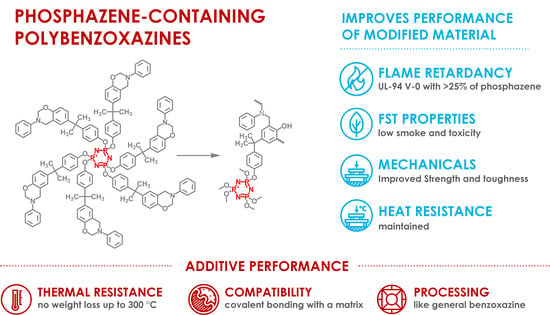
Synthesis of Phosphazene-Containing, Bisphenol A-Based Benzoxazines and Properties of Corresponding Polybenzoxazines
Abstract
1. Introduction
2. Materials and Methods
2.1. Starting Materials
2.2. Synthesis Methods
2.2.1. Synthesis of Model Monomer BA-a
2.2.2. Synthesis of Hydrohyaryloxyphosphazenes (HAP)
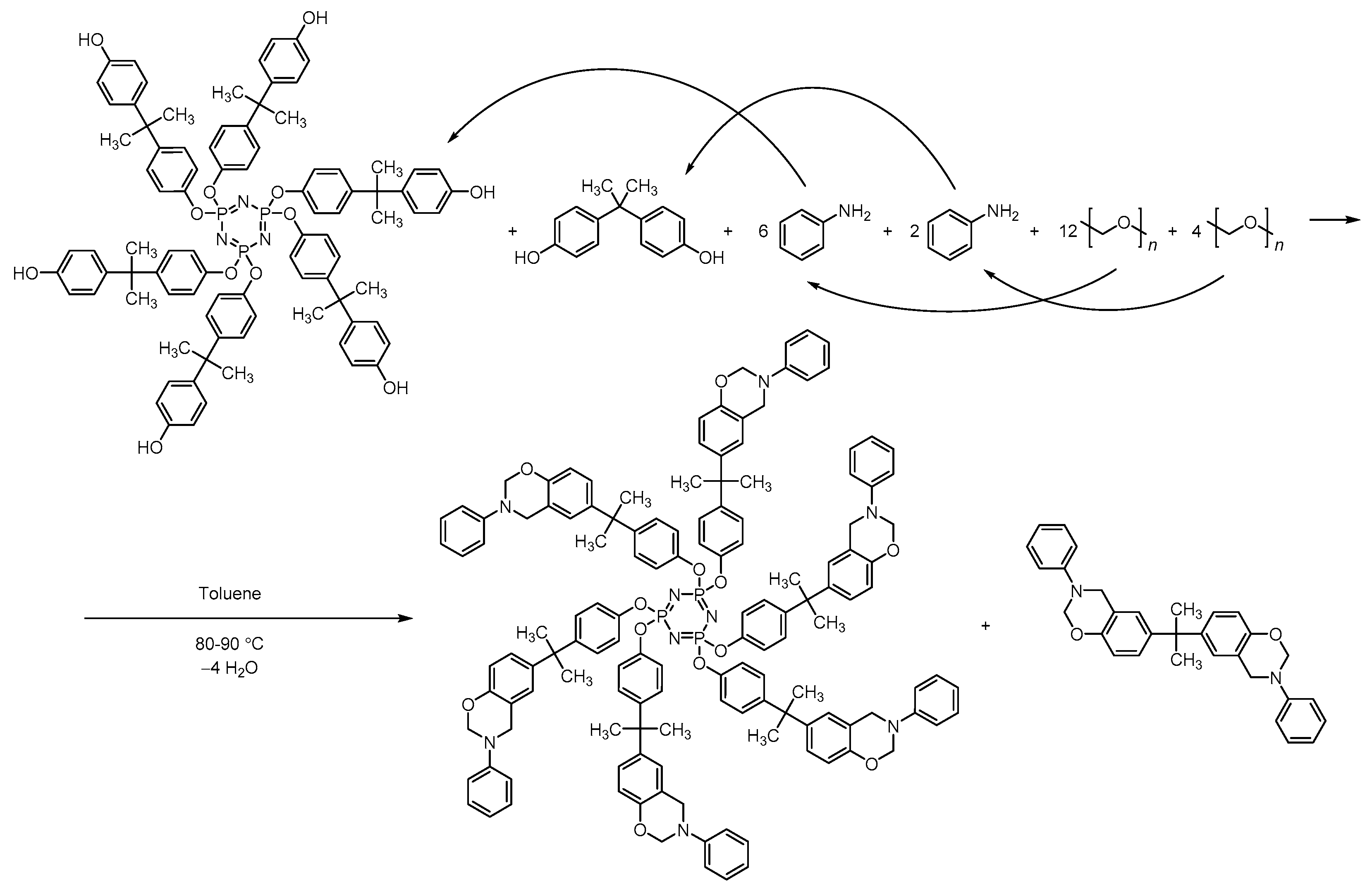
2.2.3. Synthesis of Phosphazene-Containing Benzoxazines Based on Mixtures of Hydroxyaryloxycyclotriphosphazenes and Bisphenol A
2.3. Curing of Benzoxazines
2.4. Methods of Analysis
3. Results and Discussion
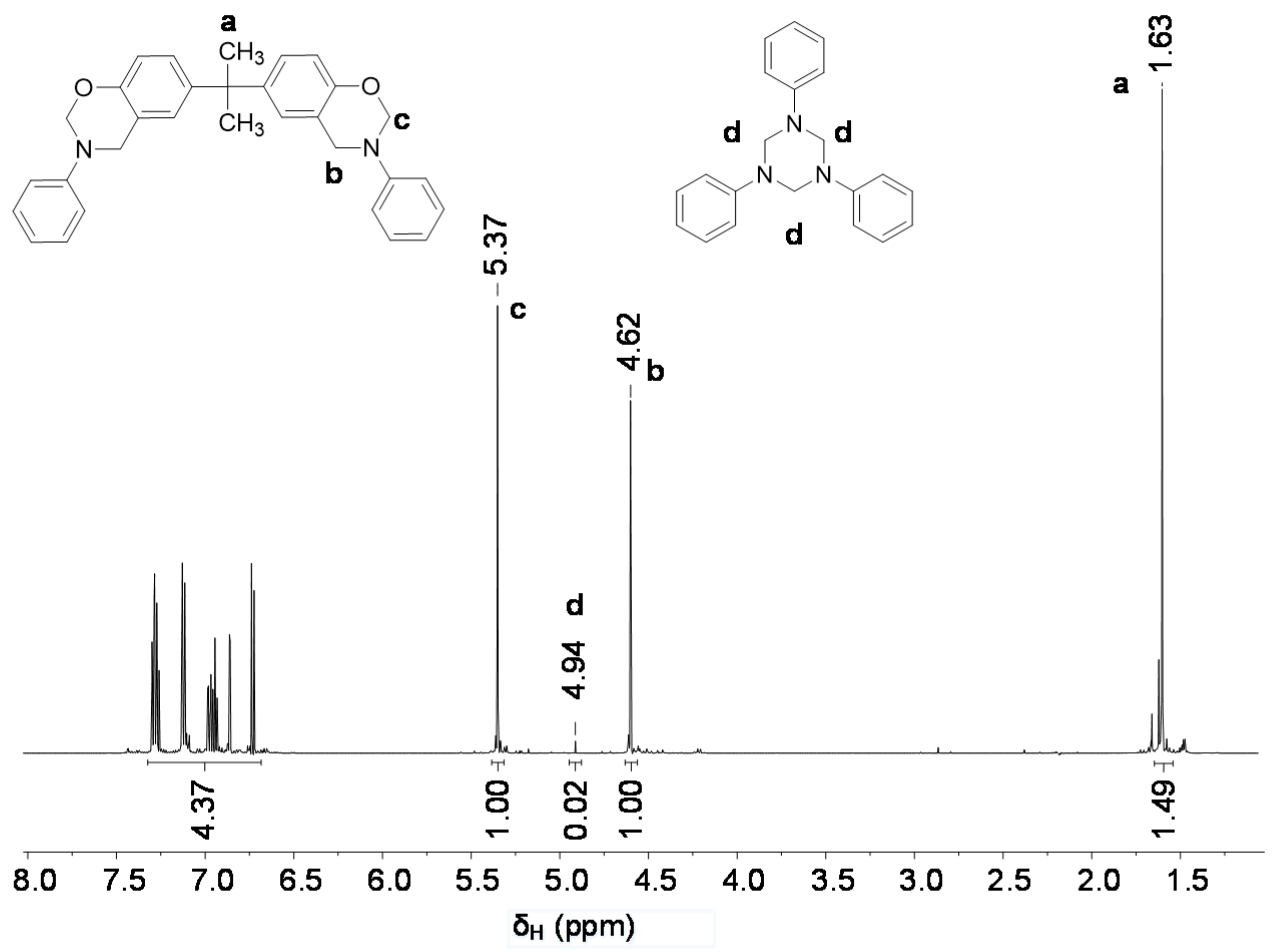
3.1. Synthesis of Model Benzoxazines
3.2. Synthesis of Hydroxyaryloxyphosphazene Precursors

3.3. Synthesis of Phosphazene-Containing Benzoxazines
3.4. Polymerization of Phosphazene-Containing Benzoxazines and Properties of the Obtained Polybenzoxazines
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Russell, V.M.; Koenig, J.L.; Low, H.Y.; Ishida, H. Study of the characterization and curing of benzoxazines using 13C solid-state nuclear magnetic resonance. J. Appl. Polym. Sci. 1998, 70, 1413–1425. [Google Scholar] [CrossRef]
- Kim, H.D.; Ishida, H. A study on hydrogen-bonded network structure of polybenzoxazines. J. Phys. Chem. A 2002, 106, 3271–3280. [Google Scholar] [CrossRef]
- Wang, C.-F.; Chiou, S.-F.; Ko, F.-H.; Chou, C.-T.; Lin, H.-C.; Huang, C.-F.; Chang, F.-C. Fabrication of Biomimetic Super-Amphiphobic Surfaces Through Plasma Modification of Benzoxazine Films. Macromol. Rapid Commun. 2006, 27, 333–337. [Google Scholar] [CrossRef]
- Takeichi, T.; Kawauchi, T.; Agag, T. High Performance Polybenzoxazines as a Novel Type of Phenolic Resin. Polym. J. 2008, 40, 1121–1131. [Google Scholar] [CrossRef]
