Synthesis, Characterization and Sorption Capacity Examination for a Novel Hydrogel Composite Based on Gellan Gum and Graphene Oxide (GG/GO)
Abstract
1. Introduction
2. Materials and Methods
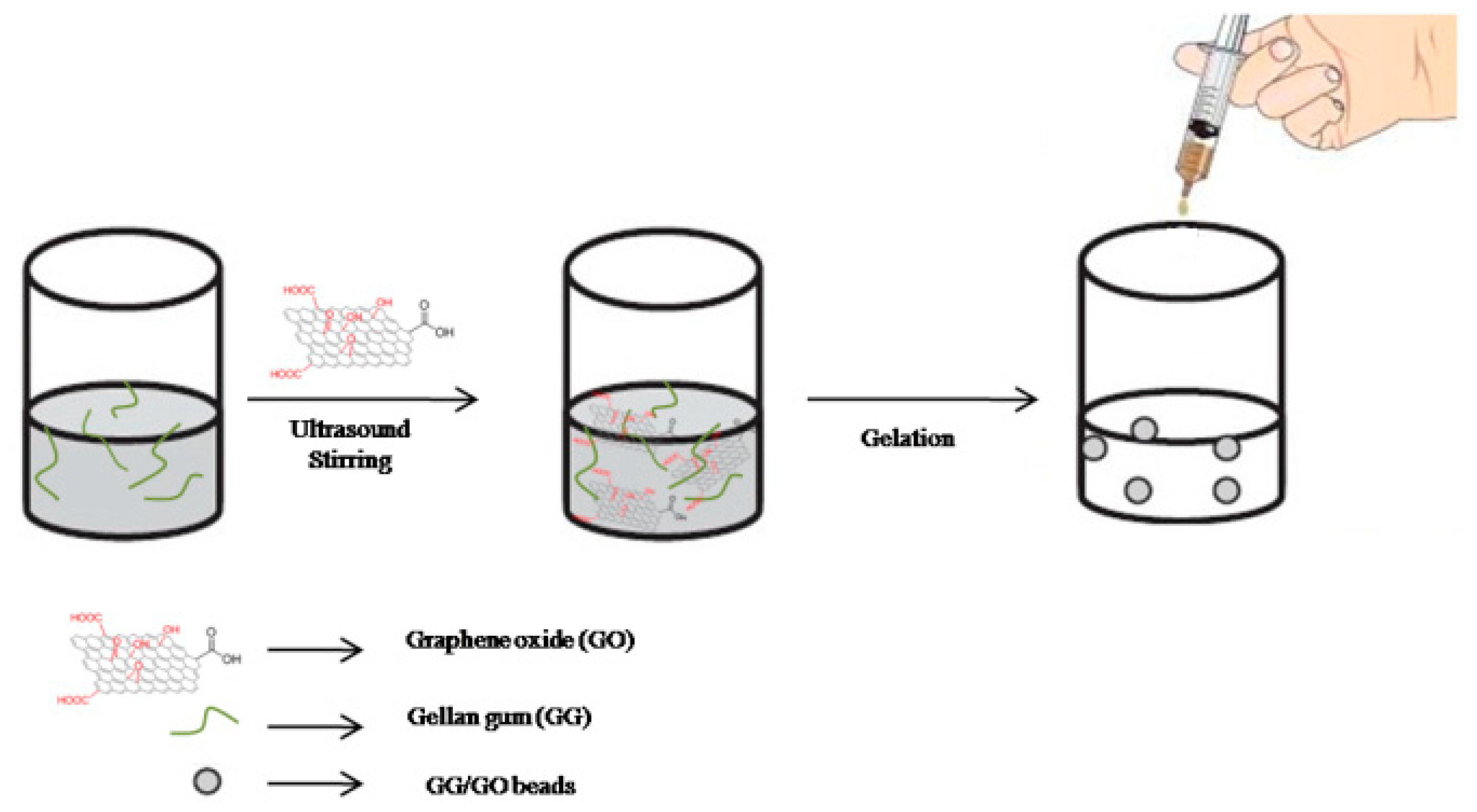
2.1. Gellan-Gum/Graphene Oxide (GG/GO) Synthesis
2.2. Removal Efficiency of Zn (II) by GG and GG/GO Composites
2.3. Sorbents Characterization
2.4. Sorption Isotherms and Kinetics
2.5. Swelling Measurements
3. Results and Discussions
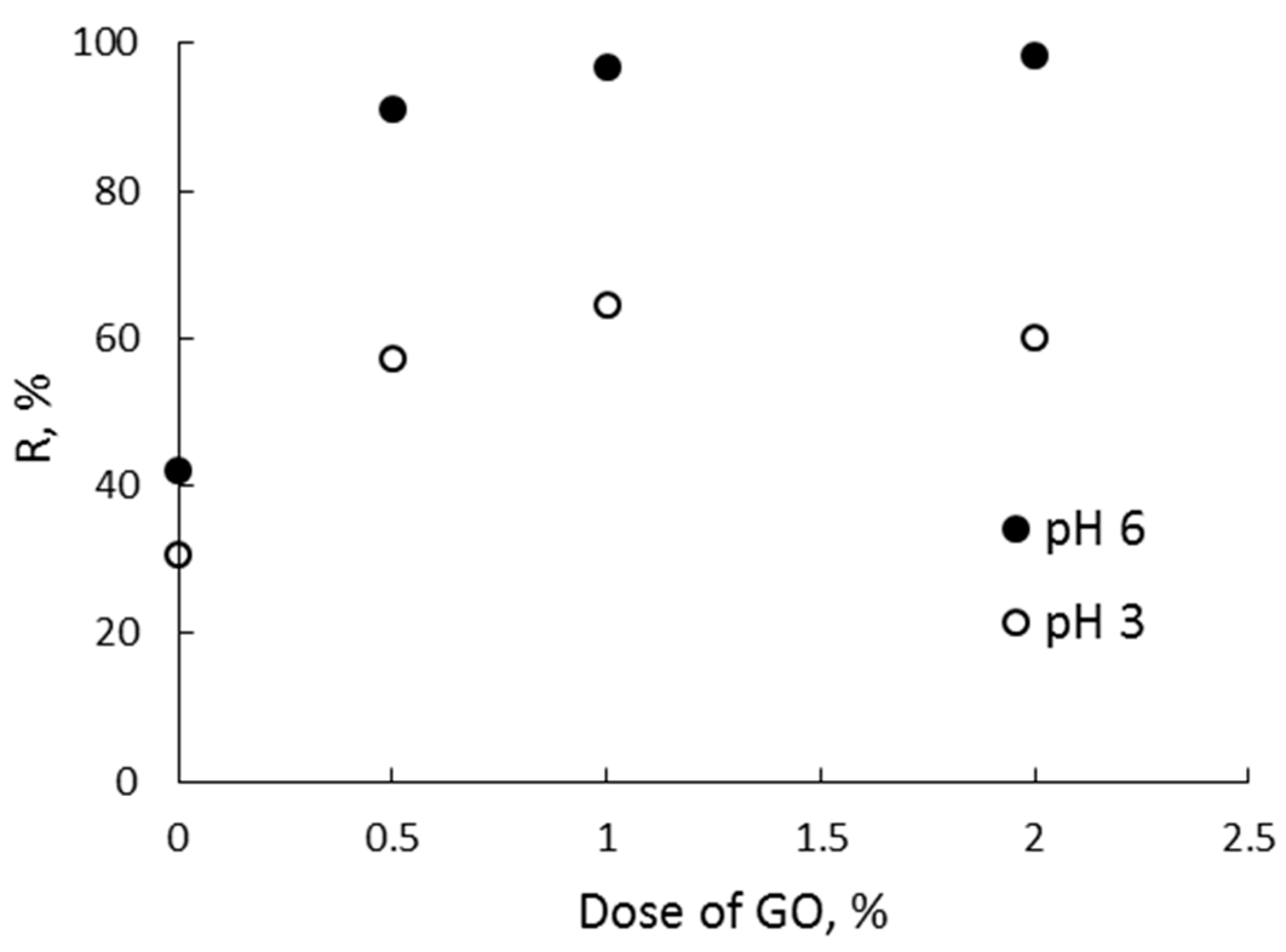
3.1. Removal Efficiency of Zn (II) by GG and GG/GO Composites
3.2. FTIR Spectroscopy
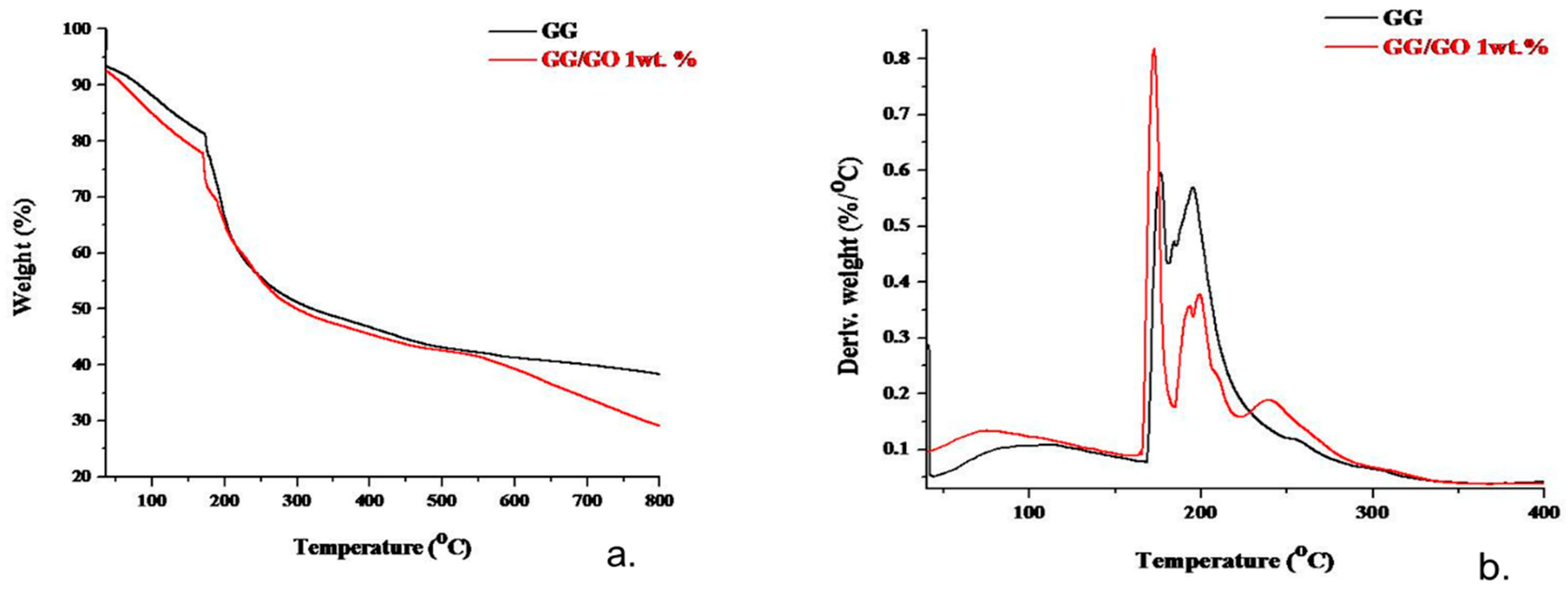
3.3. TGA Analysis
3.4. XPS Analysis
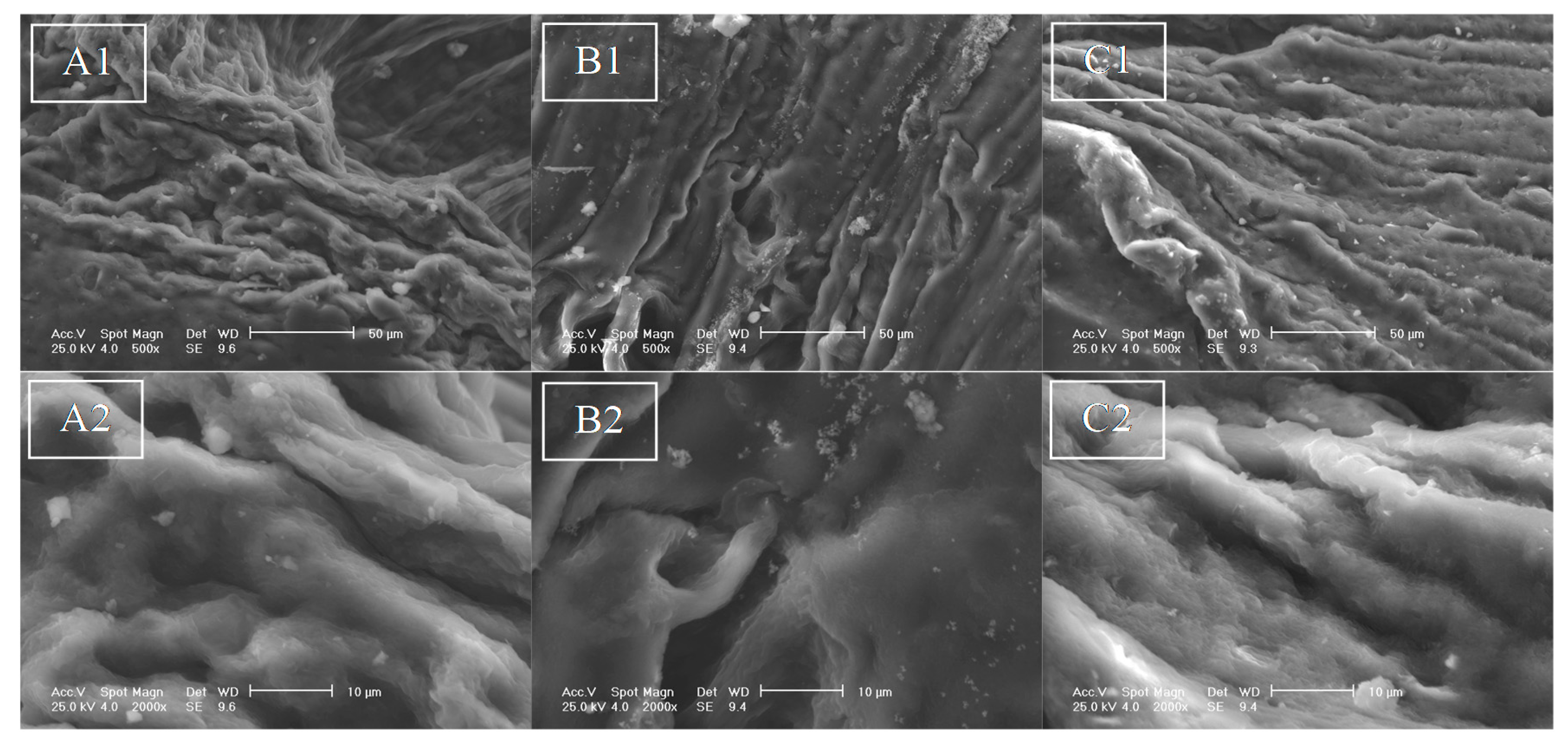
3.5. SEM-EDAX Analysis
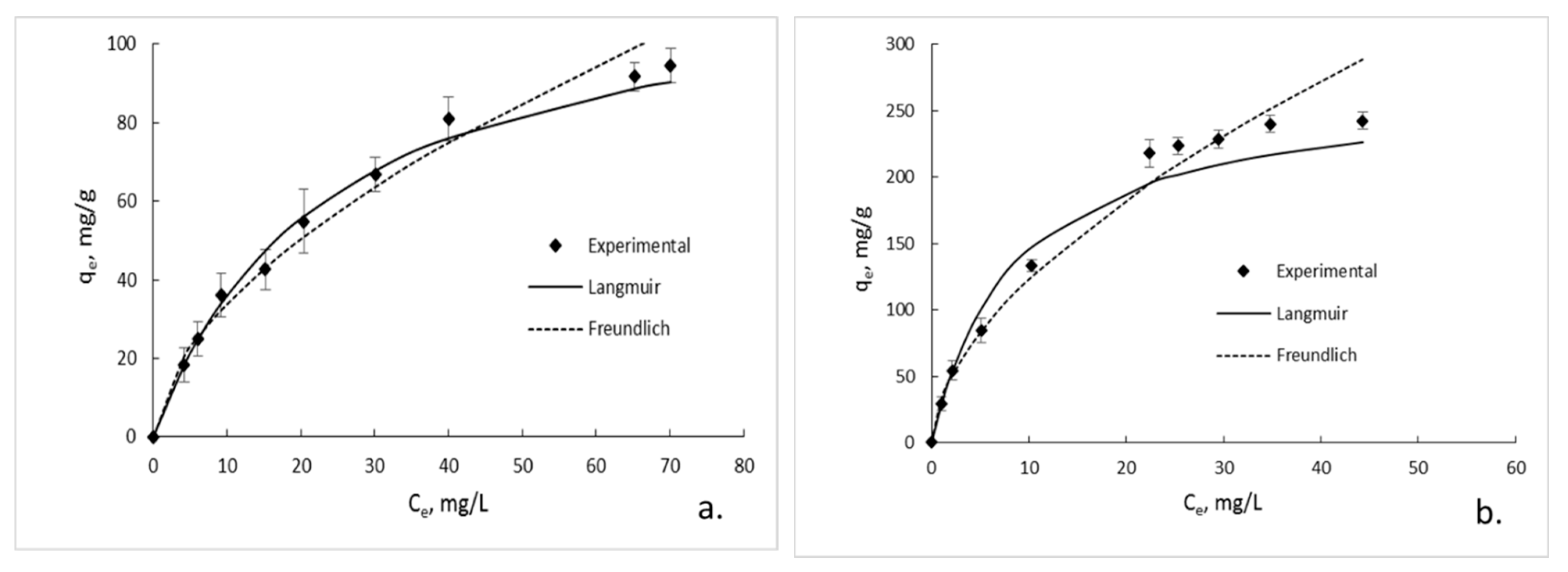
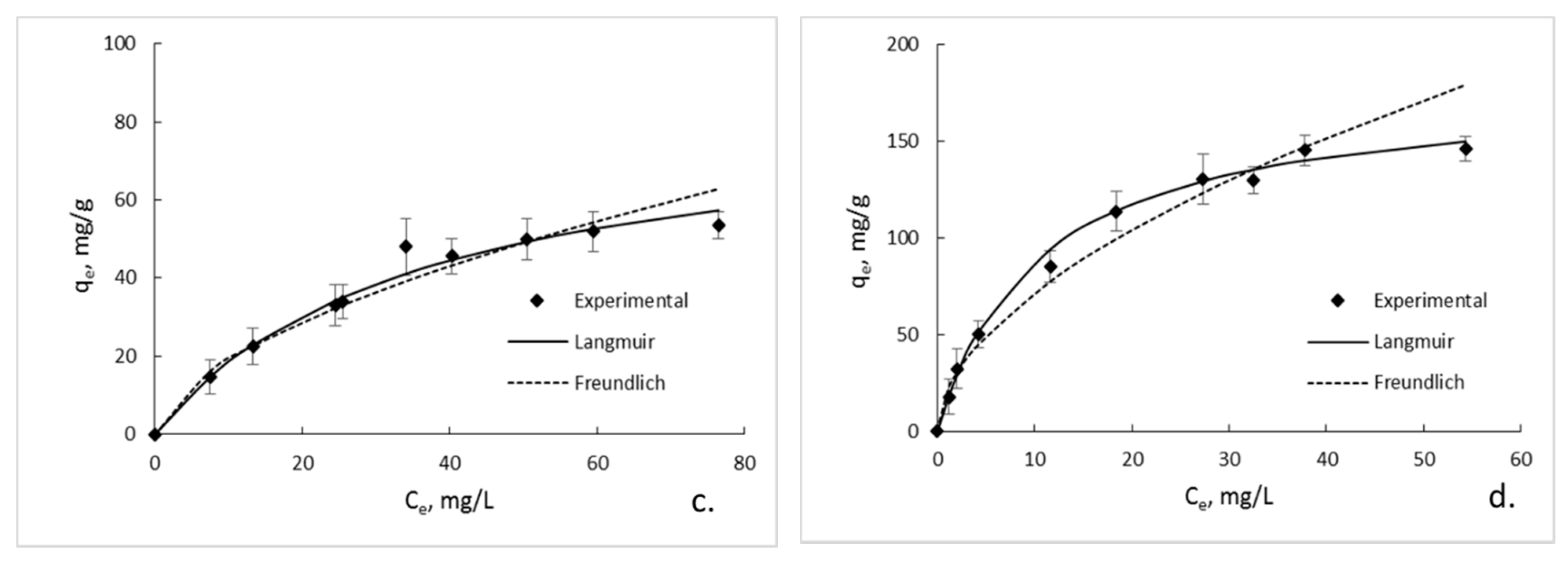
3.6. Sorption Isotherms
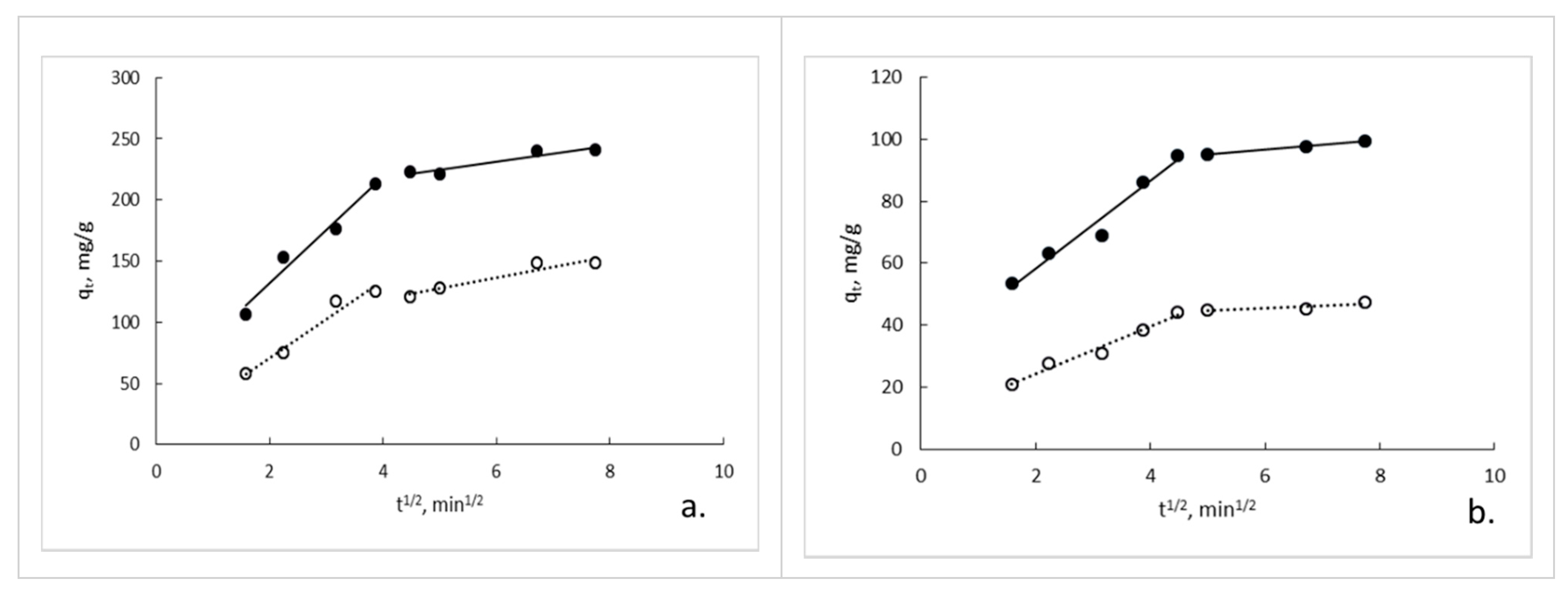
3.7. Sorption Kinetics
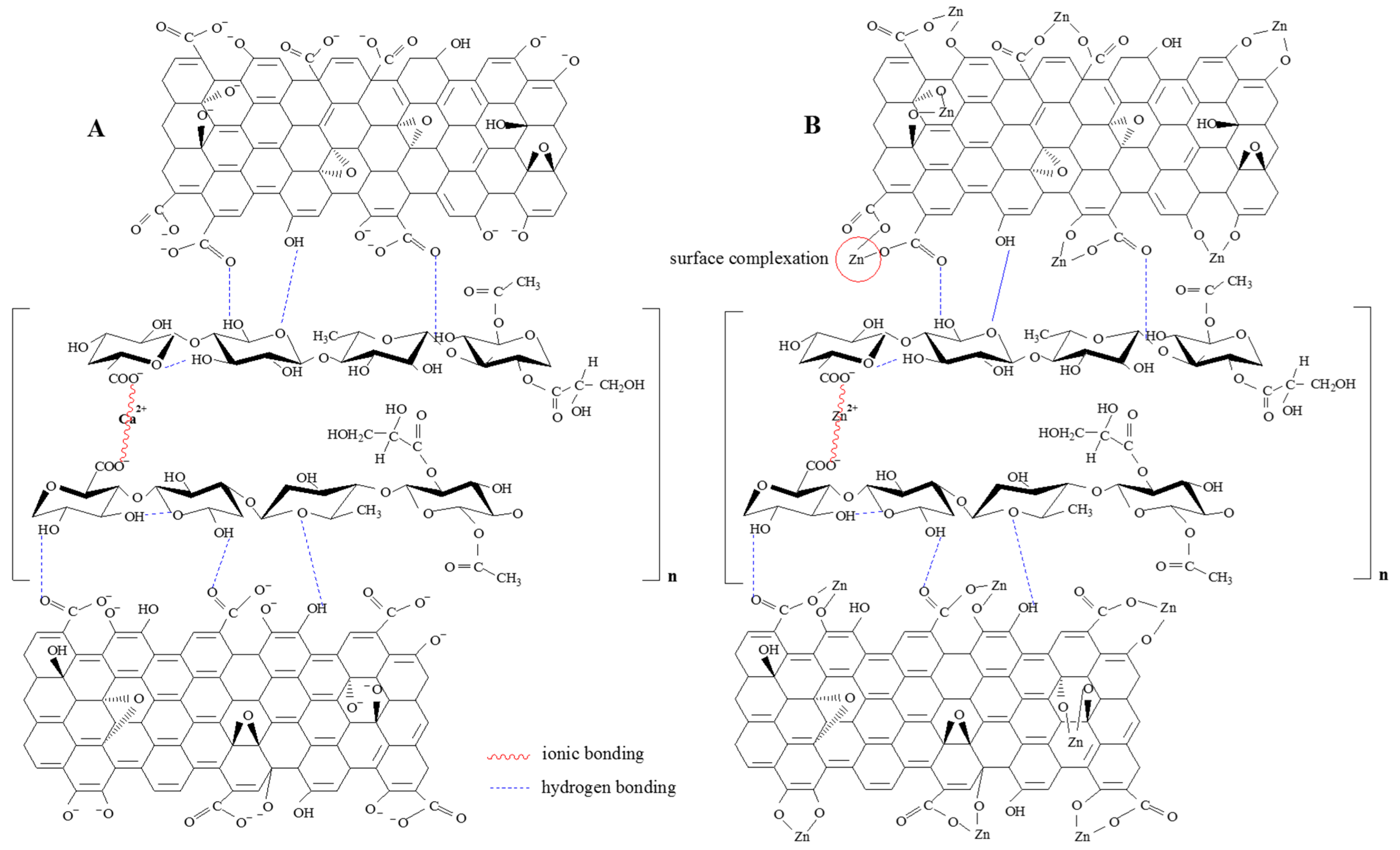
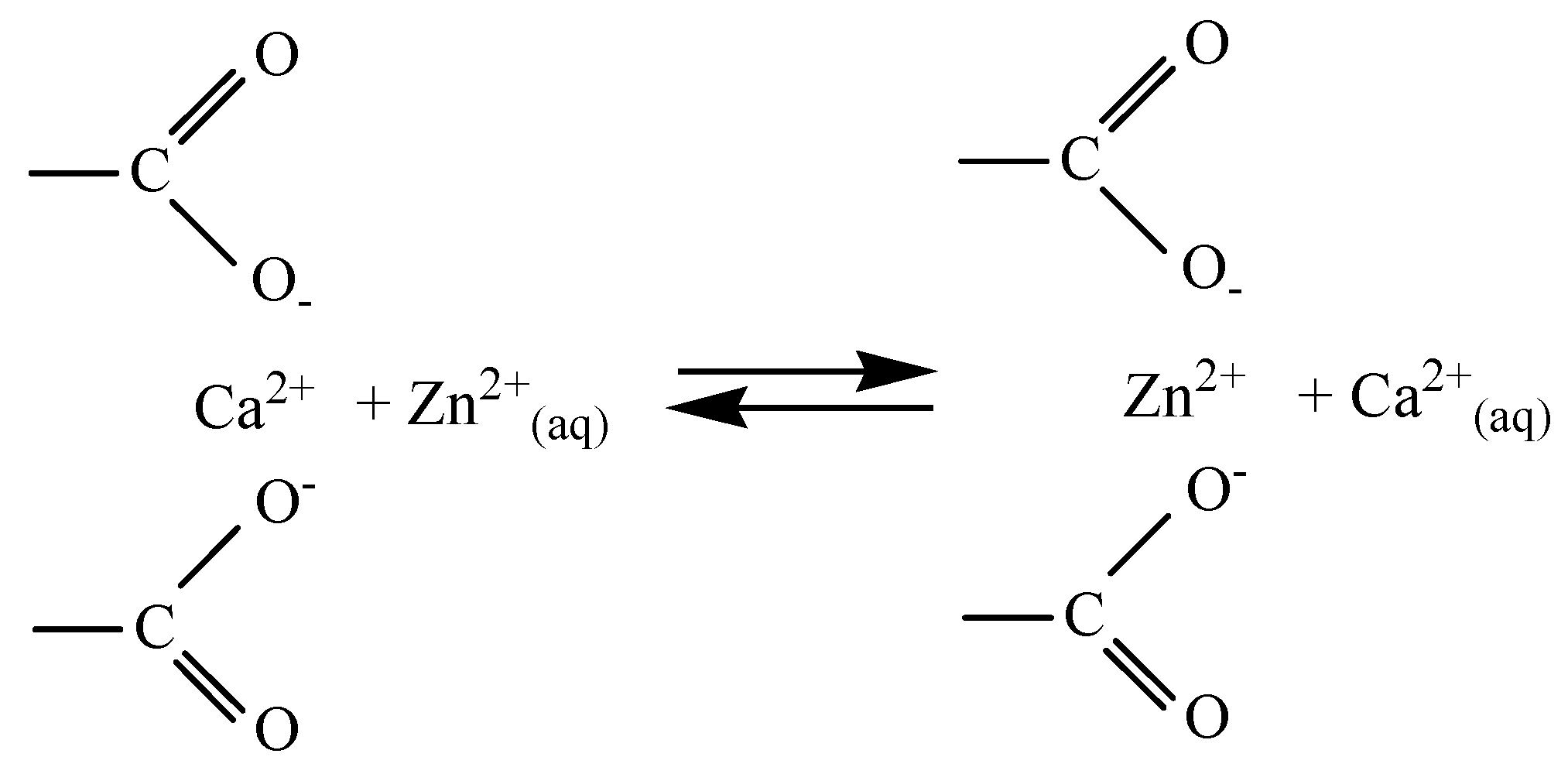
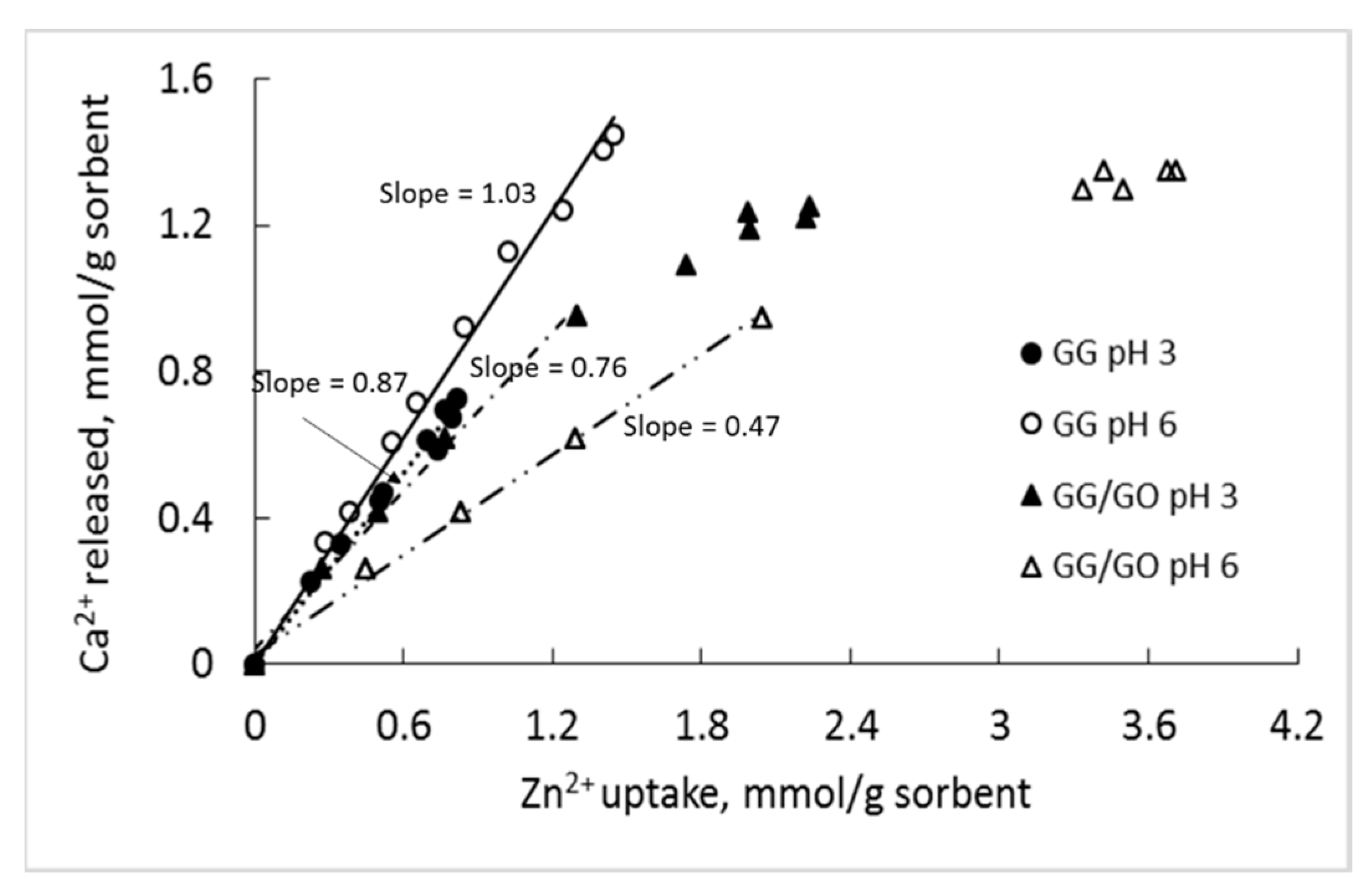
3.8. Sorption Mechanism
3.9. Desorption and Reusability Behaviors of GG/GO
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Qureshi, A.S.; Hussain, M.I.; Ismail, S.; Khan, Q.M. Evaluating heavy metal accumulation and potential health risks in vegetables irrigated with treated wastewater. Chemosphere 2016, 163, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Hayati, B.; Maleki, A.; Najafi, F.; Daraei, H.; Gharibi, F.; McKay, G. Adsorption of Pb2+, Ni2+, Cu2+, Co2+ metal ions from aqueous solution by PPI/SiO2 as new high performance adsorbent: Preparation, characterization, isotherm, kinetic, thermodynamic studies. J. Mol. Liq. 2017, 237, 428–436. [Google Scholar] [CrossRef]
- Kaličanin, B.M. Determination of very toxic metal—Cadmium in natural water samples. Desalination 2009, 249, 58–62. [Google Scholar] [CrossRef]
- Wami, A.L.; Parveen, N.; Ansari, M.O.; Ahmad, M.F.; Jameel, S.; Shadab, G.G.H.A. Zinc: An element of extensive medical importance. J. Curr. Med. Res. Pract. 2017, 7, 90–98. [Google Scholar]
- Alqadami, A.A.; Khan, M.A.; Siddiqui, M.R.; Alothman, Z.A.; Sumbul, S. A facile approach to develop industrial waste encapsulated cryogenic alginate beads to sequester toxic bivalent heavy metals. J. King Saud Univ. Sci. 2020, 32, 1444–1450. [Google Scholar] [CrossRef]
- Khan, M.A.; Alqadami, A.A.; Otero, M.; Siddiqui, M.R.; Alothman, Z.A.; Alsohaimi, I.; Rafatullah, M.; Hamedelniel, A.E. Heteroatom-doped magnetic hydrochar to remove post-transition and transition metals from water: Synthesis, characterization, and adsorption studies. Chemosphere 2019, 218, 1089–1099. [Google Scholar] [CrossRef]
- Aldawsari, A.; Khan, M.A.; Hameed, B.H.; Alqadami, A.A.; Siddiqui, M.R.; Alothman, Z.A.; Ahmed, A.Y.B.H. Mercerized mesoporous date pit activated carbon—A novel adsorbent to sequester potentially toxic divalent heavy metals from water. PLoS ONE 2017, 12, e0184493. [Google Scholar] [CrossRef]
