Reduced Graphene Oxide/Poly(Pyrrole-co-Thiophene) Hybrid Composite Materials: Synthesis, Characterization, and Supercapacitive Properties
Abstract
1. Introduction
2. Experimental
2.1. Materials and Chemicals
2.2. Preparation of Graphene Oxide (GO) and Reduced Graphene Oxide (RGO)
2.3. Synthesis of RGO/COP Composite
2.4. Materials Characterization
3. Result and Discussion
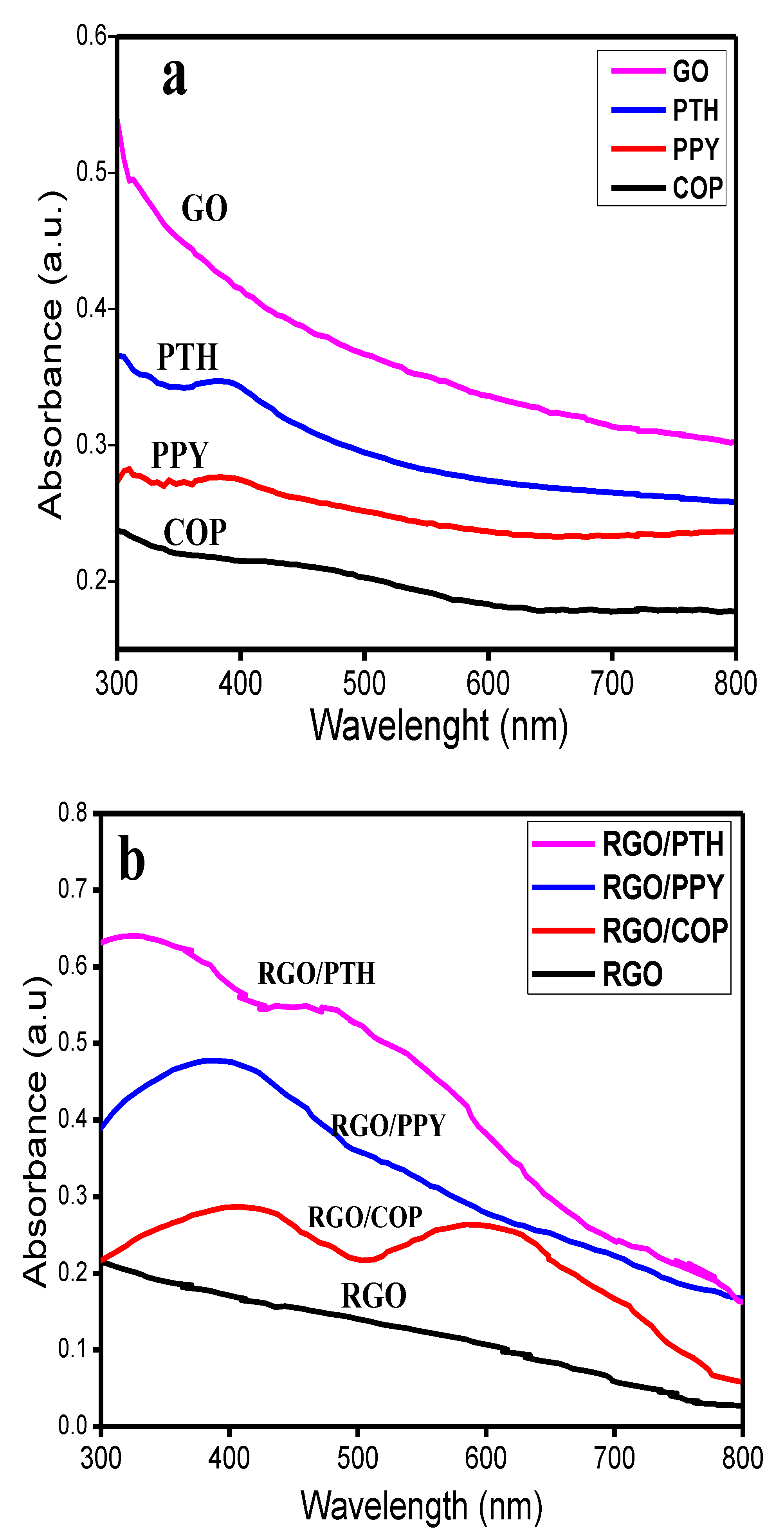
3.1. UV-Visible Spectroscopy
