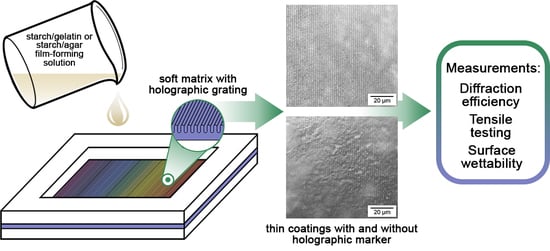
Surface Morphology Formation of Edible Holographic Marker on Potato Starch with Gelatin or Agar Thin Coatings
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
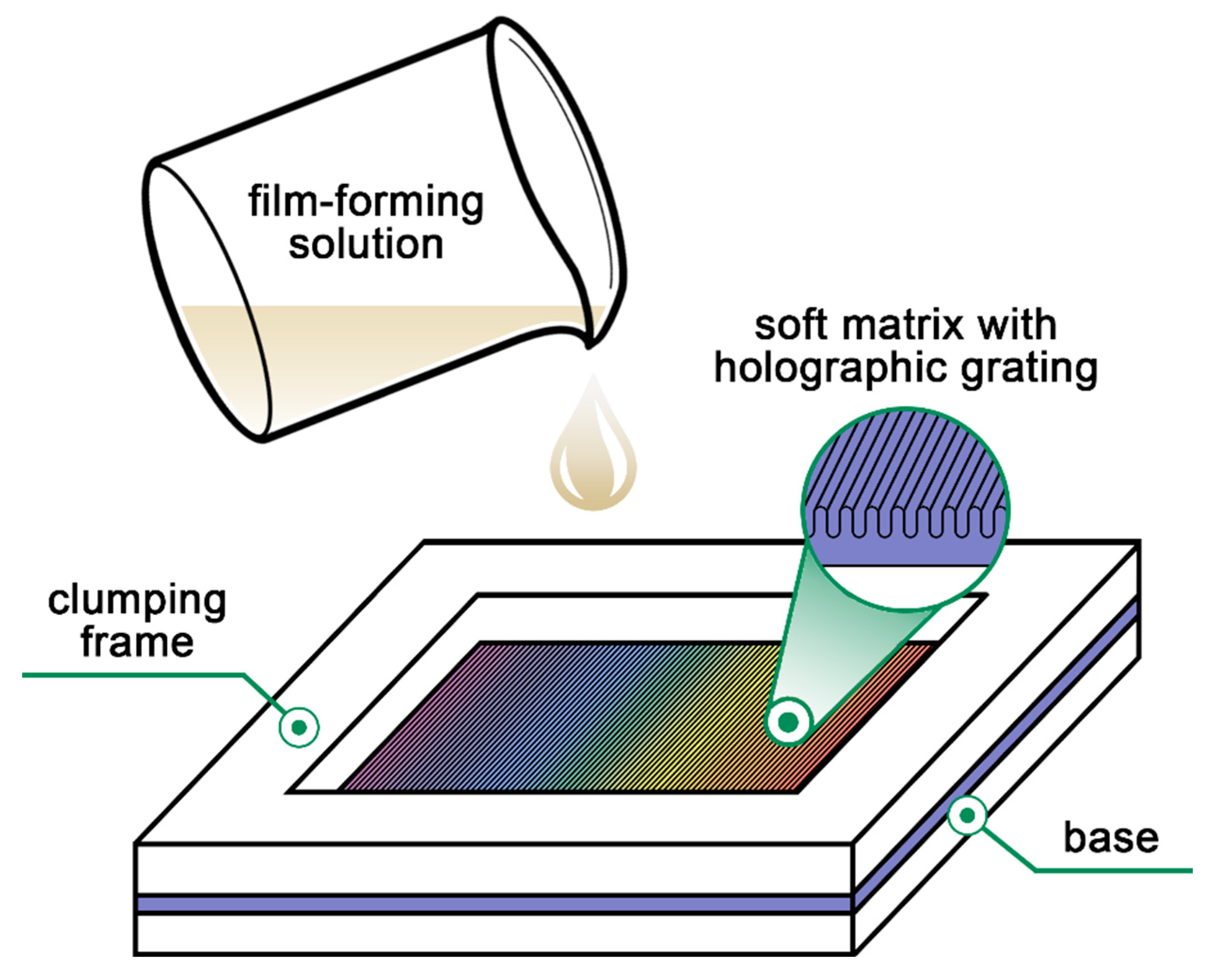
2.2. Preparation of the Film-Forming Solutions
2.3. Dynamic Viscosity Measurements of the Film-Forming Solutions
2.4. Preparation of Thin Coatings with Holographic Markers
2.5. Optical Microscopy of the Holographic Markers
2.6. Diffraction Efficiency Measurements of the Holographic Markers