- Barakat, M.A. New trends in removing heavy metals from industrial wastewater. Arab. J. Chem. 2011, 4, 361–377. [Google Scholar] [CrossRef]
- Utomo, H.D.; Tan, K.X.D.; Choong, Z.Y.D.; Yu, J.J.; Ong, J.J.; Lim, Z.B. Biosorption of heavy metal by algae biomass in surface water. J. Environ. Prot. Sci. 2016, 7, 1547–1560. [Google Scholar] [CrossRef]
- Wang, J.; Chen, C. Biosorbents for heavy metals removal and their future. Biotechnol. Adv. 2009, 27, 195–226. [Google Scholar] [CrossRef]
- Rani, G.U.; Konreddy, A.K.; Mishra, S. Novel hybrid biosorbents of agar: Swelling behaviour, heavy metal ions and dye removal efficacies. Int. J. Biol. Macromol. 2018, 117, 902–910. [Google Scholar] [CrossRef] [PubMed]
- Othmani, A.; Kesraoui, A.; Boada, R.; Seffen, M.; Valiente, M. Textile wastewater purification using an elaborated biosorbent hybrid material (Luffa–Cylindrica–Zinc Oxide) assisted by alternating current. Water 2019, 11, 1326. [Google Scholar] [CrossRef]
- Yusuf, M.; Kumar, M.; Khan, M.A.; Sillanpää, M.; Arafat, H. A review on exfoliation, characterization, environmental and energy applications of graphene and grapheme-based composites. Adv. Colloid Interface Sci. 2019, 273, 102036. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, M.; Khan, M.A.; Otero, M.; Abdullah, E.C.; Hosomi, M.; Terada, A.; Riya, S. Synthesis of CTAB intercalated graphene and its application for the adsorption of AR265 and A07 dyes from water. J. Colloid Interface Sci. 2017, 493, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, M.; Khan, M.A.; Otero, M.; Abdullah, E.C.; Elfghi, M.; Hosomi, M.; Terada, A.; Riya, S.; Ahmad, A. Dodecyl sulfate chain anchored mesoporous graphene: Synthesis and application to sequester heavy metals ions from aqueous phase. Chem. Eng. J. 2016, 304, 431–439. [Google Scholar] [CrossRef]
- Chang, A.; Babhadiashar, N.; Barrett-Catton, E.; Asuri, P. Role of nanoparticle–polymer interactions on the development of double-network hydrogel nanocomposites with high mechanical strength. Polymers 2020, 12, 470. [Google Scholar] [CrossRef]
- Thangavel, S.; Venugopal, G. Understanding the adsorption property of graphene-oxide with different degrees of oxidation levels. Powder Technol. 2014, 257, 141–148. [Google Scholar] [CrossRef]
- Vilela, D.; Parmar, J.; Zeng, Y.; Zhao, Y.; Sánchez, S. Graphene-based microbots for toxic heavy metal removal and recovery from water. Nano Lett. 2016, 16, 2860–2866. [Google Scholar] [CrossRef]
- González, J.A.; Villanueva, M.E.; Piehl, L.L.; Copello, G.J. Development of a chitin/graphene oxide hybrid composite for the removal of pollutant dyes: Adsorption and desorption study. Chem. Eng. J. 2015, 280, 41–48. [Google Scholar] [CrossRef]
