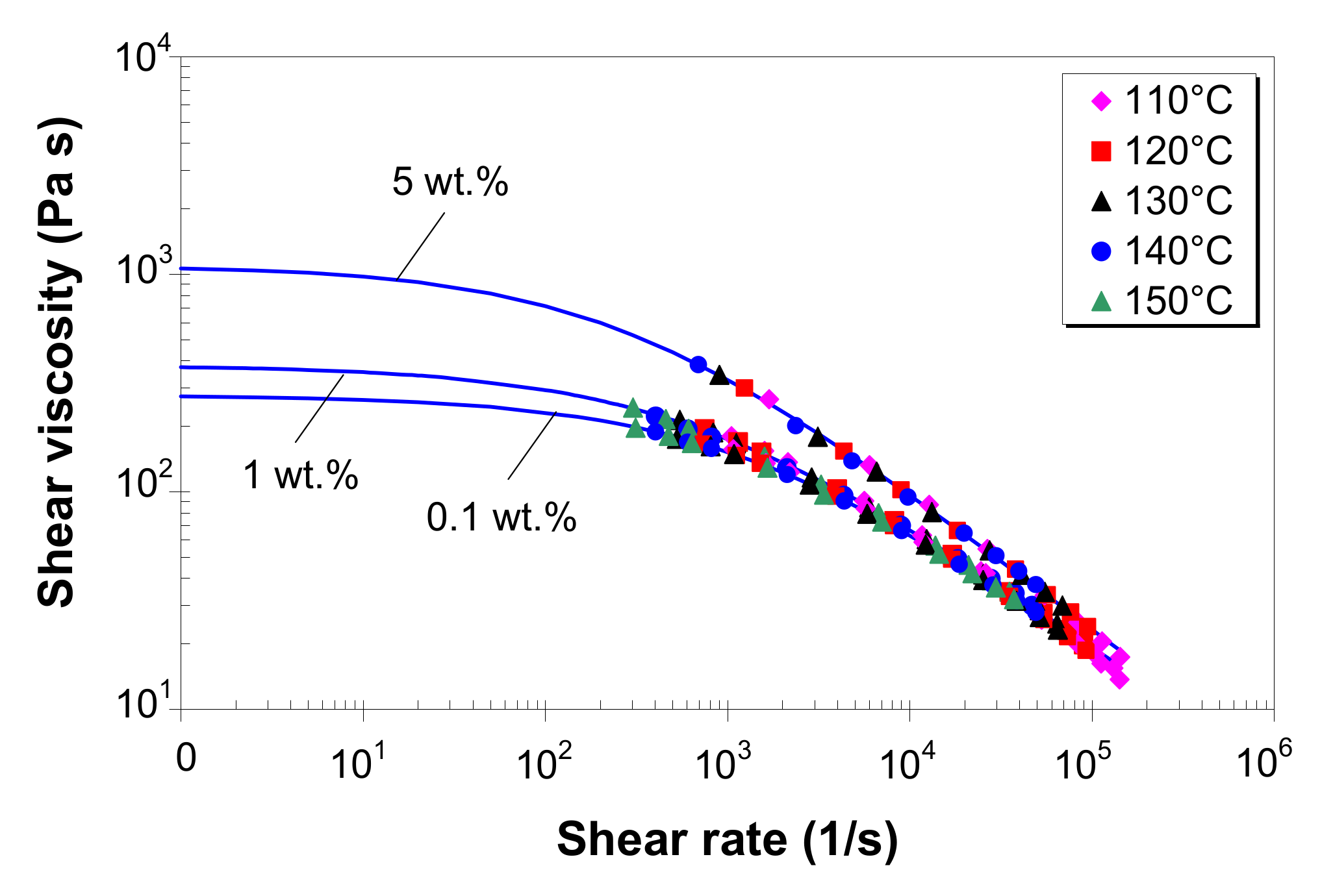
3.5. Effect of Temperature on Melt Shear Viscosity
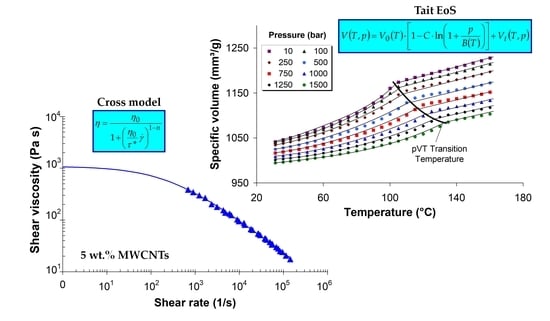
3.6. Effect of MWCNTs on Melt Shear Viscosity
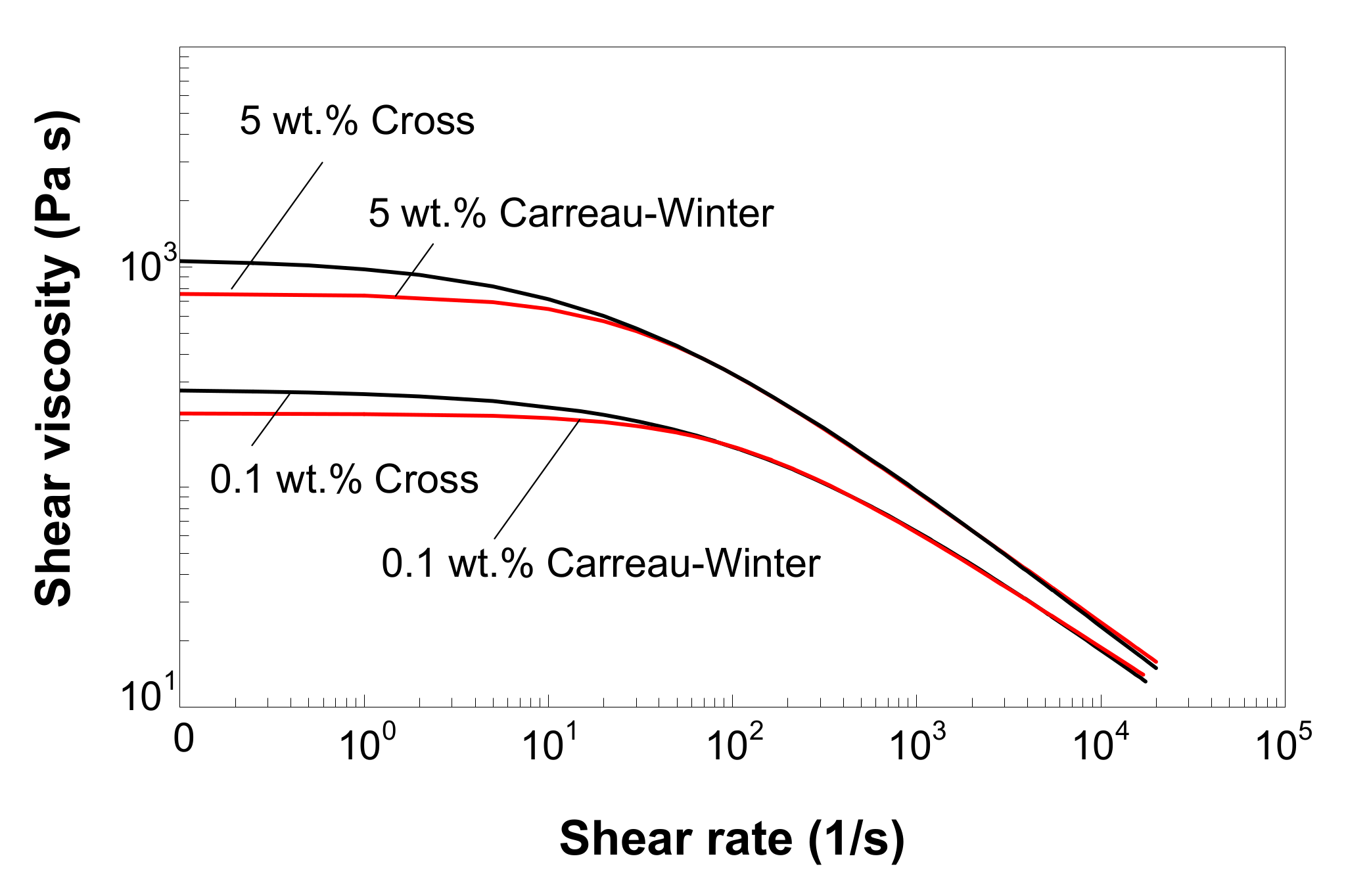
3.7. Modeling of Melt Shear Viscosity
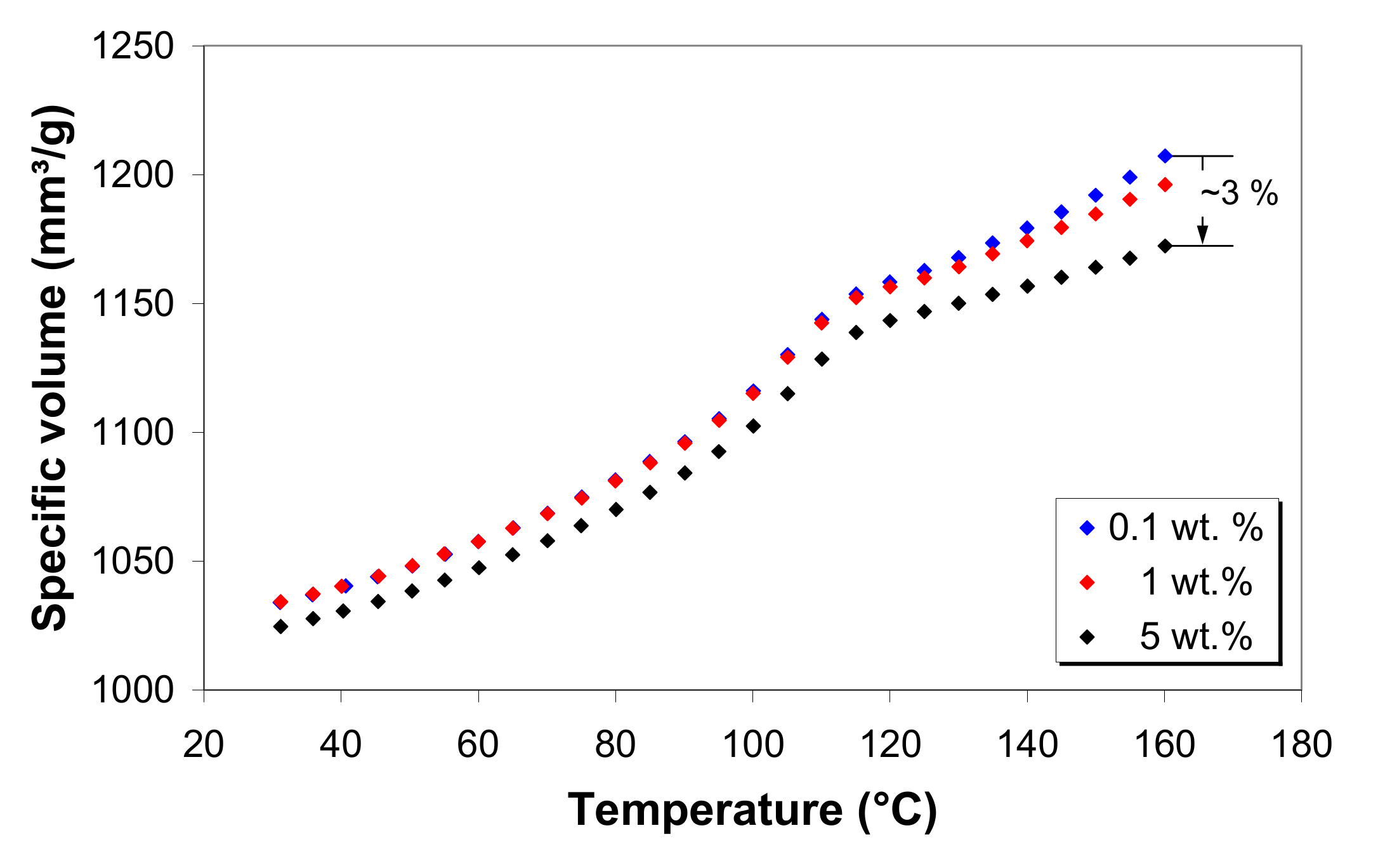
3.8. Pressure–Volume–Temperature Behavior of LDPE/MWCNT Composites
3.9. Modeling of the Specific Volume of LDPE/MWCNT Composites
3.10. Density of LDPE/MWCNT Composite
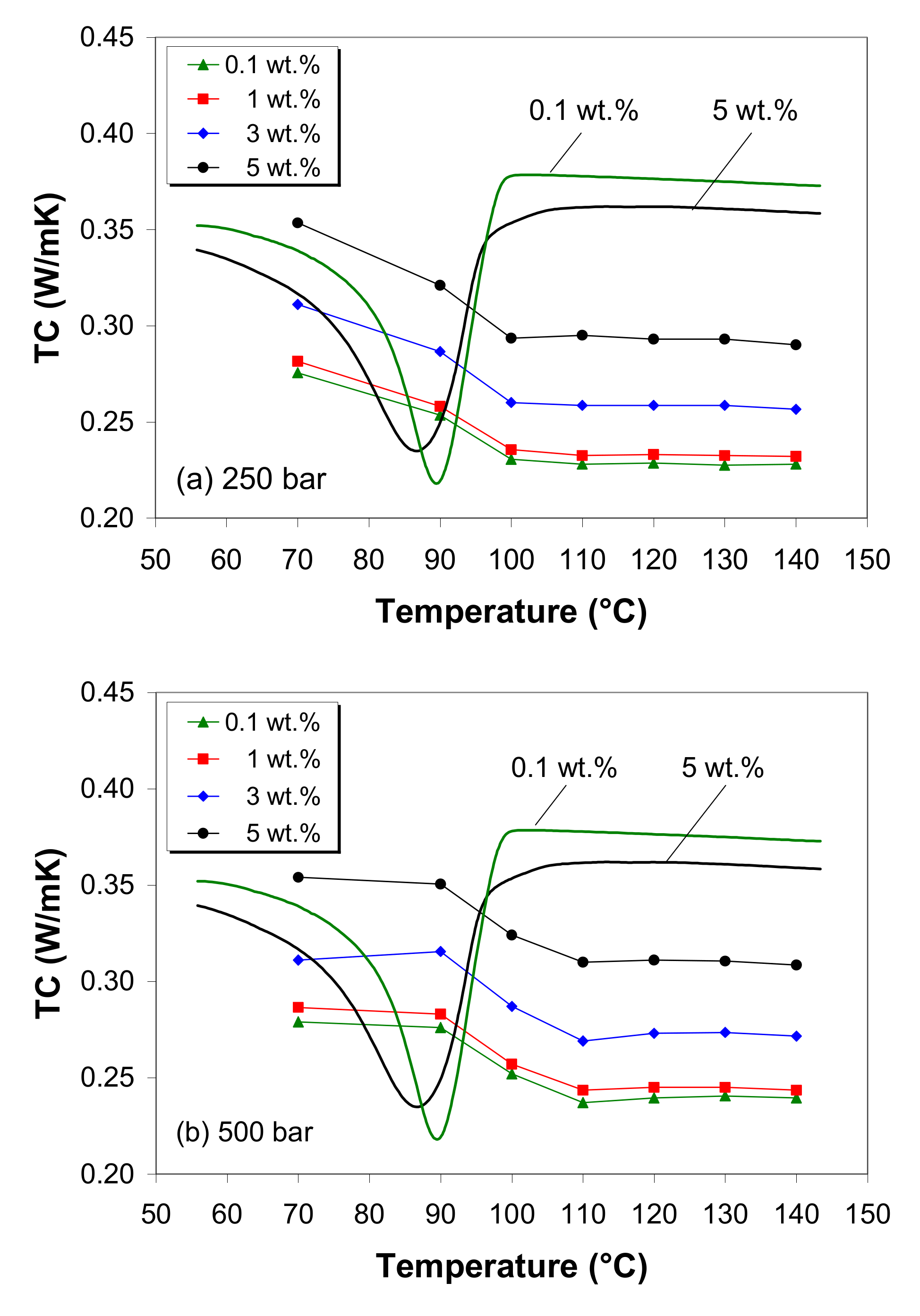
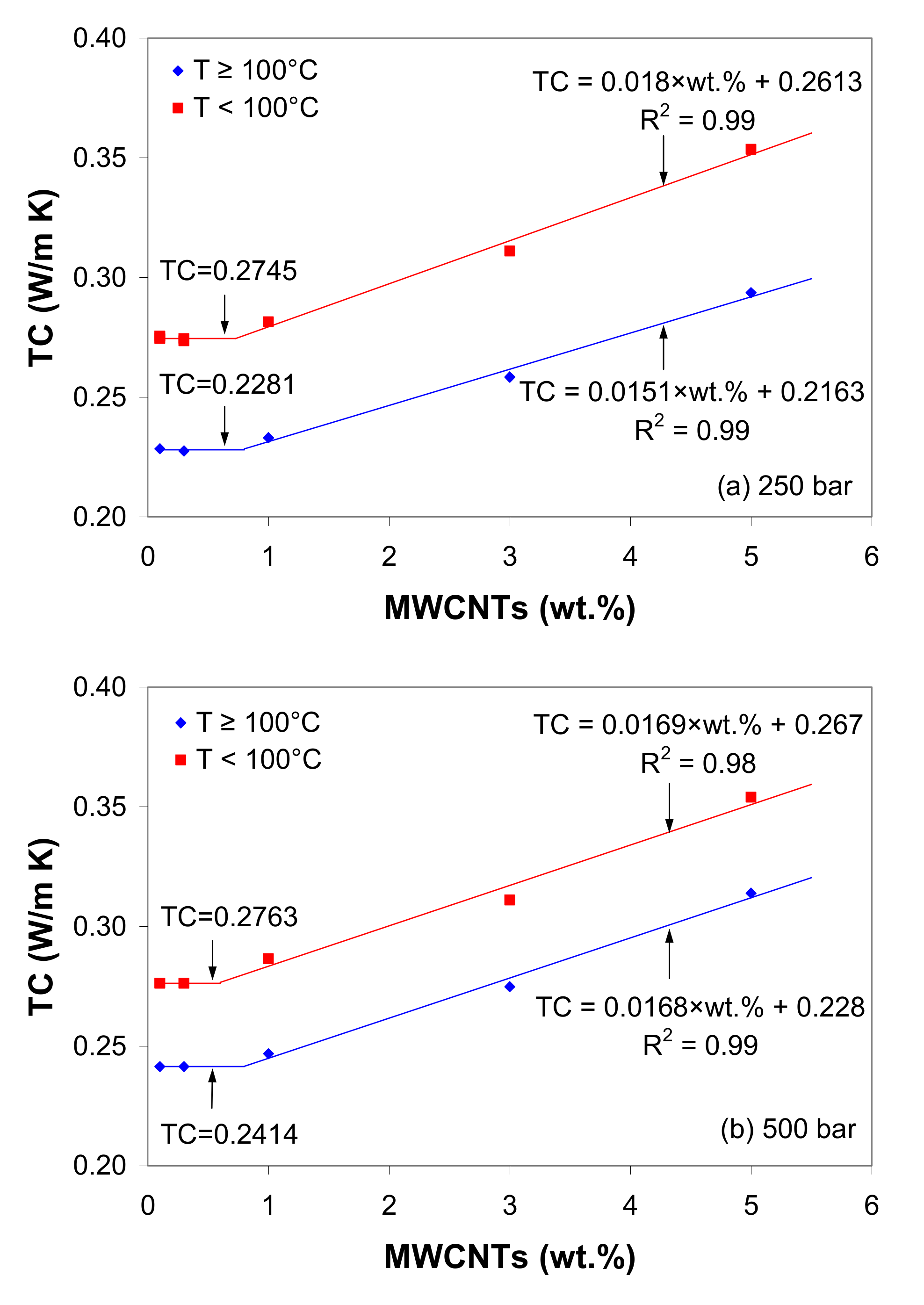
3.11. Thermal Conductivity
4. Conclusions
- The LDPE/MWCNT composite features a strong increasing melt shear viscosity with increasing MWCNT loading beyond 1 wt.%, and this effect decreases with increasing shear rates due to solid-like behavior. Moreover, a sharp decrease in the Arrhenius flow activation energy is observed at MWCNT loading higher than 3 wt.%, reflecting the strong nanotube–nanoube interactions.
- The specific volume of the LDPE/MWCNT composites decreases with increasing MWCNT loading, suggesting an improvement of the shrinkage and warpage behavior in the presence of nanotubes.
- The thermal conductivity of the LDPE/MWCNT composite is nearly independent of nanotube loading up to the thermal percolation threshold of 1 wt.% and shows a linear increasing trend with further increases in nanotube loading.
- The Carreau–Winter and Cross viscosity models and the Tait equation capture very well the experimentally measured melt shear viscosity and the specific volume, respectively.
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bikiaris, D. Microstructure and properties of polypropylene/carbon nanotube nanocomposites. Materials 2010, 3, 2884–2946. [Google Scholar] [CrossRef]
- Liu, T.X.; Huang, S. Morphology and thermal behavior of polymer/carbon nanotube composites. In Physical Properties and Applications of Polymer Nanocomposites; Tjong, S.C., Mai, Y.W., Eds.; Woodhead Publishing: Cambridge, UK, 2010; pp. 529–562. ISBN 9781845696726. [Google Scholar]
- McNally, T.; Pötschke, P. Polymer-Carbon Nanotube Composites: Preparation, Properties and Applications, 1st ed.; Woodhead Publishing: Cambridge, UK, 2011; ISBN 9780857091390. [Google Scholar]
- Han, Z.; Fina, A. Thermal conductivity of carbon nanotubes and their polymer nanocomposites: A review. Prog. Polym. Sci. 2011, 36, 914–944. [Google Scholar] [CrossRef]
- Sabu, T.; Rene, M.; Jiji, A. Rheology and Processing of Polymer Nanocomposites; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; ISBN 9781118969793. [Google Scholar]