2.7. Measurements of Thickness and Tensile Strength of the Coatings
2.8. Measurements of Contact Angle of the Coatings
3. Results and Discussion
3.1. Rheology of the Film-Forming Solutions
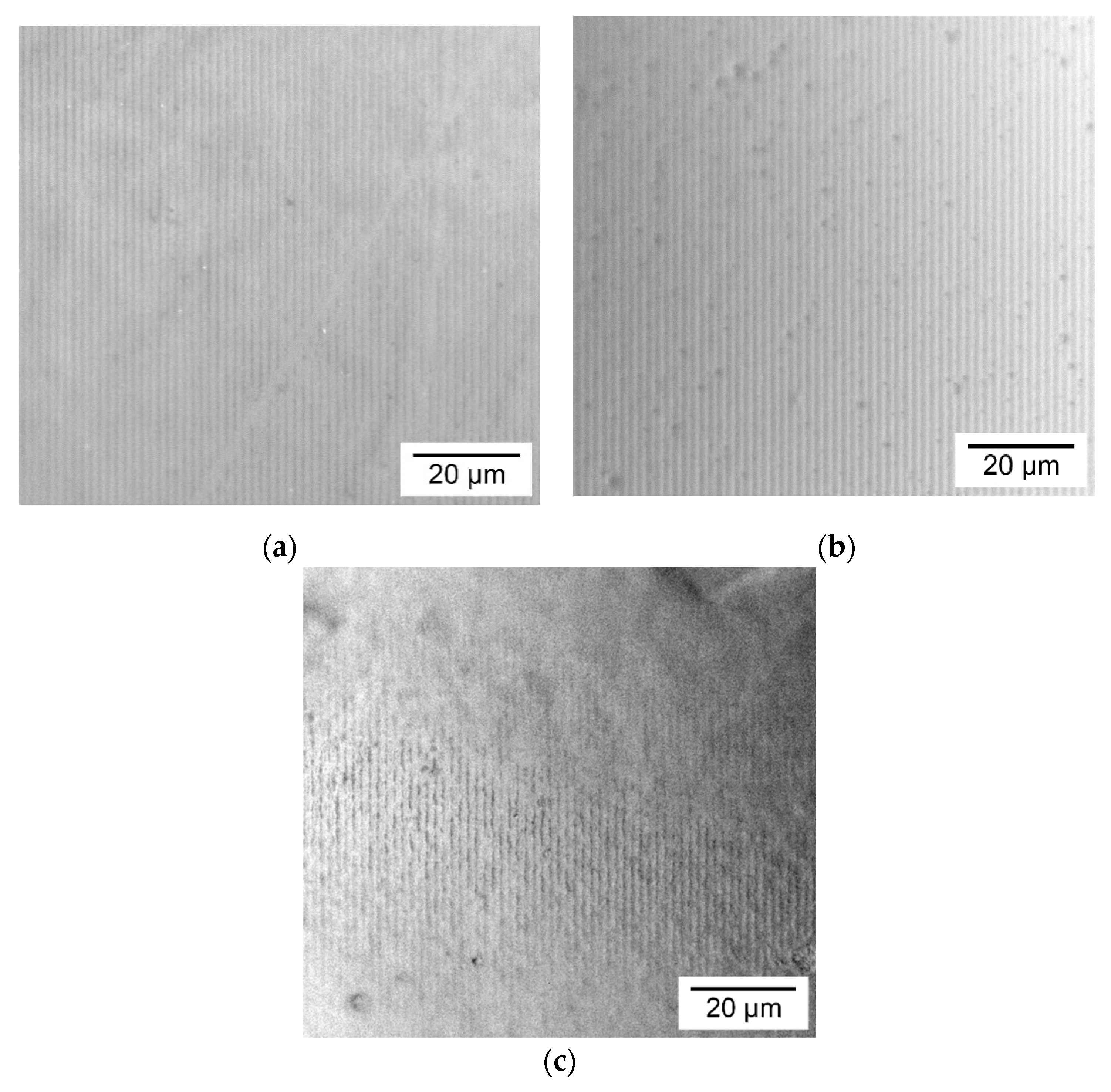
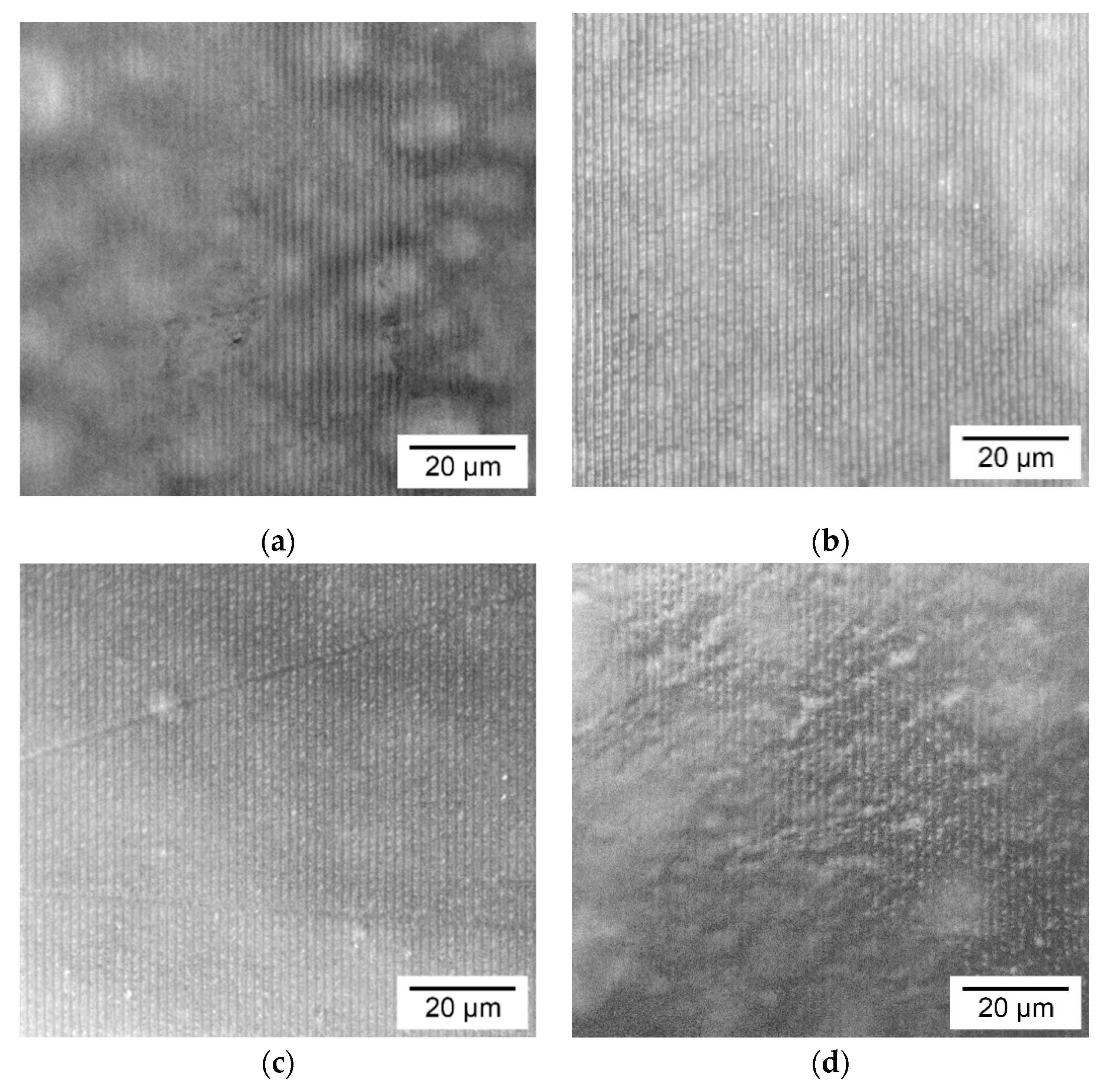
3.2. Morphology of the Holographic Markers Surface
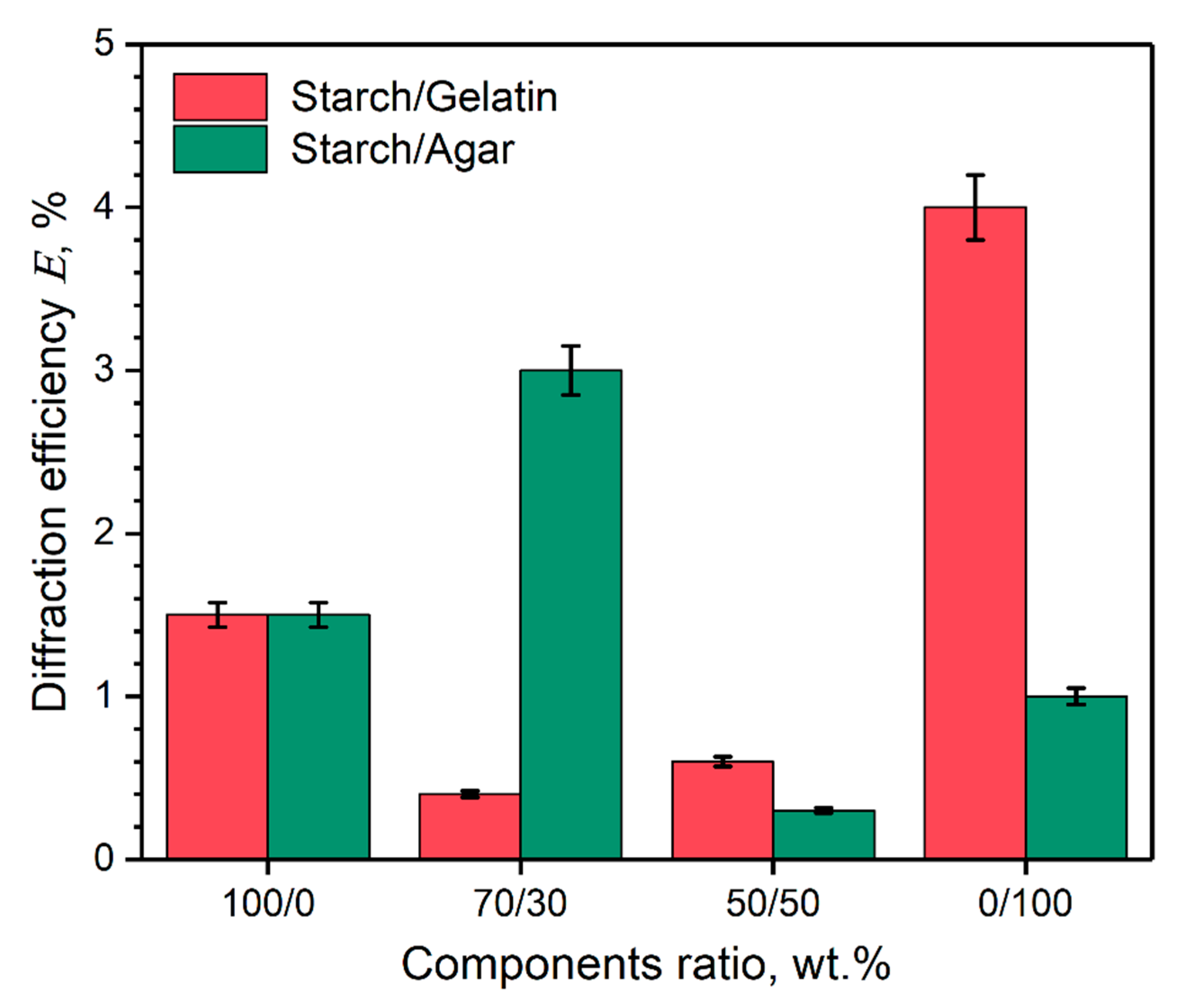
3.3. Diffraction Efficiency of the Holographic Markers
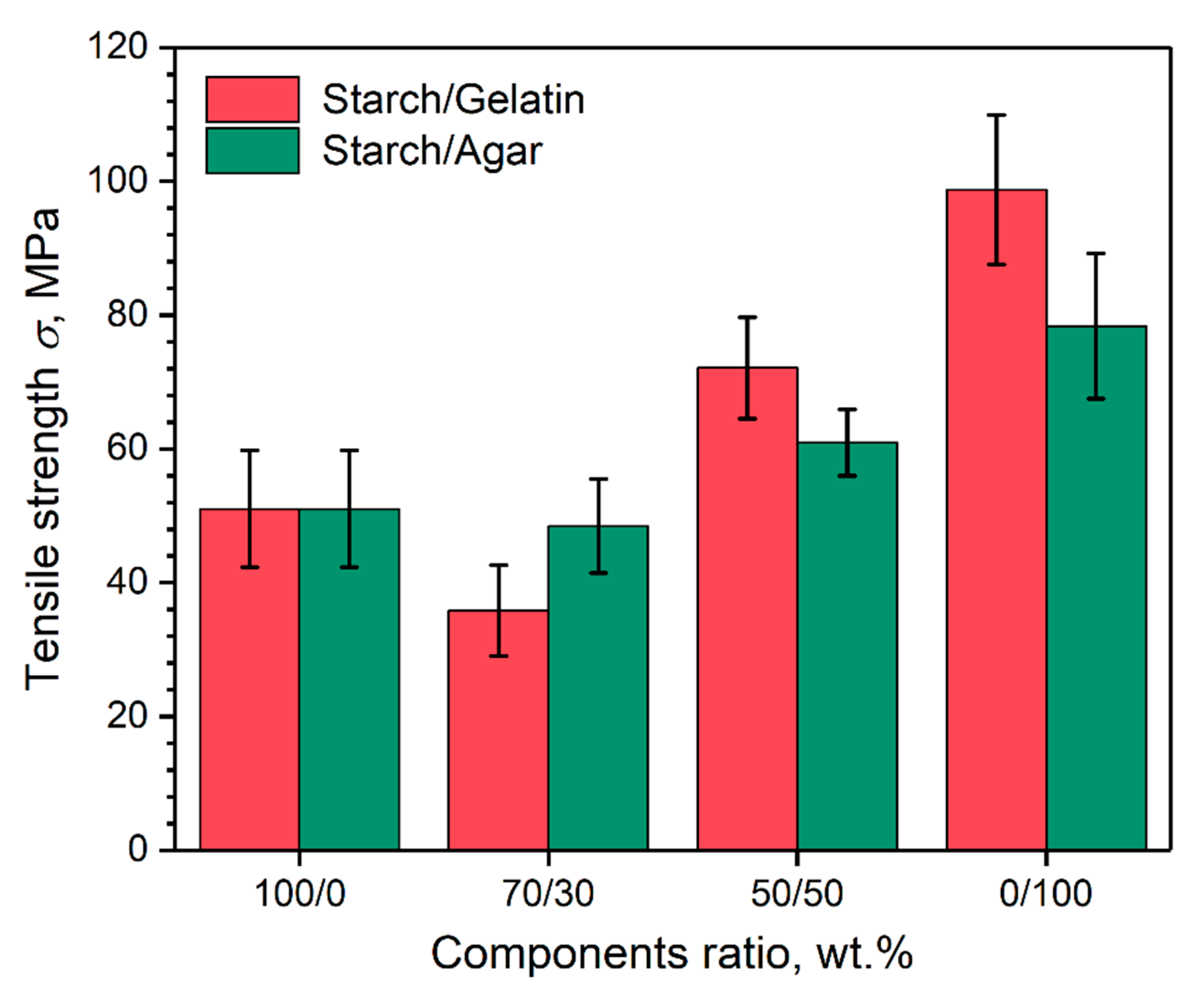