- Wang, Y.; Kou, K.; Wu, G.; Feng, A.; Zhuo, L. The effect of bis allyl benzoxazine on the thermal, mechanical and dielectric properties of bismaleimide–cyanate blend polymers. RSC Adv. 2015, 5, 58821–58831. [Google Scholar] [CrossRef]
- Jin, L.; Agag, T.; Ishida, H. Bis(benzoxazine-maleimide)s as a novel class of high performance resin: Synthesis and properties. Eur. Polym. J. 2010, 46, 354–363. [Google Scholar] [CrossRef]
- Li, S.; Zou, T. Synthesis, Characterization of New Carboxylic Acid-Containing Benzoxazine and Its Cocuring Behaviors with Bisoxazoline. J. Appl. Polym. Sci. 2012, 123. [Google Scholar] [CrossRef]
- Agag, T.; Takeichi, T. Novel Benzoxazine Monomers Containing p-Phenyl Propargyl Ether: Polymerization of Monomers and Properties of Polybenzoxazines. Macromolecules 2001, 34, 7257–7263. [Google Scholar] [CrossRef]
- Ohashi, S.; Kilbane, J.; Heyl, T.; Ishida, H. Synthesis and Characterization of Cyanate Ester Functional Benzoxazine and Its Polymer. Macromolecules 2015, 48, 8412–8417. [Google Scholar] [CrossRef]
- Zhang, H.; Lu, Z. Synthesis and characterization of novel benzoxazines containing nitrile and allyl groups and their polymers. e-Polymers 2010, 10. [Google Scholar] [CrossRef][Green Version]
- Chiu, W.-M.; Wang, Y.-X.; Tsai, P.-A.; Wu, J.-H. Preparation and characterization of novel polybenzoxazine hybrid materials. High Perform. Polym. 2016, 28, 971–983. [Google Scholar] [CrossRef]
- Mohamed, M.G.; Kuo, S.-W. Polybenzoxazine/Polyhedral Oligomeric Silsesquioxane (POSS) Nanocomposites. Polymers 2016, 8, 225. [Google Scholar] [CrossRef]