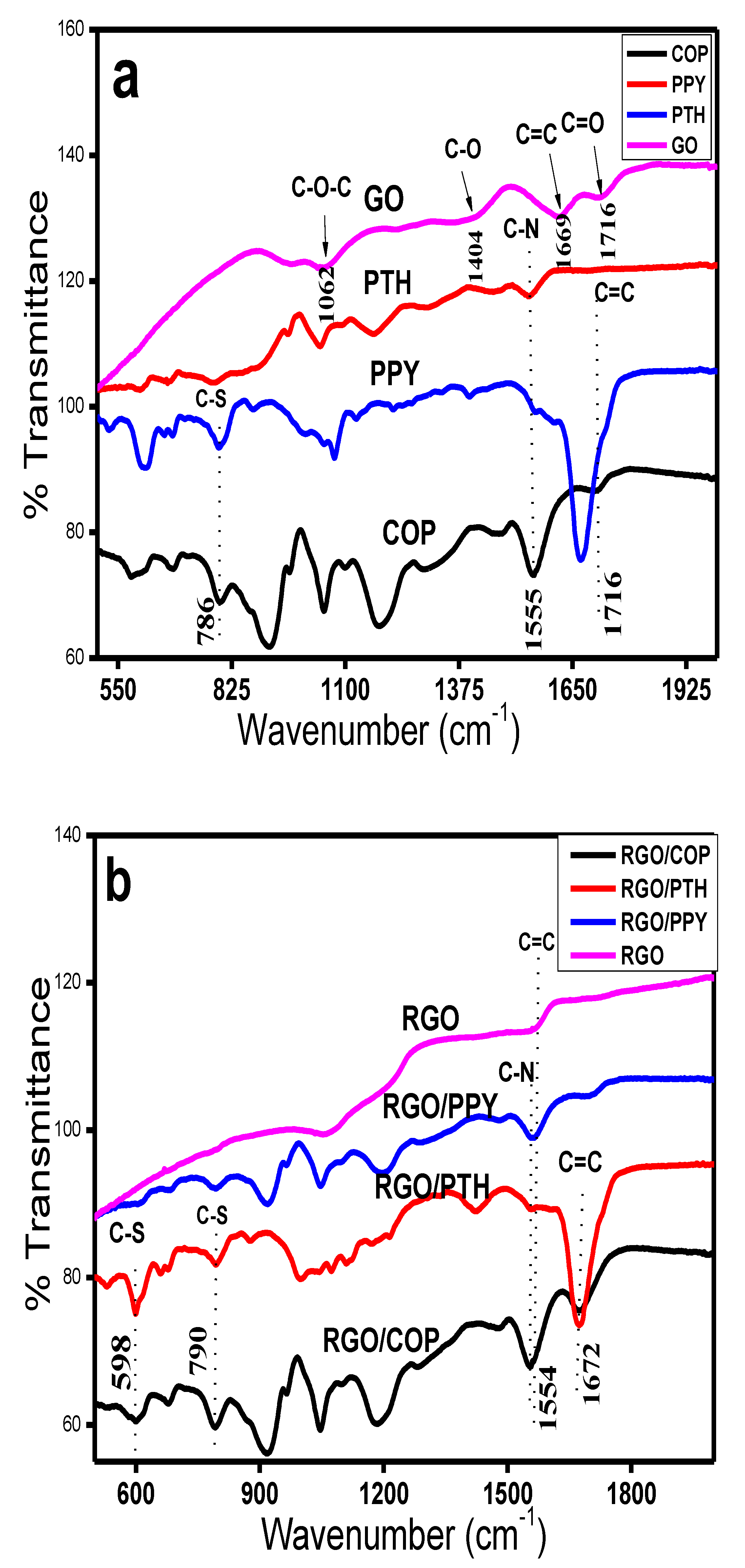
3.2. FTIR Spectroscopy
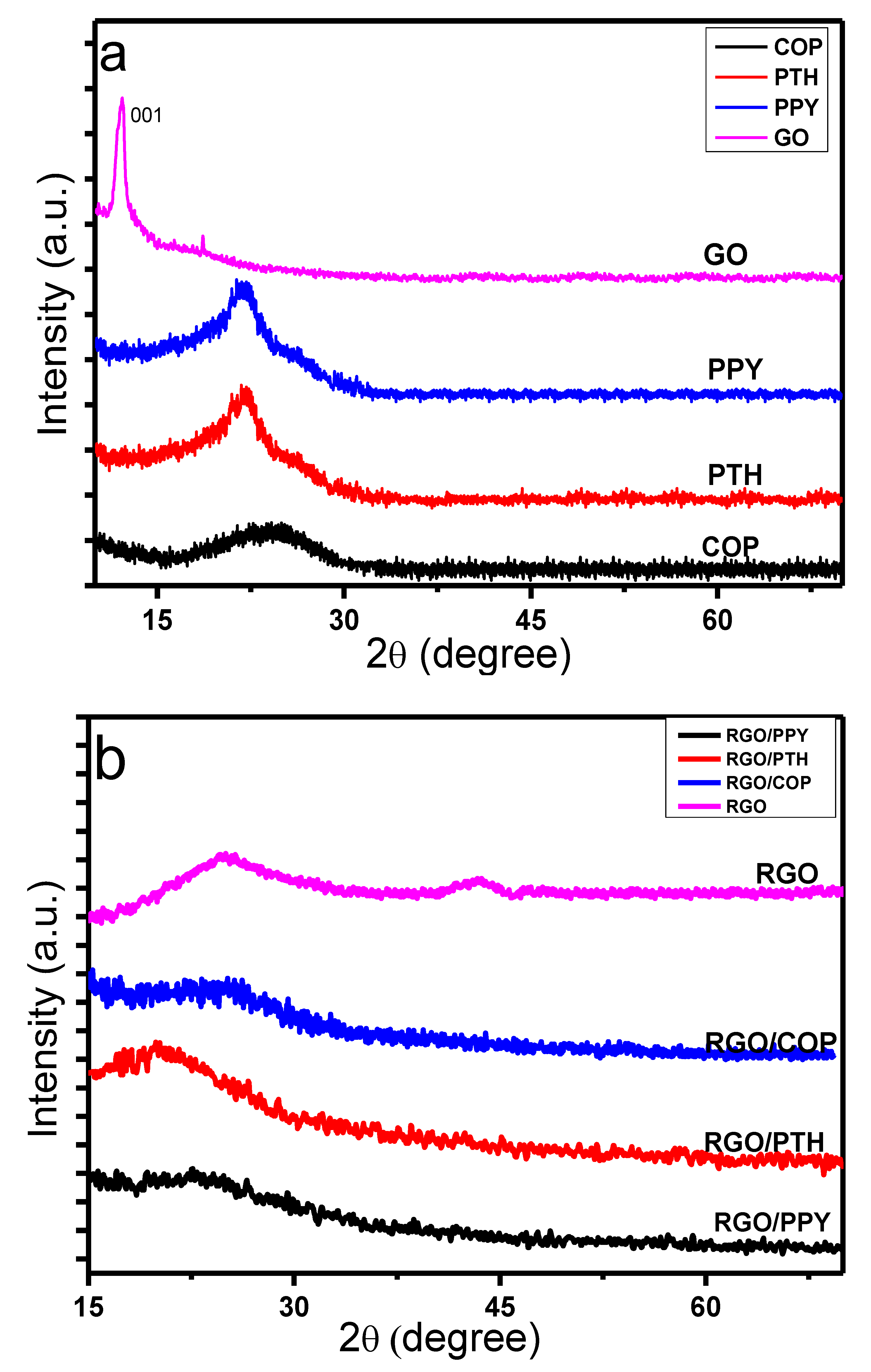
3.3. X-ray Diffraction (XRD) Study