3.4. Mechanical Tensile Strength of the Coatings
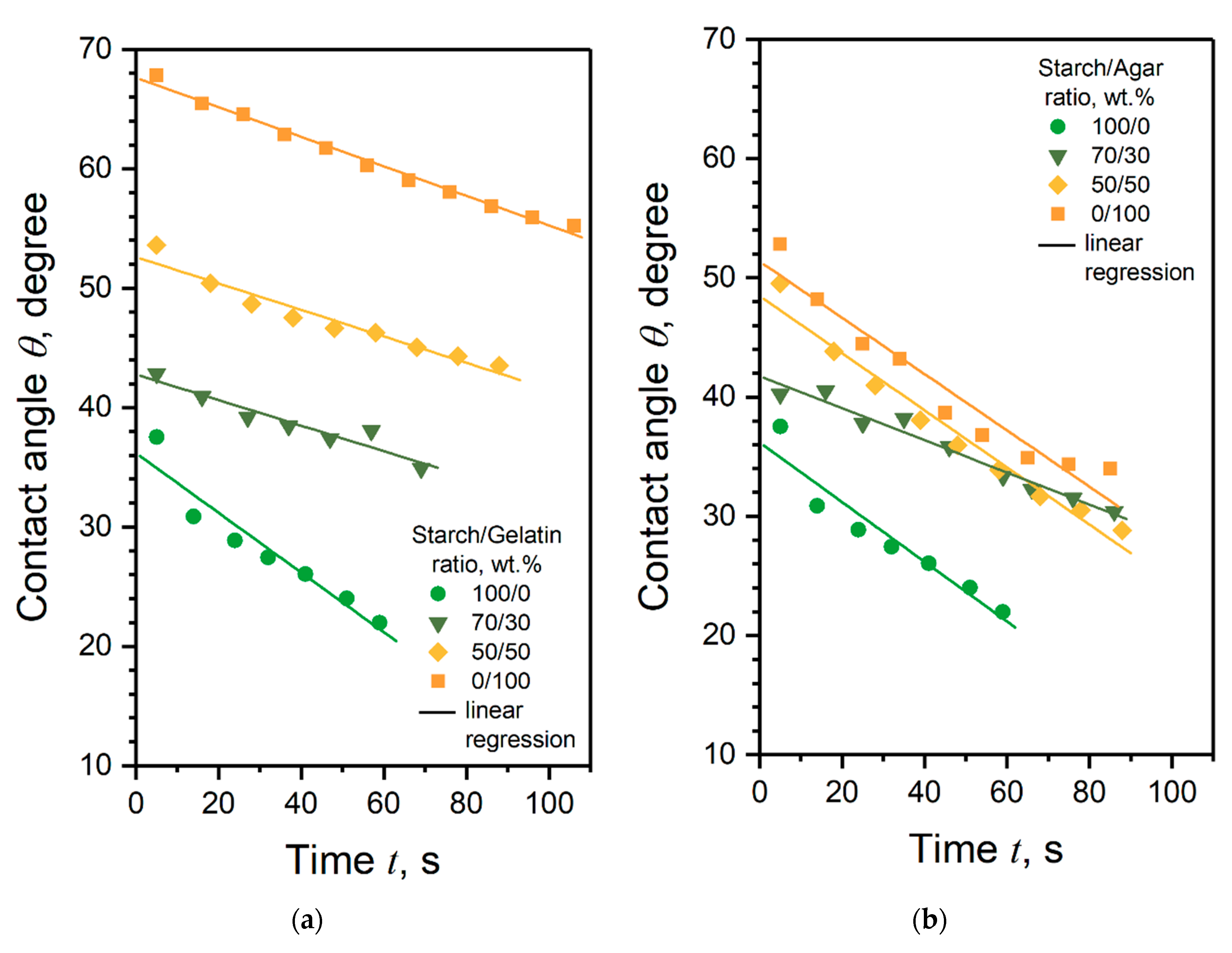
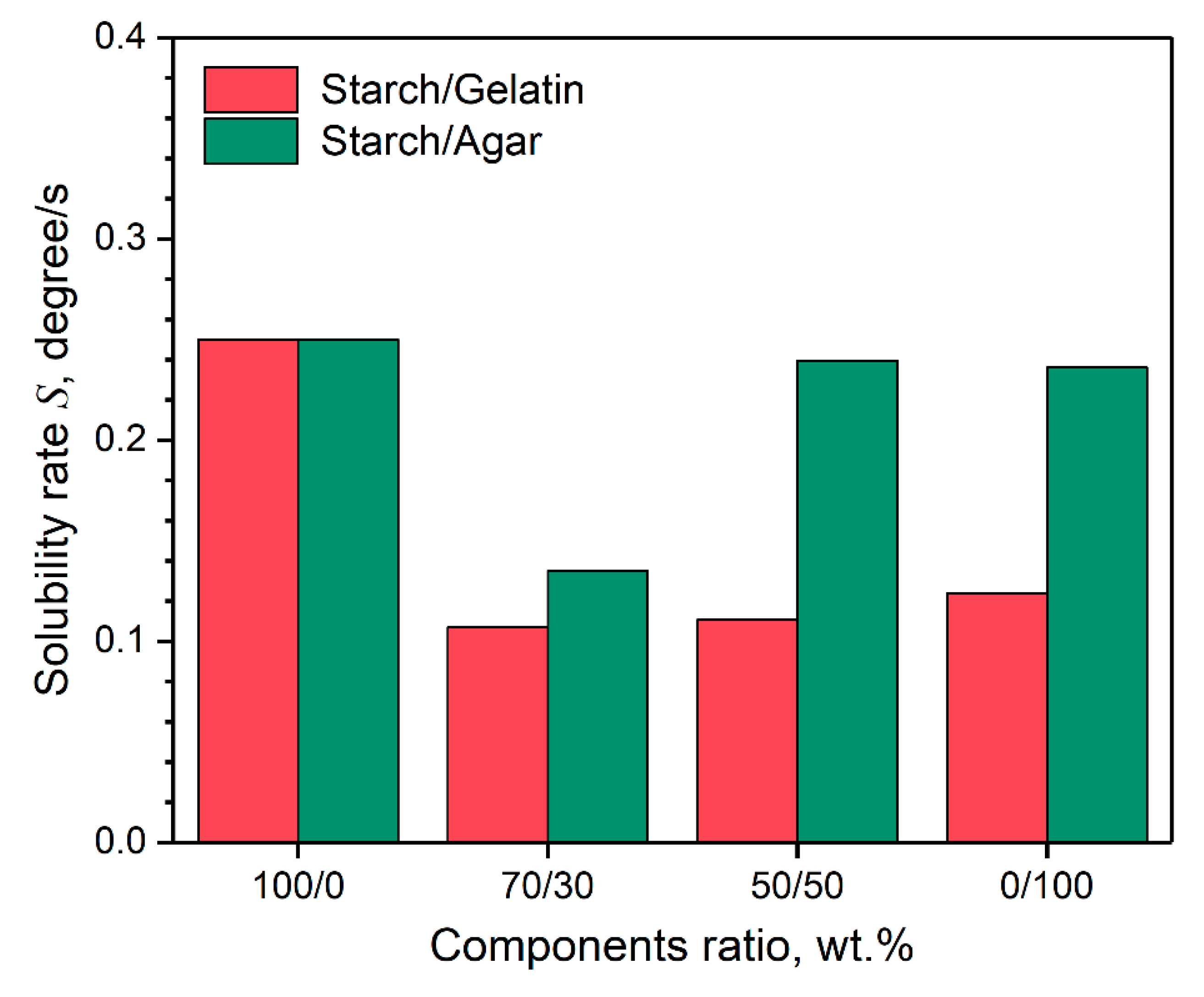
3.5. Surface Wettability of the Coatings
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dilkes-Hoffman, L.S.; Lane, J.L.; Grant, T.; Pratt, S.; Lant, P.A.; Laycock, B. Environmental impact of biodegradable food packaging when considering food waste. J. Clean. Prod. 2018, 180, 325–334. [Google Scholar] [CrossRef]