- Gao, L.; Wang, Z.; Qin, C.; Chen, Z.; Gao, M.; He, N.; Qian, X.; Zhou, Z.; Li, G. Preparation and application of iron oxide/persimmon tannin/ graphene oxide nanocomposites for efficient adsorption of erbium from aqueous solution. J. Rare Earths 2020, in press. [Google Scholar] [CrossRef]
- Gelinsky, M. Biopolymer hydrogel bioinks. In 3D Bioprinting for Reconstructive Surgery; Thomas, D.J., Jessop, Z.M., Whitaker, I.S., Eds.; Woodhead Publishing: Cambridge, MA, USA, 2018; pp. 125–136. [Google Scholar]
- Yamamoto, F.; Cunha, R.L. Acid gelation of gellan: Effect of final pH and heat treatment conditions. Carbohydr. Polym. 2007, 68, 517–527. [Google Scholar] [CrossRef]
- Omoto, T.; Uno, Y.; Asai, I. The latest technologies for the application of gellan gum. In Physical Chemistry and Industrial Application of Gellan Gum; Nishinari, K., Ed.; Springer: Berlin/Heidelberg, Germany, 1999; Volume 114, pp. 123–126. [Google Scholar]
- Jackcina Stobel Christy, E.; Sreerag, G.; Rajeswari, A.; Anitha, P. Highly crosslinked 3-D hydrogels based on graphene oxide for enhanced remediation of multi contaminant wastewater. J. Water Process Eng. 2019, 31, 100850. [Google Scholar]
- A Good Overview of Scientific, Technical and Commercial Aspects for Gellan Gum. Available online: https://www.cinogel.com/p/gellan-gum-overview.html (accessed on 7 May 2020).