- Guglielmi, M.; Colombo, P.; Brusatin, G.; Facchin, G.; Gleria, M. New materials based on the reaction of cyclo- and poly-(organo phosphazenes) with SiO2, TiO2 and ZrO2 precursors. J. Sol Gel Sci. Technol. 1994, 2, 109–114. [Google Scholar] [CrossRef]
- Krishnadevi, K.; Selvaraj, V. Development of halogen-free flame retardant phosphazene and rice husk ash incorporated benzoxazine blended epoxy composites for microelectronic applications. N. J. Chem. 2015, 39, 6555–6567. [Google Scholar] [CrossRef]
- Sun, J.; Wang, X.; Wu, D. Novel Spirocyclic Phosphazene-Based Epoxy Resin for Halogen-Free Fire Resistance: Synthesis, Curing Behaviors, and Flammability Characteristics. ACS Appl. Mater. Interfaces 2012, 4, 4047–4061. [Google Scholar] [CrossRef] [PubMed]
- Schartel, B. Phosphorus-based Flame Retardancy Mechanisms—Old Hat or a Starting Point for Future Development? Materials 2010, 3, 4710–4745. [Google Scholar] [CrossRef] [PubMed]
- Stewart, F.F. Phosphazenes. In Organophosphorus Chemistry; Allen, D.W., Loakes, D., Tebby, J.C., Eds.; Royal Society of Chemistry: London, UK, 2013; Volume 42, pp. 216–262. ISBN 978-1-84973-584-1. [Google Scholar]
- Onuchin, D.V.; Sirotin, I.S.; Sarychev, I.A.; Bornosuz, N.V.; Kireev, V.V.; Gorbunova, I.Y.; Gorbatkina, Y.A. Physicochemical Properties of Epoxy Composites Modified with Epoxyphosphazene. Polym. Sci. Ser. B 2019, 61, 286–293. [Google Scholar] [CrossRef]
- Machotova, J.; Stranska, E.; Skornok, J.; Zarybnicka, L.; Melanova, K.; Rychly, J.; Ruckerova, A. Fluorine containing self-crosslinking acrylic latexes with reduced flammability and their application as polymer binders for heterogeneous cation-exchange membranes. J. Appl. Polym. Sci. 2017, 134, 45467. [Google Scholar] [CrossRef]