- Burger, N.; Laachachi, A.; Ferriol, M.; Lutz, M.; Toniazzo, V.; Ruch, D. Review of thermal conductivity in composites: Mechanisms, parameters and theory. Prog. Polym. Sci. 2016, 61, 1–28. [Google Scholar] [CrossRef]
- Huang, C.; Qian, X.; Yang, R. Thermal conductivity of polymers and polymer nanocomposites. Mater. Sci. Eng. R Rep. 2018, 132, 1–22. [Google Scholar] [CrossRef]
- Kumanek, B.; Janas, D. Thermal conductivity of carbon nanotube networks: A review. J. Mater. Sci. 2019, 7397–7427. [Google Scholar] [CrossRef]
- McNally, T.; Pötschke, P.; Halley, P.; Murphy, M.; Martin, D.; Bell, S.E.J.; Brennan, G.P.; Bein, D.; Lemoine, P.; Quinn, J.P. Polyethylene multiwalled carbon nanotube composites. Polymer 2005, 46, 8222–8232. [Google Scholar] [CrossRef]
- Aalaie, J.; Rahmatpour, A.; Maghami, S. Preparation and characterization of linear low density polyethylene/carbon nanotube nanocomposites. J. Macromol. Sci. B 2007, 46, 877–889. [Google Scholar] [CrossRef]
- Bangarusampath, D.S.; Ruckdäschel, H.; Altstädt, V.; Sandler, J.K.W.; Garray, D.; Shaffer, M.S.P. Rheology and properties of melt-processed poly(ether ether ketone)/multi-wall carbon nanotube composites. Polymer 2009, 50, 5803–5811. [Google Scholar] [CrossRef]
- Micusik, M.; Omastova, M.; Pionteck, J.; Pandis, C.; Logakis, E.; Pissis, P. Influence of surface treatment of multiwall carbon nanotubes on the properties of polypropylene/carbon nanotubes nanocomposites. Polym. Adv. Technol. 2011, 22, 38–47. [Google Scholar] [CrossRef]
- Steinmann, W.; Vad, T.; Weise, B.; Wulfhorst, J.; Seide, G.; Gries, T.; Heidelmann, M.; Weirich, T. Extrusion of CNT-modified polymers with low viscosity—influence of crystallization and CNT orientation on the electrical properties. Polym. Polym. Compos. 2013, 21, 473–482. [Google Scholar] [CrossRef]
- Verma, P.; Verma, M.; Gupta, A.; Chauhan, S.S.; Malik, R.S.; Choudhary, V. Multi walled carbon nanotubes induced viscoelastic response of polypropylene copolymer nanocomposites: Effect of filler loading on rheological percolation. Polym. Test. 2016, 55, 1–9. [Google Scholar] [CrossRef]
- Stan, F.; Fetecau, C.; Stanciu, N.V.; Rosculet, R.T.; Sandu, I.L. Investigation of structure-property relationships in thermoplastic polyurethane/multiwalled carbon nanotube composites. In Proceedings of the ASME 2017 12th International Manufacturing Science and Engineering Conference, Los Angeles, CA, USA, 4–8 June 2017. [Google Scholar]
- Fang, C.; Yang, R.; Zhang, Z.; Zhou, X.; Lei, W.; Cheng, Y.; Zhang, W.; Wang, D. Effect of multi-walled carbon nanotubes on the physical properties and crystallization of recycled PET/TPU composites. RSC Adv. 2018, 8, 8920–8928. [Google Scholar] [CrossRef]