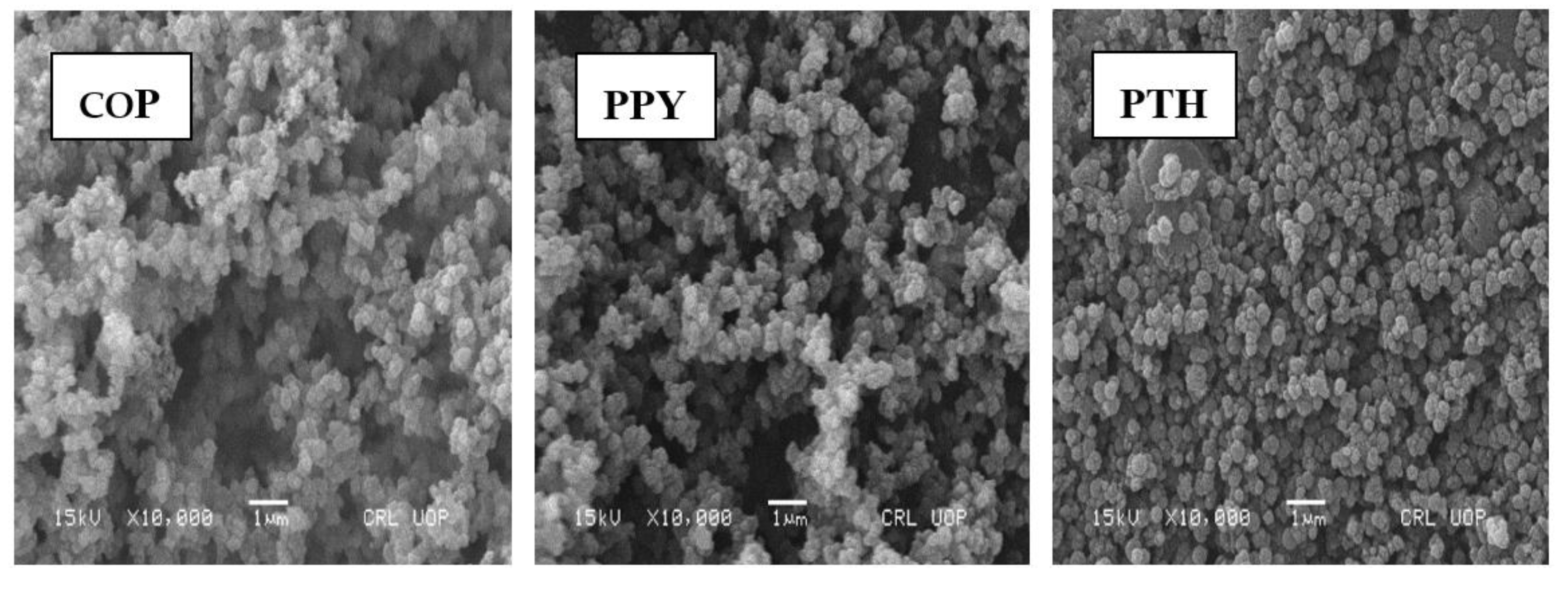
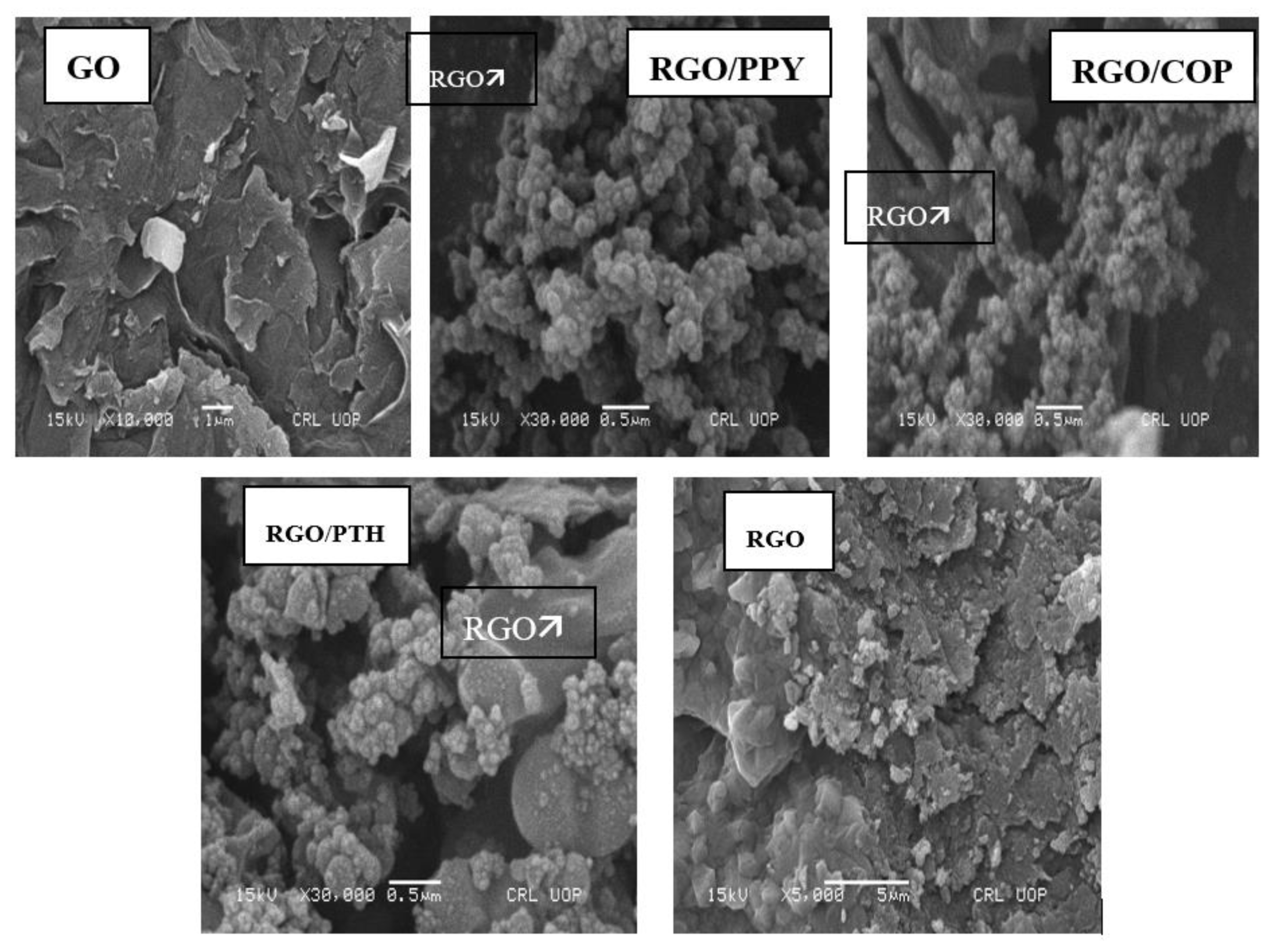
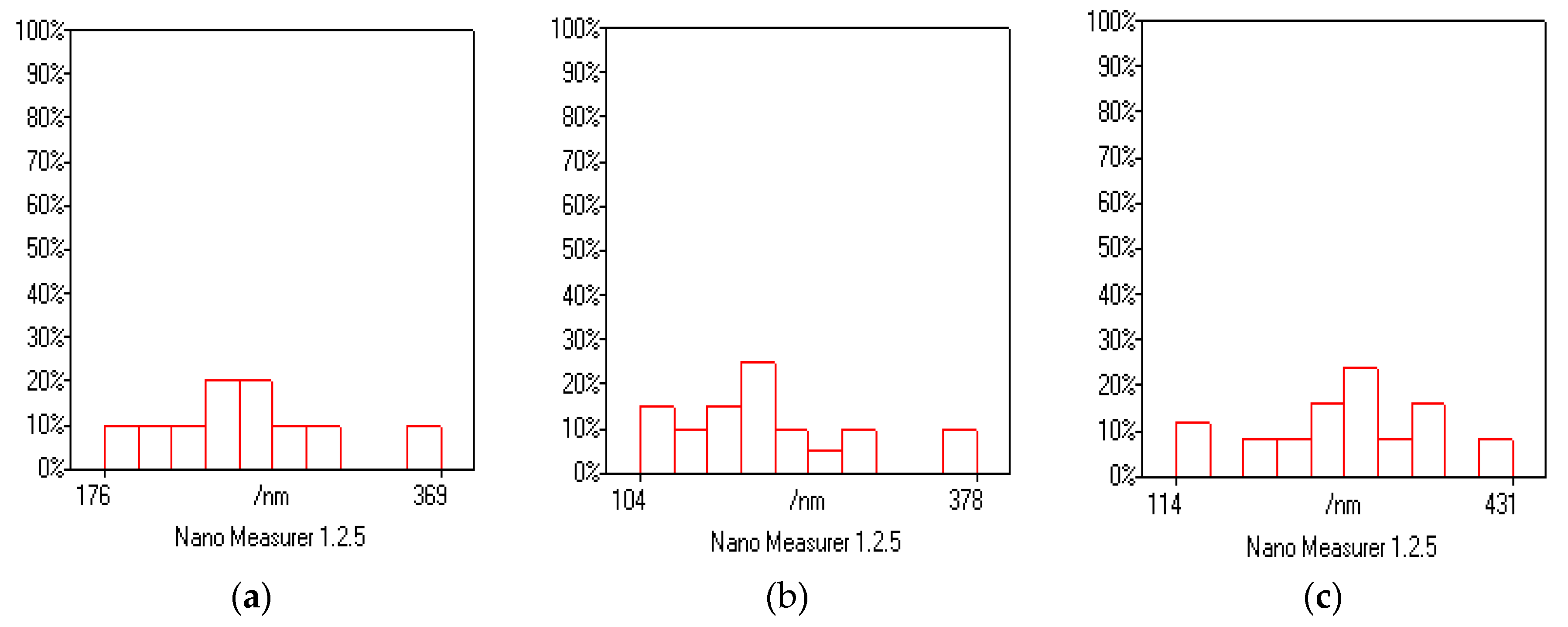