- Wu, X.; Zhou, Y.; Liu, S.-Z.; Guo, Y.-N.; Qiu, J.-J.; Liu, C.-M. Highly branched benzoxazine monomer based on cyclotriphosphazene: Synthesis and properties of the monomer and polybenzoxazines. Polymer 2011, 52, 1004–1012. [Google Scholar] [CrossRef]
- Ma, H.-X.; Zhao, C.; Qiu, J.-J.; Liu, Y.; Liu, C.-M. Synthesis of branched benzoxazine monomers with high molecular mass, wide processing window, and properties of corresponding polybenzoxazines. J. Appl. Polym. Sci. 2017, 134. [Google Scholar] [CrossRef]
- Amarnath, N.; Appavoo, D.; Lochab, B. Eco-Friendly Halogen-Free Flame Retardant Cardanol Polyphosphazene Polybenzoxazine Networks. ACS Sustain. Chem. Eng. 2018, 6, 389–402. [Google Scholar] [CrossRef]
- Tan, Z.-W.; Wu, X.; Zhang, M.; Qiu, J.-J.; Liu, C.-M. Synthesis and properties of main-chain oligomeric benzoxazine precursor containing cyclotriphosphazene units. High Perform. Polym. 2014, 26, 906–913. [Google Scholar] [CrossRef]
- Wu, X.; Liu, S.-Z.; Tian, D.-T.; Qiu, J.-J.; Liu, C.-M. Well-defined organic–inorganic hybrid benzoxazine monomers based on cyclotriphosphazene: Synthesis, properties of the monomers and polybenzoxazines. Polymer 2011, 52, 4235–4245. [Google Scholar] [CrossRef]
- Sirotin, I.S.; Bilichenko, Y.V.; Suraeva, O.V.; Solodukhin, A.N.; Kireev, V.V. Synthesis of oligomeric chlorophosphazenes in the presence of ZnCl2. Polym. Sci. Ser. B 2013, 55, 63–68. [Google Scholar] [CrossRef]
- Riddick, J.A.; Bunger, W.B.; Sakano, T.K. Organic Solvents: Physical Properties and Methods of Purification; Wiley: Hoboken, NJ, USA, 1986; ISBN 978-0-471-08467-9. [Google Scholar]
- Gelman, N.E. Methods of Quantitative Organic Elemental Microanalysis; Khimiya: Moscow, Russia, 1987. [Google Scholar]