- Gumede, T.P.; Luyt, A.S.; Perez-Camargo, R.A.; Tercjak, A.; Muller, A.J. Morphology, nucleation, and isothermal crystallization kinetics of poly(butylenes succinate) mixed with a polycarbonate/MWCNT masterbatch. Polymers 2018, 10, 424. [Google Scholar] [CrossRef]
- Stanciu, N.V.; Stan, F.; Sandu, I.L.; Susac, F.; Fetecau, C.; Rosculet, R.T. Mechanical, electrical and rheological behavior of ethylene-vinyl acetate/multi-walled carbon nanotube composites. Polymers 2019, 11, 1300. [Google Scholar] [CrossRef] [PubMed]
- Seo, M.K.; Park, S.J. Electrical resistivity and rheological behaviors of carbon nanotubes-filled polypropylene composites. Chem. Phys. Lett. 2004, 395, 44–48. [Google Scholar] [CrossRef]
- Seo, M.K.; Lee, J.-R.; Park, S.-J. Crystallization kinetics and interfacial behaviors of polypropylene composites reinforced with multi-walled carbon nanotubes. Mater. Sci. Eng. A 2005, 404, 79–84. [Google Scholar] [CrossRef]
- Hu, G.; Zhao, C.; Zhang, S.; Yang, M.; Wang, Z. Low percolation thresholds of electrical conductivity and rheology in poly(ethylene terephthalate) through the networks of multi-walled carbon nanotubes. Polymer 2006, 47, 480–488. [Google Scholar] [CrossRef]
- Abbasi, S.; Carreau, P.J.; Derdouri, A.; Moan, M. Rheological properties and percolation in suspensions of multiwalled carbon nanotubes in polycarbonate. Rheol. Acta 2009, 48, 943–959. [Google Scholar] [CrossRef]
- Singh, B.P.; Prabha; Saini, P.; Gupta, T.; Garg, P.; Kumar, G.; Pande, I.; Pande, S.; Seth, R.K.; Dhawan, S.K.; et al. Designing of multiwalled carbon nanotubes reinforced low density polyethylene nanocomposites for suppression of electromagnetic radiation. J. Nanopart. Res. 2011, 13, 7065–7074. [Google Scholar] [CrossRef]
- Huegun, A.; Fernandez, M.; Munoz, M.E.; Santamaría, A. Rheological properties and electrical conductivity of irradiated WCNT/PP nanocomposites. Compos. Sci. Technol. 2012, 72, 1602–1607. [Google Scholar] [CrossRef]
- Narimani, A.; Hemmati, M. Electrical and steady shear rheological behaviour of polypropylene composites reinforced with single-walled carbon nanotubes. Polym. Polym. Compos. 2014, 22, 533–540. [Google Scholar] [CrossRef]
- Versavaud, S.; Regnier, G.; Gouadec, G.; Vincent, M. Influence of injection molding on the electrical properties of polyamide 12 filled with multi-walled carbon nanotubes. Polymer 2014, 55, 6811–6818. [Google Scholar] [CrossRef]