- Thombare, N.; Jha, U.; Mishra, S.; Siddiqui, M.Z. Borax cross-linked guar gum hydrogels as potential adsorbents for water purification. Carbohydr. Polym. 2017, 168, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, R.; Sam Kim, S.S.; Jaewoong, L. Novel synergistic transparent -carrageenan/k xanthan gum/gellan gum hydrogel film: Mechanical, thermal and water barrier properties. Int. J. Biol. Macromol. 2018, 118, 561–568. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Obreja, A.C.; Cristea, D.; Gavrila, R.; Schiopu, V.; Dinescu, A.; Danila, M.; Comanescu, F. Isocyanate functionalized graphene/P3HT based nanocomposites. Appl. Surf. Sci. 2013, 276, 458–467. [Google Scholar] [CrossRef]
- Chaturvedi, K.; Ganguly, K.; More, U.A.; Reddy, K.R.; Dugge, T.; Naik, B.; Aminabhavi, T.M.; Noolvi, M.N. Sodium alginate in drug delivery and biomedical areas. In Natural Polysaccharides in Drug Delivery and Biomedical Applications; Hasnain, M.S., Najak, A.K., Eds.; Academic Press: Cambridge, MA, USA, 2019. [Google Scholar]
- Pandele, M.A.; Ionita, M.; Lungu, A.; Vasile, E.; Zaharia, C.; Iovu, H. Porous chitosan/graphene oxide biocomposites for tissue engineering. Carbohydr. Polym. 2013, 38, 363–370. [Google Scholar] [CrossRef]
- Zhao, W.; Qi, Y.; Wang, Y.; Xue, Y.; Xu, P.; Li, Z.; Li, Q. Morphology and thermal properties of calcium alginate/reduced graphene oxide composites. Polymers 2018, 10, 990. [Google Scholar] [CrossRef]
- Algothmi, W.M.; Bandaru, N.M.; Yu, Y.; Shapter, J.G.; Ellis, A.V. Alginate-graphene oxide hybrid gel beads: An efficient copper adsorbent material. J. Colloid Interface Sci. 2013, 397, 32–38. [Google Scholar] [CrossRef]
- Pham, V.H.; Pham, H.D.; Dang, T.T.; Hur, S.H.; Kim, E.J.; Kong, B.S.; Kim, S.; Chang, J.S. Chemical reduction of an aqueous suspension of grapheme oxide by nascent hydrogen. J. Mater. Chem. 2012, 22, 10530–10537. [Google Scholar] [CrossRef]
- Croitoru, A.; Oprea, O.; Nicoara, A.; Trusca, R.; Radu, M.; Neacsu, I.; Ficai, D.; Ficai, A.; Andronescu, E. Multifunctional platforms based on graphene oxide and natural products. Medicina (Kaunas) 2019, 55, 230. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Ehlert, G.J.; Lin, Y.; Sodano, H.A. Highly efficient synthesis of graphene nanocomposites. Nano Lett. 2012, 12, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Jiao, C.; Xiong, J.; Tao, J.; Xu, S.; Zhang, D.; Lin, H.; Chen, Y. Sodium alginate/graphene oxide aerogel with enhanced strength-toughness and its heavy metal adsorption study. Int. J. Biol. Macromol. 2016, 83, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Ruparelia, J.P.; Duttagupta, S.P.; Chatterjee, A.K.; Mukherji, S. Potential of carbon nanomaterials for removal of heavy metals from water. Desalination 2008, 232, 145–156. [Google Scholar] [CrossRef]
- Wang, H.; Yuan, X.; Wu, Y.; Huang, H.; Zeng, G.; Liu, Y.; Wang, X.; Lin, N.; Qi, Y. Adsorption characteristics and behaviors of graphene oxide for Zn(II) removal from aqueous solution. Appl. Surf. Sci. 2013, 279, 432–440. [Google Scholar] [CrossRef]
- Ramezanzadeh, M.; Asghari, M.; Ramezanzadeh, B.; Bahlakeh, G. Fabrication of an efficient system for Zn ions removal from industrial wastewater based on graphene oxide nanosheets decorated with highly crystalline polyaniline nanofibers (GO-PANI): Experimental and ab initio quantum mechanics approaches. Chem. Eng. J. 2018, 337, 385–397. [Google Scholar] [CrossRef]
- Godiya, C.B.; Cheng, X.; Deng, G.; Li, D.; Lu, X. Silk fibroin/polyethylenimine functional hydrogel for metal ion adsorption and upcycling utilization. J. Environ. Chem. Eng. 2019, 7, 102806. [Google Scholar] [CrossRef]
- Milosavljević, N.B.; Ristić, M.Đ.; Perić-Grujić, A.A.; Filipović, J.M.; Štrbac, S.B.; Rakočević, Z.L.; Kalagasidis Krušić, M.T. Sorption of zinc by novel pH-sensitive hydrogels based on chitosan, itaconic acid and methacrylic acid. J. Hazard. Mater. 2011, 192, 846–854. [Google Scholar] [CrossRef]