- Ishida, H.; Krus, C.M. Synthesis and Characterization of Structurally Uniform Model Oligomers of Polybenzoxazine. Macromolecules 1998, 31, 2409–2418. [Google Scholar] [CrossRef]
- Ishida, H. Process for preparation of benzoxazine compounds in solventless systems. U.S. Patent 5,543,516, 18 May 1994. [Google Scholar]
- Ning, X.; Ishida, H. Phenolic materials via ring-opening polymerization: Synthesis and characterization of bisphenol-A based benzoxazines and their polymers. J. Polym. Sci. Part A Polym. Chem. 1994, 32, 1121–1129. [Google Scholar] [CrossRef]
- Wang, D.; Kincaid, D.S. Method for Producing Benzoxazine Compounds. U.S. Patent Application 20140148597, 24 July 2012. [Google Scholar]
- Aizawa, T.; Hirai, Y.; Numata, S. Method for producing benzoxazine resin. U.S. Patent Application 20040138345, 12 March 2002. [Google Scholar]
- Liu, J. Synthesis, Characterization, Reaction Mechanism and Kinetics of 3,4-dihydro-2H-1,3-benzoxazine and Its Polymer. Ph.D. Thesis, Case Western Reserve University, Cleveland, OH, USA, 1995. [Google Scholar]
- Jun Ko, Y.; Kieu Giang, T.; Yoo, H.; Hwan Kim, J. Structural Study of Bisphenol-A Benzoxazine by Tandem Mass Analysis. Adv. Mater. Res. 2012, 560–561, 62–65. [Google Scholar] [CrossRef]
- Puchot, L.; Verge, P.; Fouquet, T.; Vancaeyzeele, C.; Vidal, F.; Habibi, Y. Breaking the symmetry of dibenzoxazines: A paradigm to tailor the design of bio-based thermosets. Green Chem. 2016, 18, 3346–3353. [Google Scholar] [CrossRef]
- Sirotin, I.S.; Bilichenko, Y.V.; Brigadnov, K.A.; Kireev, V.V.; Suraeva, O.V.; Borisov, R.S. Oligomeric hydroxy-aryloxy phosphazene based on cyclic chlorophosphazenes. Russ. J. Appl. Chem. 2013, 86, 1903–1912. [Google Scholar] [CrossRef]