- Kalakonda, P.; Cabrera, Y.; Judith, R.; Georgiev, G.Y.; Cebe, P.; Iannacchione, G.S. Studies of electrical and thermal conductivities of sheared multi-walled carbon nanotube with isotactic polypropylene polymer composites. Nanomater. Nanotechnol. 2015, 5, 2. [Google Scholar] [CrossRef]
- Berber, S.; Kwon, Y.K.; Tomanek, D. Unusually high thermal conductivity of carbon nanotubes. Phys. Rev. Lett. 2000, 84, 4613–4616. [Google Scholar] [CrossRef]
- Kim, P.; Shi, L.; Majumdar, A.; McEuen, P.L. Thermal transport measurements of individual multiwalled nanotubes. Phys. Rev. Lett. 2001, 87, 215502. [Google Scholar] [CrossRef]
- Biercuk, M.J.; Llaguno, M.C.; Radosavljevic, M.; Hyun, J.K.; Johnson, A.T.; Fischer, J.E. Carbon Nanotube Composites for Thermal Management. Appl. Phys. Lett. 2002, 80, 2767–2769. [Google Scholar] [CrossRef]
- Hida, S.; Hori, T.; Shiga, T.; Elliott, J.; Shiomi, J. Thermal resistance and phonon scattering at the interface between carbon nanotube and amorphous polyethylene. Int. J. Heat Mass Transfer 2013, 67, 1024–1029. [Google Scholar] [CrossRef]
- Barkauskas, J.; Mikoliunaite, L.; Paklonskaite, I.; Genys, P.; Petroniene, J.J.; Morkvenaite-Vilkonciene, I.; Ramanaviciene, A.; Samukaite-Bubniene, U.; Ramanavicius, A. Single-walled carbon nanotube based coating modified with reduced grapheme oxide for the design of amperometric biosensors. Mater. Sci. Eng. C 2019, 98, 515–523. [Google Scholar] [CrossRef]
- Kareiva, A.; Beganskiene, A.; Senvaitiene, J.; Ramanaviciene, A.; Vaitkus, R.; Barkauskas, J.; Ramanavicius, A. Evaluation of carbon-based nanostructures suitable for the development of black pigments and glazes. Colloid Surf. Asp. 2019, 580, 123718. [Google Scholar] [CrossRef]
- Shenoy, A.V. Rheology of Filled Polymer Systems; Springer: Berlin, Germany, 1999; ISBN 9789401592130. [Google Scholar]
- Morrison, F.A. Understanding Rheology; Oxford University Press: Oxford, UK, 2001; ISBN 9780195141665. [Google Scholar]
- Zheng, R.; Tanner, R.I.; Fan, X.J. Injection Molding: Integration of Theory and Modeling Methods; Springer: Berlin, Germany, 2011; ISBN 9783642212635. [Google Scholar]
- Aho, J. Rheological Characterization of Polymer Melts in Shear and Extension: Measurement Reliability and Data for Practical Processing; Tampere University of Technology: Tampere, Finland, 2011. [Google Scholar]
- Aho, J.; Boetker, J.P.; Baldursdottir, S.; Rantanen, J. Rheology as a tool for evaluation of melt processability of innovative dosage forms. Int. J. Pharm. 2015, 494, 623–624. [Google Scholar] [CrossRef]
- Palza, H.; Reznik, B.; Kappes, M.; Hennrich, F.; Naue, I.F.C.; Wilhelm, M. Characterization of melt flow instabilities in polyethylene/carbon nanotube composites. Polymer 2010, 51, 3753–3761. [Google Scholar] [CrossRef]