- Mkandawire, M.; Aryee, A.N. Resurfacing and modernization of edible packaging material technology. Curr. Opin. Food Sci. 2018, 19, 104–112. [Google Scholar] [CrossRef]
- Sohail, M.; Sun, D.-W.; Zhu, Z. Recent developments in intelligent packaging for enhancing food quality and safety. Crit. Rev. Food Sci. Nutr. 2018, 58, 2650–2662. [Google Scholar] [CrossRef] [PubMed]
- Kalpana, S.; Priyadarshini, S.R.; Maria Leena, M.; Moses, J.A.; Anandharamakrishnan, C. Intelligent packaging: Trends and applications in food systems. Trends Food Sci. Technol. 2019, 93, 145–157. [Google Scholar] [CrossRef]
- Begleiter, E. Edible Holography: The Application of Holographic Techniques to Food Processing. In Proceedings of the SPIE 1461, Practical Holography V, San Jose, CA, USA, 1 July 1991. [Google Scholar] [CrossRef]
- Begleiter, E. Edible Holographic Products, Particularly Pharmaceuticals and Methods and Apparatus for Producing Same. Patent USA No US7083805B2, 1 August 2006. Priority 14.09.2005. Available online: https://patents.google.com/patent/US7083805B2/en (accessed on 13 May 2020).