- Dunkers, J.; Ishida, H. Vibrational assignments of 3-alkyl-3,4-dihydro-6-methyl-2H-1,3-benzoxazines in the fingerprint region. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 1995, 51, 1061–1074. [Google Scholar] [CrossRef]
- Onuchin, D.V.; Sirotin, I.S.; Pavlova, G.A.; Filatov, S.N.; Kireev, V.V.; Kerber, M.L.; Gorbunova, I.Y. Features of Curing of a Diane Epoxy Oligomer Modified with Epoxyphosphazene. Polym. Sci. Ser. B 2018, 60, 182–187. [Google Scholar] [CrossRef]
- Van Krevelen, D.W.; Krevelen, D.W.; Nijenhuis, K. Properties of Polymers: Their Correlation with Chemical Structure; Their Numerical Estimation and Prediction from Additive Group Contributions; Elsevier: Amsterdam, The Netherlands, 2009; ISBN 978-0-08-054819-7. [Google Scholar]
- Tan, Z.-W.; Wu, X.; Zhang, M.; Qiu, J.-J.; Liu, C.-M. Performances improvement of traditional polybenzoxazines by copolymerizing with cyclotriphosphazene-based benzoxazine monomers. Polym. Bull. 2015, 72, 1417–1431. [Google Scholar] [CrossRef]
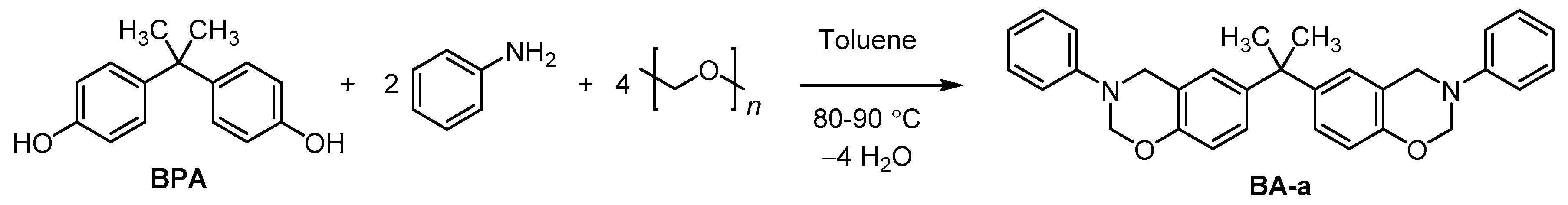



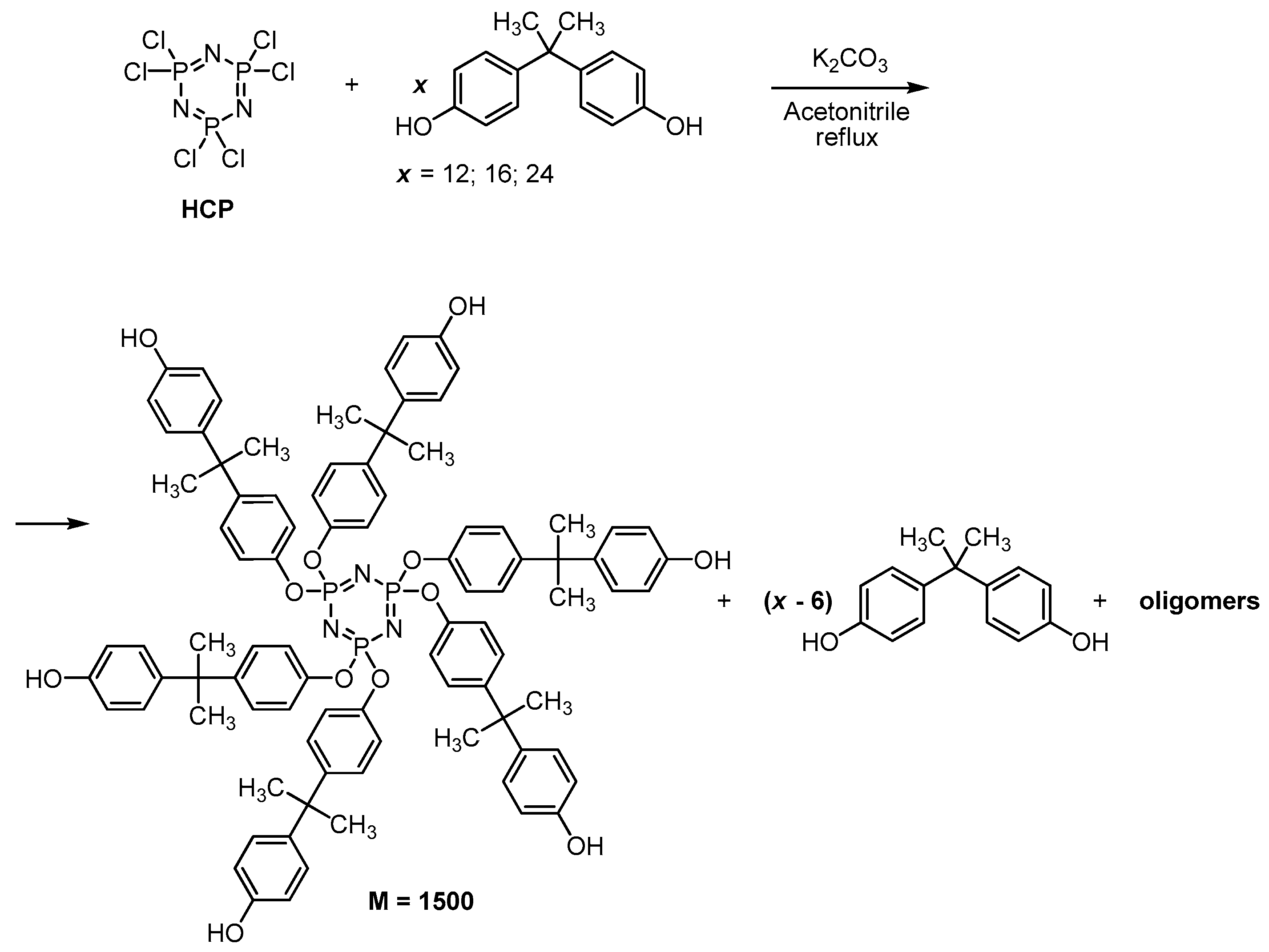





| Parameter | Unit | Molar Ratio of HCP: Bisphenol A | ||
|---|---|---|---|---|
| 1:24 | 1:16 | 1:12 | ||
| The content of the phosphazene component in a mixture with bisphenol A 1 | wt% | 27 | 40 | 52 |
| Designation of benzoxazines synthesized on these mixtures | - | BP-1 | BP-2 | BP-3 |
| Bisphenol A | g | 62.97 | 42.0 | 31.5 |
| mole | 0.276 | 0.184 | 0.138 | |
| Aniline | g | 2.78 | 2.57 | 2.35 |
| mole | 0.0298 | 0.0276 | 0.252 | |
| Paraformaldehyde | g | 2.068 | 1.840 | 1.746 |
| mole | 0.063 | 0.061 | 0.058 | |