- Stan, F.; Stanciu, N.V.; Fetecau, C. Melt rheological properties of ethylene-vinyl acetate/multi-walled carbon nanotube composites. Compos. Part B 2017, 110, 20–31. [Google Scholar] [CrossRef]
- Stanciu, N.V.; Stan, F.; Fetecau, C. Melt shear rheology and pVT behavior of polypropylene/multi-walled carbon nanotube composites. Mater. Plast. 2018, 55, 482–487. [Google Scholar] [CrossRef]
- Thiebaud, F.; Gelin, J.C. Characterization of rheological behaviors of polypropylene/carbon nanotubes composites and modeling their flow in a twin-screw mixer. Compos. Sci. Technol. 2010, 70, 647–656. [Google Scholar] [CrossRef]
- Liang, J.Z.; Chen, C.Y.; Zou, C.P.; Tsui, C.P.; Tang, C.Y.; Zhang, S.D. Melt flow behavior of polypropylene composites filled with multi-walled carbon nanotubes during extrusion. Polym. Test. 2015, 45, 41–46. [Google Scholar] [CrossRef]
- Gracia-Fernandez, C.; Gomez-Barreiro, S.; Lopez-Beceiro, J.; Naya, S.; Artiaga, R. Characterization of MWCNT/TPU systems by large amplitude oscillation shear. J. Therm. Anal. Calorim. 2014, 115, 1727–1731. [Google Scholar] [CrossRef]
- Chang, R.Y.; Chen, C.H.; Su, K.S. Modifying the Tait equation with cooling-rate effects to predict the pressure-volume-temperature behaviors of amorphous polymers: Modeling and experiments. Polym. Eng. Sci. 1996, 36, 1789–1795. [Google Scholar] [CrossRef]
- Wang, J. PVT properties of polymers for injection molding. In Some Critical Issues for Injection Molding; Wang, J., Ed.; IntechOpen: London, UK, 2012; ISBN 9789535102977. [Google Scholar]
- Fischer, J.M. Handbook of Molded Part Shrinkage and Warpage, 2nd ed.; Elsevier Inc.: New York, NY, USA, 2013. [Google Scholar]
- Sun, X.; Su, X.; Tibbenham, P.; Mao, J.; Tao, J. The application of modified PVT data on the warpage prediction of injection molded part. J. Polym. Res. 2016, 23, 86. [Google Scholar] [CrossRef]
- Zoller, P. Pressure-volume-temperature relationship of solid and molten polypropylene and poly(butene-1). J. Appl. Polym. Sci. 1979, 23, 1057–1061. [Google Scholar] [CrossRef]
- Zoller, P. A study of the pressure-volume-temperature relationships of four related amorphous polymers: Polycarbonate, polyarylate, phenoxy, and polysulfone. J. Polym. Sci. 1982, 20, 1453–1464. [Google Scholar] [CrossRef]
- Mekhilef, N. Viscoelastic and pressure–volume–temperature properties of poly(vinylidene fluoride) and poly(vinylidene fluoride)–hexafluoropropylene copolymers. J. Appl. Polym. Sci. 2001, 80, 230–241. [Google Scholar] [CrossRef]