- Souda, P.; Sreejith, L. Magnetic hydrogel for better adsorption of heavy metals from aqueous solutions. J. Environ. Chem. Eng. 2015, 3, 1882–1891. [Google Scholar] [CrossRef]
- Peng, W.; Li, H.; Liu, Y.; Song, S. Comparison of Pb (II) adsorption onto graphene oxide prepared from natural graphites: Diagramming the Pb (II) adsorption sites. Appl. Surf. Sci. 2016, 364, 620–627. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, S.; Zhang, L.; Wang, C.; Zhang, B. Kinetic, isotherm, and thermodynamic studies for Ag(I) adsorption using carboxymethyl functionalized poly(glycidyl methacrylate). Polymers 2018, 10, 1090. [Google Scholar] [CrossRef] [PubMed]
- Xiong, C.; Wang, S.; Zhang, L.; Li, Y.; Zhou, Y.; Peng, J. Preparation of 2-aminothiazole-functionalized poly(glycidyl methacrylate) microspheres and their excellent gold ion adsorption properties. Polymers 2018, 10, 159. [Google Scholar] [CrossRef] [PubMed]
- Mohan, S.; Kumar, V.; Singh, D.K.; Hasan, S.H. Effective removal of lead ions using graphene oxide-MgO nanohybrid from aqueous solution: Isotherm, kinetic and thermodynamic modeling of adsorption. J. Environ. Chem. Eng. 2017, 5, 2259–2273. [Google Scholar] [CrossRef]
- Peng, W.; Li, H.; Liu, Y.; Song, S. A review on heavy metal ions adsorption from water by graphene oxide and its composites. Mol. Liq. 2017, 230, 496–504. [Google Scholar] [CrossRef]
- Arshada, F.; Selvaraj, M.; Zain, J.; Banat, F.; Haija, M.A. Polyethylenimine modified graphene oxide hydrogel composite as an efficient adsorbent for heavy metal ions. Sep. Purif. Technol. 2019, 209, 870–880. [Google Scholar] [CrossRef]
- Harland, C.E. Ion exchange: Theory and Practice, 1st ed.; The Royal Society of Chemistry: Cambridge, UK, 1994. [Google Scholar]
- Zhao, J.; Wang, C.; Wang, S.; Zhang, L.; Zhang, B. Selective recovery of Au(III) from wastewater by a recyclable magnetic Ni0.6Fe2.4O4 nanoparticels with mercaptothiadiazole: Interaction models and adsorption mechanisms. J. Clean. Prod. 2019, 236, 117605. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, C.; Wang, S.; Zhou, Y. Experimental and DFT study of selective adsorption mechanisms of Pb(II) by UiO-66-NH2 modified with 1,8-dihydroxyanthraquinone. J. Ind. Eng. Chem. 2020, 83, 111–122. [Google Scholar] [CrossRef]
- Almeida, F.A.; Sato, A.C.K. Structure of gellan gum–hydrolyzed collagen particles: Effect of starch addition and coating layer. Food Res. Int. 2019, 121, 394–403. [Google Scholar] [CrossRef]
- Hoor, Y.Q.; Au, P.-I.; Mubarak, N.M.; Khalid, M.; Jagadish, P.; Walvekar, R.; Abdullah, E.C. Surface force arising from adsorbed graphene oxide in kaolinite suspensions. Colloids Surf. A Physicochem. Eng. Asp. 2020, 592, 124592. [Google Scholar] [CrossRef]
- Krishna, K.A.; Vishalakski, B. Gellan gum-based novel composite hydrogel: Evaluation as adsorbent for cationic dyes. J. Appl. Polym. Sci. 2017, 134, 45527–45536. [Google Scholar] [CrossRef]
- Stela Dragan, E.S.; Humelnicu, D.; Dinu, M.V.; Olariu, R.I. Kinetics, equilibrium modeling, and thermodynamics on removal of Cr(VI) ions from aqueous solution using novel composites with strong base anion exchanger microspheres embedded into chitosan/poly(vinyl amine) cryogels. Chem. Eng. J. 2017, 330, 675–691. [Google Scholar] [CrossRef]
- Humelnicu, D.; Lazar, M.M.; Ignat, M.; Dinu, L.A.; Dragan, E.S.; Dinu, M.V. Removal of heavy metal ions from multi-component aqueous solutions by eco-friendly and low-cost composite sorbents with anisotropic pores. J. Hazard. Mater. 2020, 381, 120980. [Google Scholar] [CrossRef] [PubMed]
- Lazar, M.M.; Dinu, L.A.; Silion, M.; Dragan, E.S.; Dinu, M.V. Could the porous chitosan-based composite materials have a chance to a “NEW LIFE” after Cu(II) ion binding? Int. J. Biol. Macromol. 2019, 131, 134–146. [Google Scholar] [CrossRef]

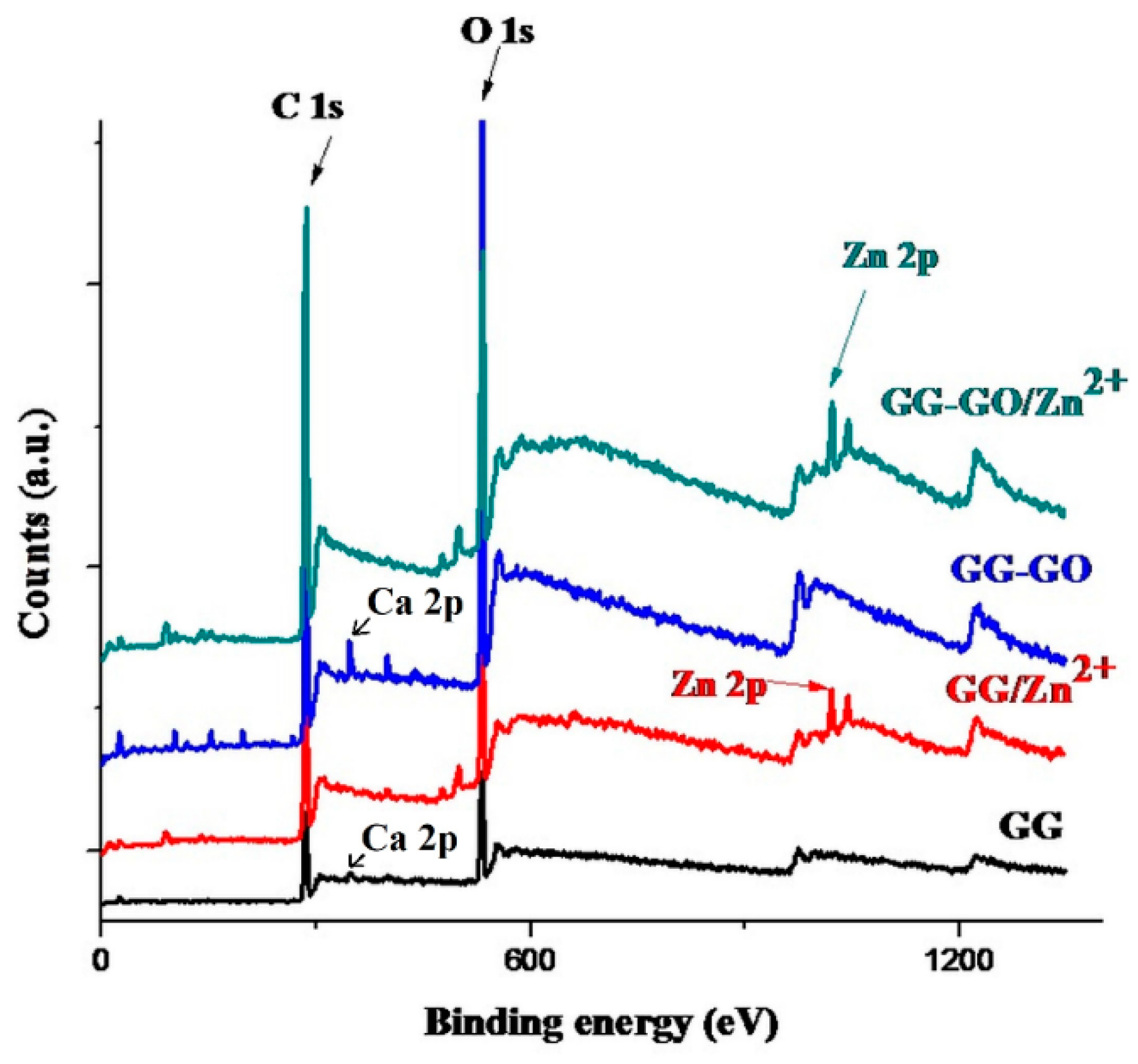
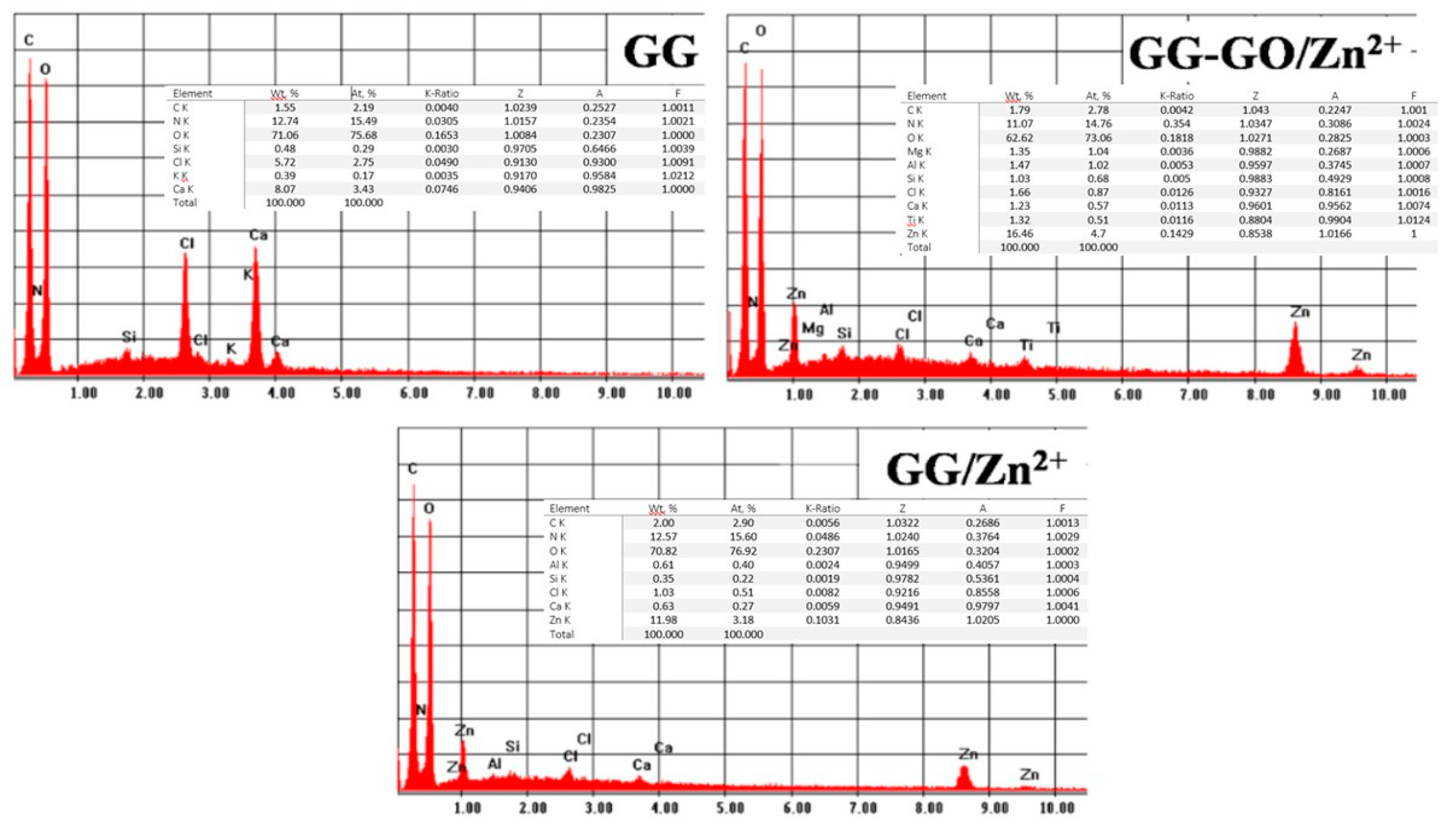