| Sample | Phosphazene Content, % | Relative 1H Signal Intensity | |||
|---|---|---|---|---|---|
| Benzoxazine Ring | -CH2-N- (Oligomer) 1 | -CH3 of Bisphenol A | |||
| -CH2-O- | -CH2-N- | ||||
| δH = 5.37 (s) | δH =4.62 (s) | δH = 4.48 (s) | δH = 1.63 (s) | ||
| BA-a | 0 | 1.00 | 1.00 | 0.02 | 1.49 |
| BP-1 | 27 | 1.00 | 1.01 | 0.13 | 2.05 |
| BP-2 | 40 | 1.01 | 0.99 | 0.17 | 2.39 |
| BP-3 | 52 | 1.00 | 1.01 | 0.29 | 3.19 |
| Sample | Mn | Mw | Mw/Mn | Content (wt%) | |
|---|---|---|---|---|---|
| Phosphorus 1 | Phosphazene Containing Fraction 2 | ||||
| BA-a | 480 | 610 | 1.27 | - | - |
| BP-1 | 570 | 1240 | 2.20 | 0.88/1.09 | 20.9/25.8/19.9 |
| BP-2 | 570 | 1830 | 3.23 | 1.36/1.61 | 32.3/38.1/24.7 |
| BP-3 | 910 | 5800 | 6.38 | 1.87/2.47 | 44.3/58.4/49.8 |
| Sample | Exotherm Characteristics | Relative Benzoxazine Cycles Content 1 | ||||
|---|---|---|---|---|---|---|
| Temperature °C | Polymerization Heat (J/g) | |||||
| Beginning | Maximal Value | End | Calculated | Found 2 | ||
| BP-3 | 181 | 215 | 248 | 247 | 75.0 | 71.0 |
| BP-2 | 209 | 227 | 250 | 283 | 82.0 | 82.0 |
| BP-1 | 223 | 234 | 247 | 297 | 87.5 | 86.0 |
| BA-a | 233 | 242 | 252 | 346 | 100.0 | 100.0 |
| Parameter | Sample | |||
|---|---|---|---|---|
| BA-a | BP-1 | BP-2 | BP-3 | |
| Glass transition temperature (°C) 1 | 168/137 | 173/132 | 168/145 | 169/136 |
| LOI 2 | 31/23 | 32/29 | 32/31 | 32/32 |
| UL-94 Rating | V-1 | V-1 | V-0 | V-0 |
| Tensile strength (MPa) | 60 | 65 | 84 | 75 |
| Parameter | In Air | In Argon | ||||||
|---|---|---|---|---|---|---|---|---|
| BA-a | BP-1 | BP-2 | BP-3 | BA-a | BP-1 | BP-2 | BP-3 | |
| Temperature of weight loss (°C) | ||||||||