- Park, H.E.; Lim, S.T.; Laun, H.M.; Dealy, J.M. Measurement of pressure coefficient of melt viscosity: Drag flow versus capillary flow. Rheol. Acta 2008, 47, 1023–1038. [Google Scholar] [CrossRef]
- Tao, R.; Simon, S.L. Pressure-volume-temperature and glass transition behavior of silica-polystyrene nanocomposites. J. Polym. Sci. Pol. Phys. 2015, 53, 1131–1138. [Google Scholar] [CrossRef]
- Pionteck, J. Determination of pressure dependence of polymer phase transitions by pVT analysis. Polymers 2018, 10, 578. [Google Scholar] [CrossRef] [PubMed]
- Avalos-Belmontes, F.; Ramos-deValle, L.F.; Ramirez-Vargas, E.; Sanchez-Valdes, S.; Mendez-Nonel, J.; Zitzumbo-Guzman, R. Nucleating effect of carbon nanoparticles and their influence on the thermal and chemical stability of polypropylene. J. Nanomater. 2012. [Google Scholar] [CrossRef]
- Schmidt, F.; Pirc, N.; Mongeau, M.; Chinesta, F. Efficient mold cooling optimization by using model reduction. Int. J. Mater. Form. 2011, 4, 73–82. [Google Scholar] [CrossRef][Green Version]
- Suplicz, A.; Kovacs, J.G. Development of thermally conductive polymer materials and their investigation. Mater. Sci. Forum 2012, 729, 80–84. [Google Scholar] [CrossRef]
- Choi, T.Y.; Poulikakos, D. Measurement of thermal conductivity of individual multiwalled carbon nanotubes by the 3 − ω method. Appl. Phys. Lett. 2005, 87, 013108. [Google Scholar] [CrossRef]
- Zhou, T.Y.; Tsui, G.C.P.; Liang, J.Z.; Zou, S.Y.; Tang, C.Y.; Miskovic-Stankovic, V. Thermal properties and thermal stability of PP/MWCNT composites. Compos. Part B-Eng. 2016, 90, 107–114. [Google Scholar] [CrossRef]
- Marconnet, A.M.; Yamamoto, N.; Panzer, M.A.; Wardle, B.L.; Goodson, K.E. Thermal conduction in aligned carbon nanotube-polymer nanocomposites in high packing density. ACS Nano 2011, 5, 4818–4825. [Google Scholar] [CrossRef]
- Lucyshyn, T.; Knapp, G.; Kipperer, M.; Holzer, C. Determination of the transition temperature at different cooling rates and its influence on prediction of shrinkage and warpage in injection molding simulation. J. Appl. Polym. Sci. 2012, 123, 1162–1168. [Google Scholar] [CrossRef]
- Spina, R.; Spekowius, M.; Dahlmann, R.; Hopmann, C. Analysis of polymer crystallization and residual stresses in injection molded parts. Int. J. Precis. Eng. Manuf. 2014, 15, 89–96. [Google Scholar] [CrossRef]
- Spina, R.; Spekowius, M.; Hopmann, C. Multiphysics simulation of thermoplastic polymer crystallization. Mater. Des. 2016, 95, 455–469. [Google Scholar] [CrossRef]
- Moldflow: Plastic Injection and Compression Mold Simulation for Design and Manufacturing. Available online: https://www.autodesk.com/products/moldflow/overview (accessed on 6 February 2020).