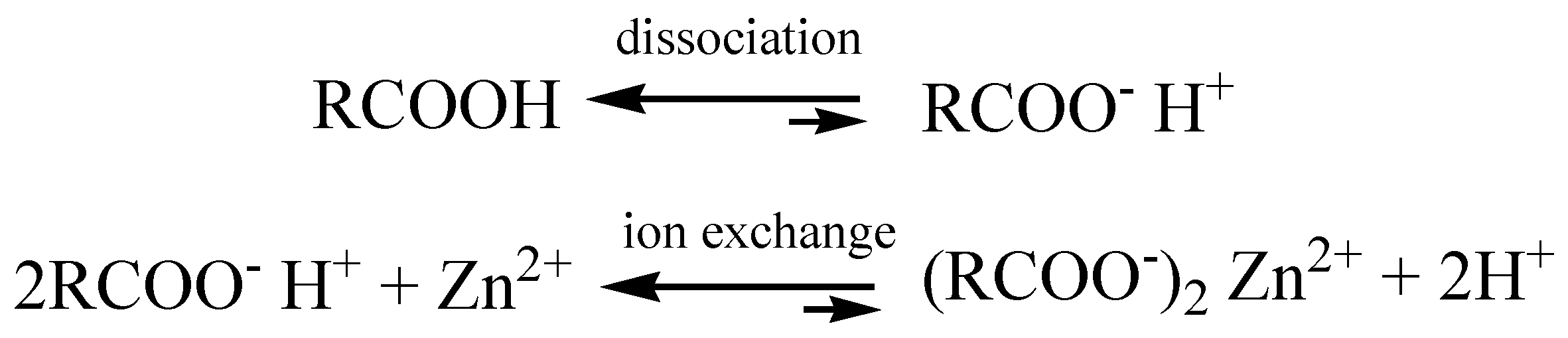
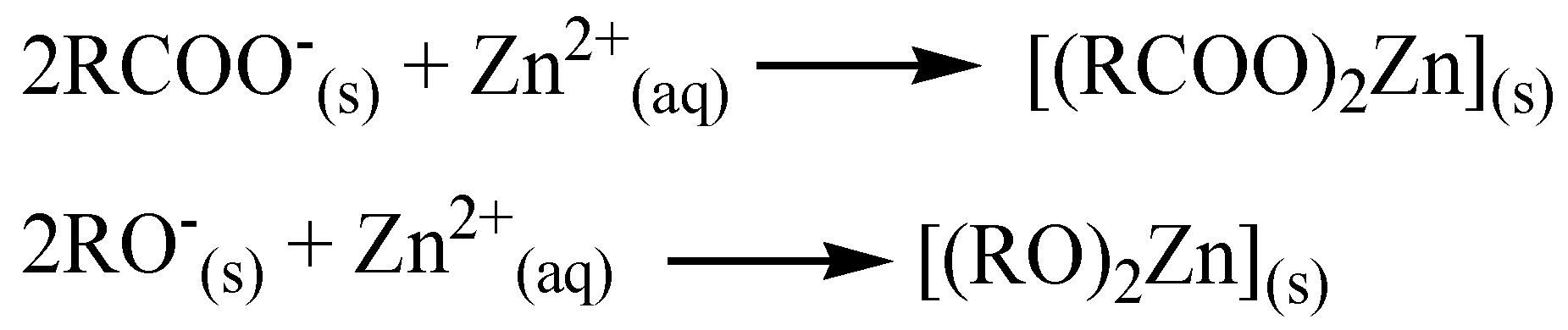
| Sample | C 1s (%) | O 1s (%) | Zn 2p (%) |
|---|---|---|---|
| GG GG/Zn2+ GG/GO GG-GO/Zn2+ | 69.88 75.22 62.02 78.32 | 30.12 21.04 31.31 20.33 | 0 1.24 0 1.35 |
| Sorbent | Parameters | |||||
|---|---|---|---|---|---|---|
| Langmuir | Freundlich | |||||
| qm (mg/g) | KL (L/mg) | R2 | 1/n | KF (mg1−n. Ln/g) | R2 | |
| GG; pH 6 GG/GO; pH 6 GG; pH 3 GG/GO; pH 3 | 120.48 272.27 84.03 178.57 | 0.043 0.116 0.028 0.096 | 0.9946 0.9935 0.9921 0.9914 | 0.573 0.575 0.583 0.545 | 9.057 32.564 5.015 20.306 | 0.9798 0.9841 0.9471 0.9675 |
| Sorbent | Sorption Capacity, mg/g | Reference |
|---|---|---|
| Activated Carbon (adsorbent dose 0.5 g/L, pH 6.5) | 40.86 | [38] |
| Nanoporous carbon (adsorbent dose 0.5 g/L, pH 6.5) | 130.76 | |
| Graphene oxide (adsorbent dose 2 mg/mL, pH 7) | 246.00 | [39] |
| Graphene oxide (adsorbent amount 2.5 mg, GO 0.0025 ppm, pH 7) | 287.86 | [40] |
| Graphene oxide-Polyaniline (adsorbent amount 2.5 mg, GO 2.5 mg, pH 7) | 297.62 | |
| Silk fibroin/polyethylenimine hydrogel (adsorbent dose 0.5 g/L, pH 5.5) | 125.00 | [41] |
| Chitosan, itaconic acid, and methacrylic acid based hydrogel (adsorbent amount 0.035 g, pH 5.5) | 105.50 | [42] |
| Poly(acrylate-acrylic acid-co-maleic acid) and poly(2-acrylamido-2-methyl-1-propanesulfonic acid) based hydrogel (adsorbent amount 0.01 g) | 267.73 | [43] |
| Magnetic Poly(acrylate-acrylic acid-co-maleic acid) and poly(2-acrylamido-2-methyl-1-propanesulfonic acid) based hydrogel (adsorbent amount 0.01 g) | 289.12 | |
| Gellan Gum (liquid-to-solid mass ratio of 50/1, pH 6)Gellan Gum/Graphene oxide 1 wt % (liquid-to-solid mass ratio of 50/1, pH 6) | 120.48272.27 | This article |
| Kinetic Model | Parameters | GG pH 6 | GG/GO pH 6 | GG pH 3 | GG/GO pH 3 |
|---|---|---|---|---|---|
| Pseudo-first order | qe k1, min−1 R2 | 163.47 0.155 0.9671 | 207.05 0.132 0.8832 | 38.16 0.147 0.8580 | 76.52 0.143 0.9623 |
| Pseudo-second order | qe k2, g mg−1 min−1 R2 | 156.25 0.0045 0.9919 | 250.00 0.0040 0.9917 | 49.02 0.0012 0.9923 | 103.09 0.0017 0.9958 |
| Intraparticle diffusion (Stage 1) | kid1, mg g−1 min−1/2 C1 R2 | 31.544 8.167 0.9596 | 43.488 44.513 0.9555 | 7.637 8.993 0.9759 | 14.124 29.962 0.9623 |
| Intraparticle diffusion (Stage 2) | kid2, mg g−1 min−1/2 C2 R2 | 8.911 83.143 0.9297 | 6.440 192.89 0.8932 | 0.766 40.766 0.6950 | 10.532 87.400 0.9948 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Modrogan, C.; Pandele, A.M.; Bobirică, C.; Dobrotǎ, D.; Dăncilă, A.M.; Gârleanu, G.; Orbuleţ, O.D.; Borda, C.; Gârleanu, D.; Orbeci, C. Synthesis, Characterization and Sorption Capacity Examination for a Novel Hydrogel Composite Based on Gellan Gum and Graphene Oxide (GG/GO). Polymers 2020, 12, 1182. https://doi.org/10.3390/polym12051182
Modrogan C, Pandele AM, Bobirică C, Dobrotǎ D, Dăncilă AM, Gârleanu G, Orbuleţ OD, Borda C, Gârleanu D, Orbeci C. Synthesis, Characterization and Sorption Capacity Examination for a Novel Hydrogel Composite Based on Gellan Gum and Graphene Oxide (GG/GO). Polymers. 2020; 12(5):1182. https://doi.org/10.3390/polym12051182
Chicago/Turabian StyleModrogan, Cristina, Andreea Mădălina Pandele, Constantin Bobirică, Dan Dobrotǎ, Annette Madelene Dăncilă, Gabriel Gârleanu, Oanamari Daniela Orbuleţ, Claudia Borda, Delia Gârleanu, and Cristina Orbeci. 2020. "Synthesis, Characterization and Sorption Capacity Examination for a Novel Hydrogel Composite Based on Gellan Gum and Graphene Oxide (GG/GO)" Polymers 12, no. 5: 1182. https://doi.org/10.3390/polym12051182
APA StyleModrogan, C., Pandele, A. M., Bobirică, C., Dobrotǎ, D., Dăncilă, A. M., Gârleanu, G., Orbuleţ, O. D., Borda, C., Gârleanu, D., & Orbeci, C. (2020). Synthesis, Characterization and Sorption Capacity Examination for a Novel Hydrogel Composite Based on Gellan Gum and Graphene Oxide (GG/GO). Polymers, 12(5), 1182. https://doi.org/10.3390/polym12051182