| Beginning | 290 | 302 | 312 | 315 | 285 | 303 | 320 | 303 |
| T5% | 318 | 330 | 338 | 336 | 314 | 324 | 338 | 336 |
| T10% | 351 | 365 | 366 | 363 | 347 | 349 | 363 | 359 |
| Char yield (wt%) | ||||||||
| at 600 °C | 22 | 32 | 35 | 35 | 35 | 40 | 39 | 38 |
| at 700 °C | 0 | 9 | 18 | 19 | 34 | 38 | 38 | 36 |
| at 800 °C | 0 | 1 | 2 | 2 | 33 | 37 | 36 | 35 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sirotin, I.S.; Sarychev, I.A.; Vorobyeva, V.V.; Kuzmich, A.A.; Bornosuz, N.V.; Onuchin, D.V.; Gorbunova, I.Y.; Kireev, V.V. Synthesis of Phosphazene-Containing, Bisphenol A-Based Benzoxazines and Properties of Corresponding Polybenzoxazines. Polymers 2020, 12, 1225. https://doi.org/10.3390/polym12061225
Sirotin IS, Sarychev IA, Vorobyeva VV, Kuzmich AA, Bornosuz NV, Onuchin DV, Gorbunova IY, Kireev VV. Synthesis of Phosphazene-Containing, Bisphenol A-Based Benzoxazines and Properties of Corresponding Polybenzoxazines. Polymers. 2020; 12(6):1225. https://doi.org/10.3390/polym12061225
Chicago/Turabian StyleSirotin, Igor S., Igor A. Sarychev, Viktoria V. Vorobyeva, Anastasia A. Kuzmich, Natalia V. Bornosuz, Denis V. Onuchin, Irina Yu. Gorbunova, and Vyacheslav V. Kireev. 2020. "Synthesis of Phosphazene-Containing, Bisphenol A-Based Benzoxazines and Properties of Corresponding Polybenzoxazines" Polymers 12, no. 6: 1225. https://doi.org/10.3390/polym12061225
APA StyleSirotin, I. S., Sarychev, I. A., Vorobyeva, V. V., Kuzmich, A. A., Bornosuz, N. V., Onuchin, D. V., Gorbunova, I. Y., & Kireev, V. V. (2020). Synthesis of Phosphazene-Containing, Bisphenol A-Based Benzoxazines and Properties of Corresponding Polybenzoxazines. Polymers, 12(6), 1225. https://doi.org/10.3390/polym12061225