- Toropova, A.P.; Fokina, M.I. Shellac hologram. In Proceedings of the 6th International School and Conference “Saint Petersburg OPEN 2019”: Optoelectronics, Photonics, Engineering and Nanostructures, Saint Petersburg, Russian, 22–25 April 2019. [Google Scholar] [CrossRef]
- Naydenova, I.; Jallapuram, R.; Toal, V.; Martin, S. A visual indication of environmental humidity using a color changing hologram recorded in a self-developing photopolymer. Appl. Phys. Lett. 2008, 92, 031109. [Google Scholar] [CrossRef]
- Palmer, C. Diffraction Grating Handbook, 8th ed.; MKS Instruments, Inc.: New York, NY, USA, 2020. [Google Scholar]
- Vorzobova, N.D.; Bulgakova, V.G.; Moskalenko, A.I.; Pavlovets, I.M.; Denisyuk, I.; Burunkova, Y.E. Development of periodic and three-dimensional structures in acrylic-monomer photopolymer materials by holographic methods. Radiophys. Quantum Electron. 2015, 57, 595–602. [Google Scholar] [CrossRef]
- Olivares-Pérez, A.; Ponce-Lee, E.L.; Fuentes-Tapia, I. Sugar (sucrose) holograms. Opt. Mater. 2004, 26, 5–10. [Google Scholar] [CrossRef]
- Mejias-Brizuela, N.Y.; Olivares-Pérez, A.; Páez-Trujillo, G.; Fuentes-Tapia, I. Sugar holograms with erioglaucine and tartrazine. In Proceedings of the Organic 3D Photonics Materials and Devices, San Diego, CA, USA, 18 September 2007. [Google Scholar] [CrossRef]
- Ordóñez-Padilla, M.J.; Olivares-Pérez, A.; Dorantes-García, V.; Vallejo-Mendoza, R.; Fuentes-Tapia, I. Study of holograms made with saccharides and iron ions. In Proceedings of the Practical Holography XXVI: Materials and Applications, San Francisco, CA, USA, 9 February 2012. [Google Scholar] [CrossRef]
- Mejias-Brizuela, N.Y.; Olivares-Pérez, A.; Páez-Trujillo, G.; Hernández-Garay, M.P.; Fontanilla-Urdaneta, R.; Fuentes-Tapia, I. Hydrophobic sugar holograms. In Proceedings of the Practical Holography XXII: Materials and Applications, San Jose, CA, USA, 25 February 2008. [Google Scholar] [CrossRef]
- Olivares-Pérez, A.; Ponce-Lee, E.L.; Fuentes-Tapia, I.; Juárez-Pérez, J.L. Glucose-fructose holograms. In Proceedings of the Practical Holography XVIII: Materials and Applications, San Jose, CA, USA, 29 June 2004. [Google Scholar] [CrossRef]
- Pinto-Iguanero, B.; Olivares-Perez, A.; Mendez-Alvarado, A.W.; Fuentes-Tapia, I.; Trevino-Palacios, C.G. Non-hydroscopic vanilla doped dichromated gelatin holographic material. Opt. Mater. 2003, 22, 397–404. [Google Scholar] [CrossRef]
- Grande-Grande, A.; Mejias-Brizuela, N.Y.; Olivares-Pérez, A.; Paez-Trujillo, G.; Fuentes-Tapia, I. Holograms with corn honey and erioglaucine dye. In Proceedings of the Practical Holography XXII: Materials and Applications, San Jose, CA, USA, 25 February 2008. [Google Scholar] [CrossRef]
- Pérez-Salinas, P.; Mejias-Brizuela, N.Y.; Olivares-Perez, A.; Grande-Grande, A.; Páez-Trujillo, G.; Hernández-Garay, M.P.; Fuentes-Tapia, I. Holograms with egg albumin. In Proceedings of the Practical Holography XXII: Materials and Applications, San Jose, CA, USA, 25 January 2008. [Google Scholar] [CrossRef]
- Mejias-Brizuela, N.Y.; Olivares-Pérez, A.; Ortiz-Gutiérrez, M. Replication of holograms with corn syrup by rubbing. Materials 2012, 5, 1462–1476. [Google Scholar] [CrossRef]
- Begleiter, E. Edible Articles that Include Edible Optical Elements and Methods for Producing Same. Patent USA No US20040170725A1, 2 September 2004. Priority 08.07.2002. Available online: https://patents.google.com/patent/US20040170725A1/en (accessed on 13 May 2020).
- Omenetto, F.; Kaplan, D.L. Edible Holographic Silk Products. International Patent No WO2009155397A2, 23 December 2009. Priority 18.06.2008. Available online: https://patents.google.com/patent/US20110135697A1/en (accessed on 13 May 2020).
- Begleiter, E. Holographic Products. Patent USA No US4668523A, 26 May 1987. Priority 06.03.1985. Available online: https://patents.google.com/patent/US4668523A/en (accessed on 13 May 2020).