- Moldex3D Products Overview. Available online: https://www.moldex3d.com/en/products/software/moldex3d/ (accessed on 6 February 2020).
- Product Datasheet for LDPE ExxonMobilTM LD 655. Available online: https://exxonmobilchemical.ulprospector.com (accessed on 6 February 2020).
- Product Datasheet for NC7000™ Industrial Multiwall Carbon Nanotubes. Available online: https://www.nanocyl.com/product/nc7000/ (accessed on 6 February 2020).
- Göttfert. WinRheo II Software Documentation; Göttfert: Buchen, Germany, 2018. [Google Scholar]
- Sinha Ray, S. Crystallization behavior, kinetics and morphology of environmentally friendly polymer nanocomposites using biodegradable polymer matrices and clay/carbon nanotube (CNT) reinforcements. In Environmentally Friendly Polymer Nanocomposites; Sinha Ray, S., Ed.; Woodhead Publishing: Cambridge, UK, 2013; pp. 346–384. ISBN 9780857097774. [Google Scholar]
- Sinha Ray, S.; Maiti, P.; Okamoto, M.; Yamada, K.; Ueda, K. New polylactide-layered silicate nanocomposites. 1. Preparation, characterization, and properties. Macromolecules 2002, 35, 3104–3110. [Google Scholar] [CrossRef]
- Hristov, V.; Vlachopoulos, J. Effects of polymer molecular weight and filler particle size on flow behavior of wood polymer composites. Polym. Compos. 2008, 29, 831–839. [Google Scholar] [CrossRef]
- Moldenaers, P.; Vermant, J.; Mewis, J. Origin of nonlinearities in the Bagley plots of thermotropic copolyesters. J. Rheol. 1996, 40, 203–219. [Google Scholar] [CrossRef][Green Version]
- Mezger, T.G. The Rheology Handbook, 2nd ed.; Vincentz Network: Hanover, Germany, 2006; ISBN 3878701748. [Google Scholar]
- Zuidema, H.; Peters, G.W.M.; Meijer, H.E.H. Influence of cooling rate on pVT-data of semi-crystalline polymers. J. Appl. Polym. Sci. 2001, 82, 1170–1186. [Google Scholar] [CrossRef]
- Zhang, S.; Minus, M.L.; Zhu, L.; Wong, C.-P.; Kumar, S. Polymer transcrystallinity induced by carbon nanotubes. Polymer 2008, 49, 1356–1364. [Google Scholar] [CrossRef]
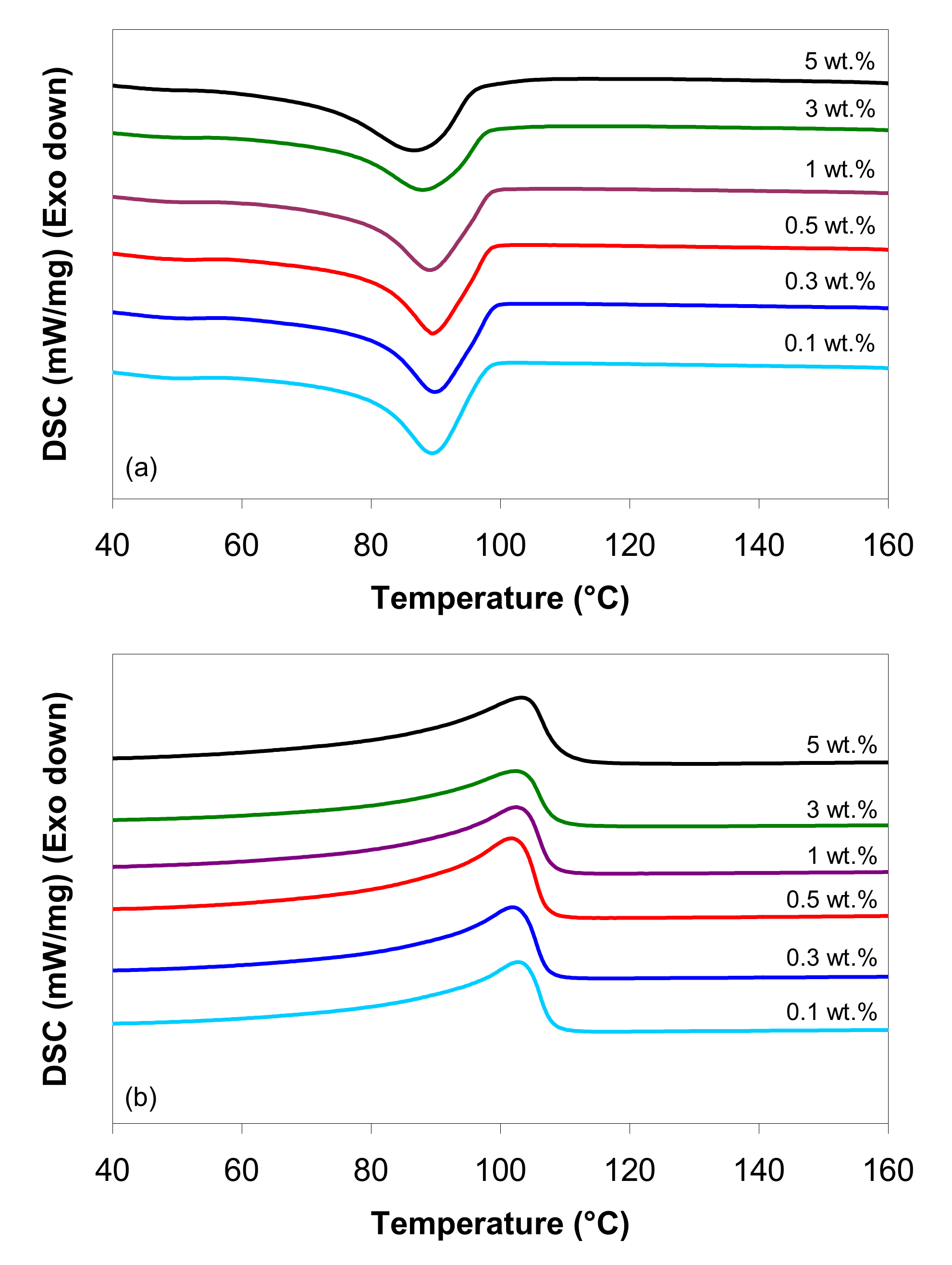





| MWCNTs (wt.%) | 1st Heating Scan | Cooling Scan | 2nd Heating Scan | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 0.1 | 104.8 | 66.3 | 22.6 | 89.7 | 87.0 | 76.54 | 102.8 | 78.9 | 27.0 |
| 0.3 | 104.7 | 71.6 | 24.5 | 89.9 | 83.2 | 74.41 | 102.0 | 82.4 | 28.2 |
| 0.5 | 103.8 | 84.7 | 29.1 | 89.7 | 91.7 | 76.45 | 101.8 | 88.8 | 30.5 |
| 1 | 105.4 | 68.8 | 23.7 | 89.2 | 82.2 | 76.35 | 102.4 | 80.3 | 27.7 |
| 3 | 106.9 | 60.1 | 21.1 | 88.0 | 67.7 | 73.65 | 102.4 | 67.7 | 23.8 |
| 5 | 104.4 | 72.7 | 26.1 | 86.7 | 85.2 | 67.24 | 103.3 | 86.3 | 31.0 |
| Parameters | MWCNTs (wt.%) | |||||
|---|---|---|---|---|---|---|
| 0.1 | 0.3 | 0.5 | 1 | 3 | 5 | |
| (Pa·s) | 211.04 | 276.09 | 271.01 | 259.91 | 280.30 | 653.36 |
| (s) | 0.008 | 0.013 | 0.012 | 0.009 | 0.007 | 0.020 |
| 0.565 | 0.550 | 0.552 | 0.574 | 0.619 | 0.626 | |
| 0.999 | 0.999 | 0.999 | 0.999 | 0.988 | 0.999 | |
| Parameters | MWCNTs (wt.%) | |||||
|---|---|---|---|---|---|---|
| 0.1 | 0.3 | 0.5 | 1 | 3 | 5 | |
| (Pa·s) | 276.55 | 381.76 | 380.72 | 332.03 | 395.09 | 1093.52 |
| (Pa) | 38,364.58 | 28,160.93 | 28,595.41 | 40,465.30 | 42,938.44 | 29,112.84 |
| (s) | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.04 |
| 0.379 | 0.398 | 0.400 | 0.362 | 0.347 | 0.354 | |
| 1.00 | 1.00 | 0.999 | 0.997 | 0.989 | 0.999 | |
| MWCNTs (wt.%) | Temperature (°C) | pVT Density (g/cm3) | Bulk Density (kg/m3) | |
|---|---|---|---|---|
| 10 bar | 1500 bar | |||
| 0.1 | 30 | 0.951 | 0.997 | 0.892 ± 0.0054 |
| 160 | 0.790 | 0.883 | ||
| 0.3 | 30 | 0.954 | 1.000 | 0.895 ± 0.0033 |
| 160 | 0.790 | 0.884 | ||
| 0.5 | 30 | 0.938 | 0.984 | 0.896 ± 0.0046 |
| 160 | 0.784 | 0.880 | ||
| 1 | 30 | 0.950 | 0.996 | 0.896 ± 0.0043 |
| 160 | 0.798 | 0.889 | ||
| 3 | 30 | 0.947 | 0.993 | 0.905 ± 0.0075 |
| 160 | 0.802 | 0.893 | ||
| 5 | 30 | 0.960 | 1.005 | 0.916 ± 0.0035 |
| 160 | 0.814 | 0.906 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stanciu, N.-V.; Stan, F.; Fetecau, C. Experimental Investigation of the Melt Shear Viscosity, Specific Volume and Thermal Conductivity of Low-Density Polyethylene/Multi-Walled Carbon Nanotube Composites Using Capillary Flow. Polymers 2020, 12, 1230. https://doi.org/10.3390/polym12061230
Stanciu N-V, Stan F, Fetecau C. Experimental Investigation of the Melt Shear Viscosity, Specific Volume and Thermal Conductivity of Low-Density Polyethylene/Multi-Walled Carbon Nanotube Composites Using Capillary Flow. Polymers. 2020; 12(6):1230. https://doi.org/10.3390/polym12061230
Chicago/Turabian StyleStanciu, Nicoleta-Violeta, Felicia Stan, and Catalin Fetecau. 2020. "Experimental Investigation of the Melt Shear Viscosity, Specific Volume and Thermal Conductivity of Low-Density Polyethylene/Multi-Walled Carbon Nanotube Composites Using Capillary Flow" Polymers 12, no. 6: 1230. https://doi.org/10.3390/polym12061230
APA StyleStanciu, N.-V., Stan, F., & Fetecau, C. (2020). Experimental Investigation of the Melt Shear Viscosity, Specific Volume and Thermal Conductivity of Low-Density Polyethylene/Multi-Walled Carbon Nanotube Composites Using Capillary Flow. Polymers, 12(6), 1230. https://doi.org/10.3390/polym12061230