- Toxqui-López, S.; Calixte, L.; Olivares-Pérez, A.; Padilla, A.V.; Ponce-Lee, E.L. Pineapple holograms. In Proceedings of the Practical Holography XX: Materials and Applications, San Jose, CA, USA, 27 February 2006. [Google Scholar] [CrossRef]
- Toropova, A.; Uspenskaia, T.; Fokina, M.; Smekhov, A. Edible gelatin hologram. In Proceedings of the 19th International Multidisciplinary Scientific GeoConference SGEM 2019, Sofia, Bulgaria, 30 June–6 July 2019. [Google Scholar] [CrossRef]
- Jiang, T.; Duan, Q.; Zhu, J.; Liu, H.; Yu, L. Starch-based biodegradable materials: Challenges and opportunities. Adv. Ind. Eng. Polym. Res. 2020, 3, 8–18. [Google Scholar] [CrossRef]
- Kalra, B.; Gross, R.A. Biodegradable polymers for the environment. Science 2002, 297, 803–807. [Google Scholar] [CrossRef]
- Lu, D.R.; Xiao, C.M.; Xu, S.J. Starch-based completely biodegradable polymer materials. Express Polym. Lett. 2009, 3, 366–375. [Google Scholar] [CrossRef]
- Zhang, Y.; Ding, L.; Gu, J.; Tan, H.; Zhu, L. Preparation and properties of a starch-based wood adhesive with high bonding strength and water resistance. Carbohydr. Polym. 2015, 115, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Jeżowski, P.; Kowalczewski, P.Ł. Starch as a green binder for the formulation of conducting glue in supercapacitors. Polymers 2019, 11, 1648. [Google Scholar] [CrossRef] [PubMed]
- Acosta, S.; Jiménez, A.; Cháfer, M.; González-Martínez, C.; Chiralt, A. Physical properties and stability of starch-gelatin based films as affected by the addition of esters of fatty acids. Food Hydrocoll. 2015, 49, 135–143. [Google Scholar] [CrossRef]
- Garcia, V.A.S.; Borges, J.G.; Maciel, V.B.V.; Mazalli, M.R.; Lapa-Guimaraes, J.G.; Vanin, F.M.; Carvalho, R.A. Gelatin/starch orally disintegrating films as a promising system for vitamin C delivery. Food Hydrocoll. 2018, 79, 127–135. [Google Scholar] [CrossRef]
- Al-Hassan, A.A.; Norziah, M.H. Starch–gelatin edible films: Water vapor permeability and mechanical properties as affected by plasticizers. Food Hydrocoll. 2012, 26, 108–117. [Google Scholar] [CrossRef]
- Al-Hassan, A.A.; Norziah, M.H. Effect of transglutaminase induced crosslinking on the properties of starch/gelatin films. Food Packag. Shelf Life 2017, 13, 15–19. [Google Scholar] [CrossRef]
- Jumaidin, R.; Sapuan, S.M.; Jawaid, M.; Ishak, M.R.; Sahari, J. Characteristics of thermoplastic sugar palm starch/agar blend: Thermal, tensile, and physical properties. Int. J. Biol. Macromol. 2016, 89, 575–581. [Google Scholar] [CrossRef]
- Wongphan, P.; Harnkarnsujarit, N. Characterization of starch, agar and maltodextrin blends for controlled dissolution of edible films. Int. J. Biol. Macromol. 2020, 156, 80–93. [Google Scholar] [CrossRef]
- Phan The, D.; Debeaufort, F.; Voilley, A.; Luu, D. Biopolymer interactions affect the functional properties of edible films based on agar, cassava starch and arabinoxylan blends. J. Food Eng. 2009, 90, 548–558. [Google Scholar] [CrossRef]
- Wu, Y.; Geng, F.; Chang, P.R.; Yu, J.; Ma, X. Effect of agar on the microstructure and performance of potato starch film. Carbohydr. Polym. 2009, 76, 299–304. [Google Scholar] [CrossRef]
- Ostwald, W. Ueber die rechnerische Darstellung des Strukturgebietes der Viskosität. Kolloid Z. 1929, 47, 176–187. [Google Scholar] [CrossRef]
- Nagar, M.; Sharanagat, V.S.; Kumar, Y.; Singh, L.; Mani, S. Influence of xanthan and agar-agar on thermo-functional, morphological, pasting and rheological properties of elephant foot yam (Amorphophallus paeoniifolius) starch. Int. J. Biol. Macromol. 2019, 136, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Liu, X.; Yu, L.; Shanks, R.; Petinaks, E.; Liu, H. Phase composition and interface of starch–gelatin blends studied by synchrotron FTIR micro-spectroscopy. Carbohydr. Polym. 2013, 95, 649–653. [Google Scholar] [CrossRef] [PubMed]
- Podshivalov, A.; Zakharova, M.; Glazacheva, E.; Uspenskaya, M. Gelatin/potato starch edible biocomposite films: Correlation between morphology and physical properties. Carbohydr. Polym. 2017, 157, 1162–1172. [Google Scholar] [CrossRef]
- Zhang, N.; Liu, H.; Yu, L.; Liu, X.; Zhang, L.; Chen, L.; Shanks, R. Developing gelatin–starch blends for use as capsule materials. Carbohydr. Polym. 2013, 92, 455–461. [Google Scholar] [CrossRef]
- Acosta, S.; Chiralt, A.; Santamarina, P.; Rosello, J.; González-Martínez, C.; Cháfer, M. Antifungal films based on starch-gelatin blend, containing essential oils. Food Hydrocoll. 2016, 61, 233–240. [Google Scholar] [CrossRef]
- Wang, W.; Wang, K.; Xiao, J.; Liu, Y.; Zhao, Y.; Liu, A. Performance of high amylose starch-composited gelatin films influenced by gelatinization and concentration. Int. J. Biol. Macromol. 2017, 94, 258–265. [Google Scholar] [CrossRef]
- Jumaidin, R.; Sapuan, S.M.; Jawaid, M.; Ishak, M.R.; Sahari, J. Effect of seaweed on mechanical, thermal, and biodegradation properties of thermoplastic sugar palm starch/agar composites. Int. J. Biol. Macromol. 2017, 99, 265–273. [Google Scholar] [CrossRef]
- Ordóñez-Padilla, M.J.; Olivares-Pérez, A.; Ortiz-Gutiérrez, M.; Juárez-Ramírez, J.C. Albumin holograms with iron ions. In Proceedings of the Practical Holography XXVIII: Materials and Applications, San Francisco, CA, USA, 25 February 2014. [Google Scholar] [CrossRef]
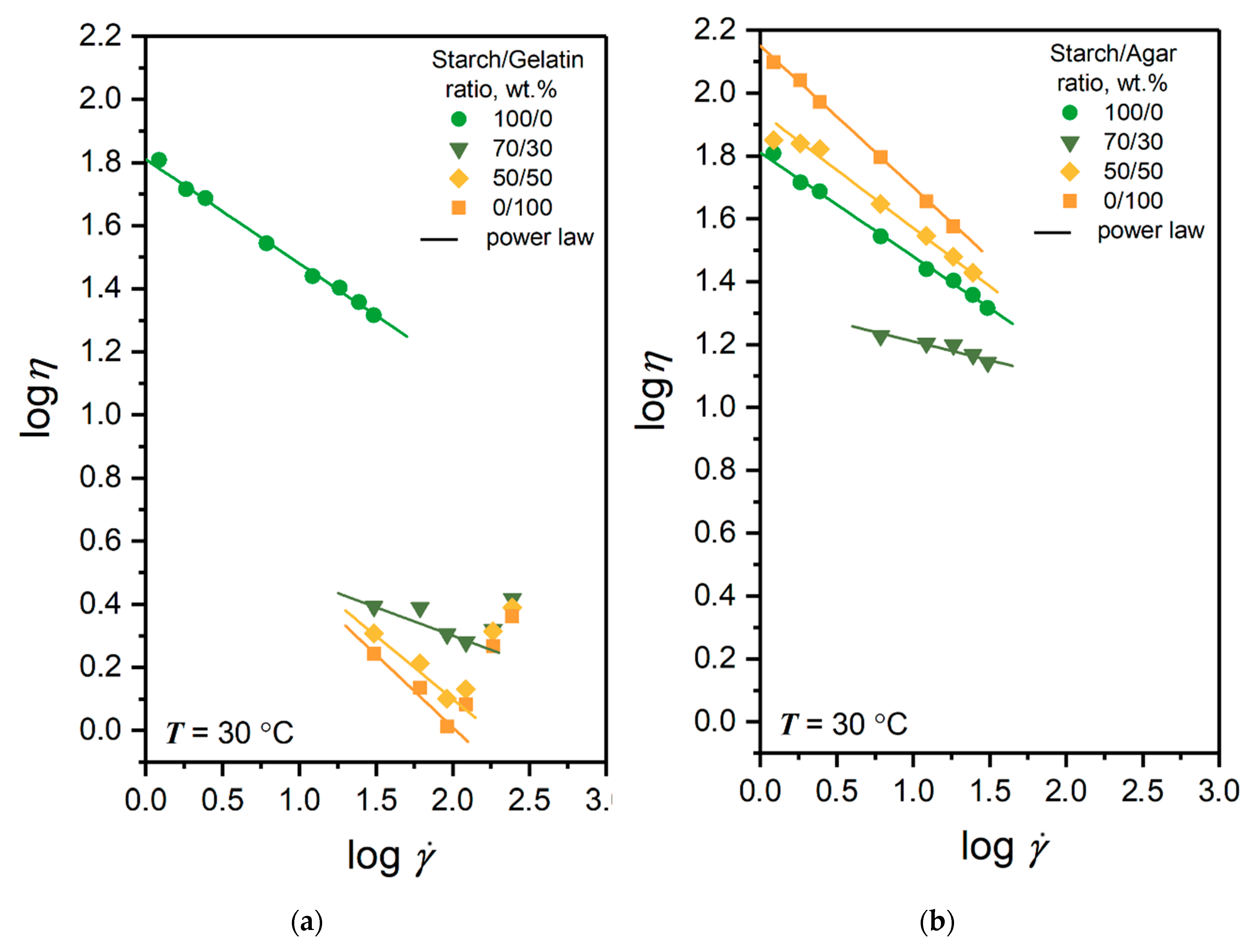
| Components Ratio in Solution, wt.% | n | K, Pa·sn |
|---|---|---|
| Starch/Gelatin | ||
| 100/0 | 0.67 | 064.56 |
| 70/30 | 0.82 | 004.57 |
| 50/50 | 0.60 | 007.94 |
| 0/100 | 0.54 | 008.51 |
| Starch/Agar | ||
| 100/0 | 0.67 | 064.56 |
| 70/30 | 0.88 | 021.38 |
| 50/50 | 0.63 | 087.09 |
| 0/100 | 0.55 | 141.25 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Podshivalov, A.; Toropova, A.; Fokina, M.; Uspenskaya, M. Surface Morphology Formation of Edible Holographic Marker on Potato Starch with Gelatin or Agar Thin Coatings. Polymers 2020, 12, 1123. https://doi.org/10.3390/polym12051123
Podshivalov A, Toropova A, Fokina M, Uspenskaya M. Surface Morphology Formation of Edible Holographic Marker on Potato Starch with Gelatin or Agar Thin Coatings. Polymers. 2020; 12(5):1123. https://doi.org/10.3390/polym12051123
Chicago/Turabian StylePodshivalov, Aleksandr, Alexandra Toropova, Maria Fokina, and Mayya Uspenskaya. 2020. "Surface Morphology Formation of Edible Holographic Marker on Potato Starch with Gelatin or Agar Thin Coatings" Polymers 12, no. 5: 1123. https://doi.org/10.3390/polym12051123
APA StylePodshivalov, A., Toropova, A., Fokina, M., & Uspenskaya, M. (2020). Surface Morphology Formation of Edible Holographic Marker on Potato Starch with Gelatin or Agar Thin Coatings. Polymers, 12(5), 1123. https://doi.org/10.3390/polym12051123