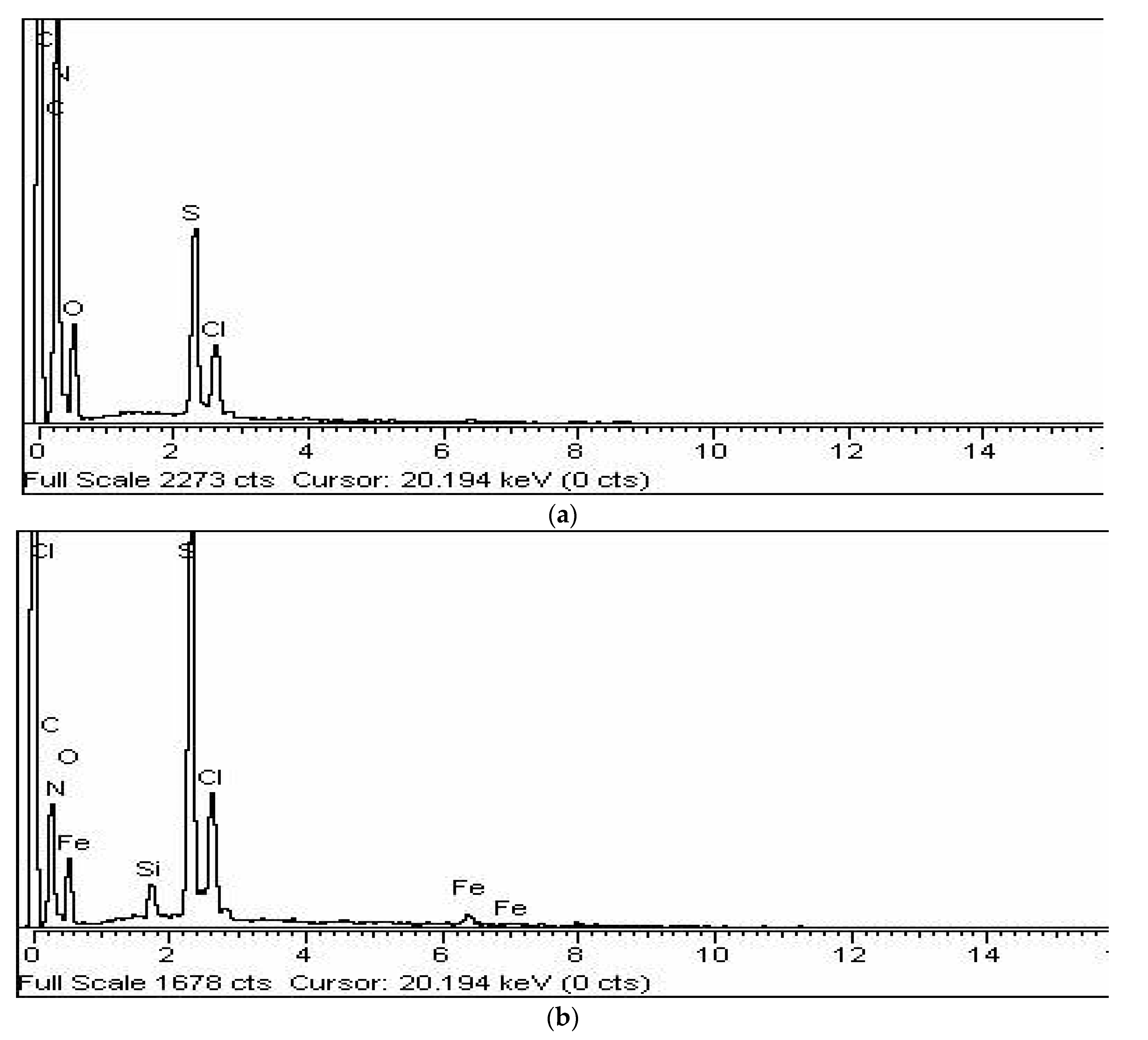
3.4. Scanning Electron Microscopy (SEM) and Energy Dispersive X-ray (EDX) Analysis
3.5. Electrochemical Analysis
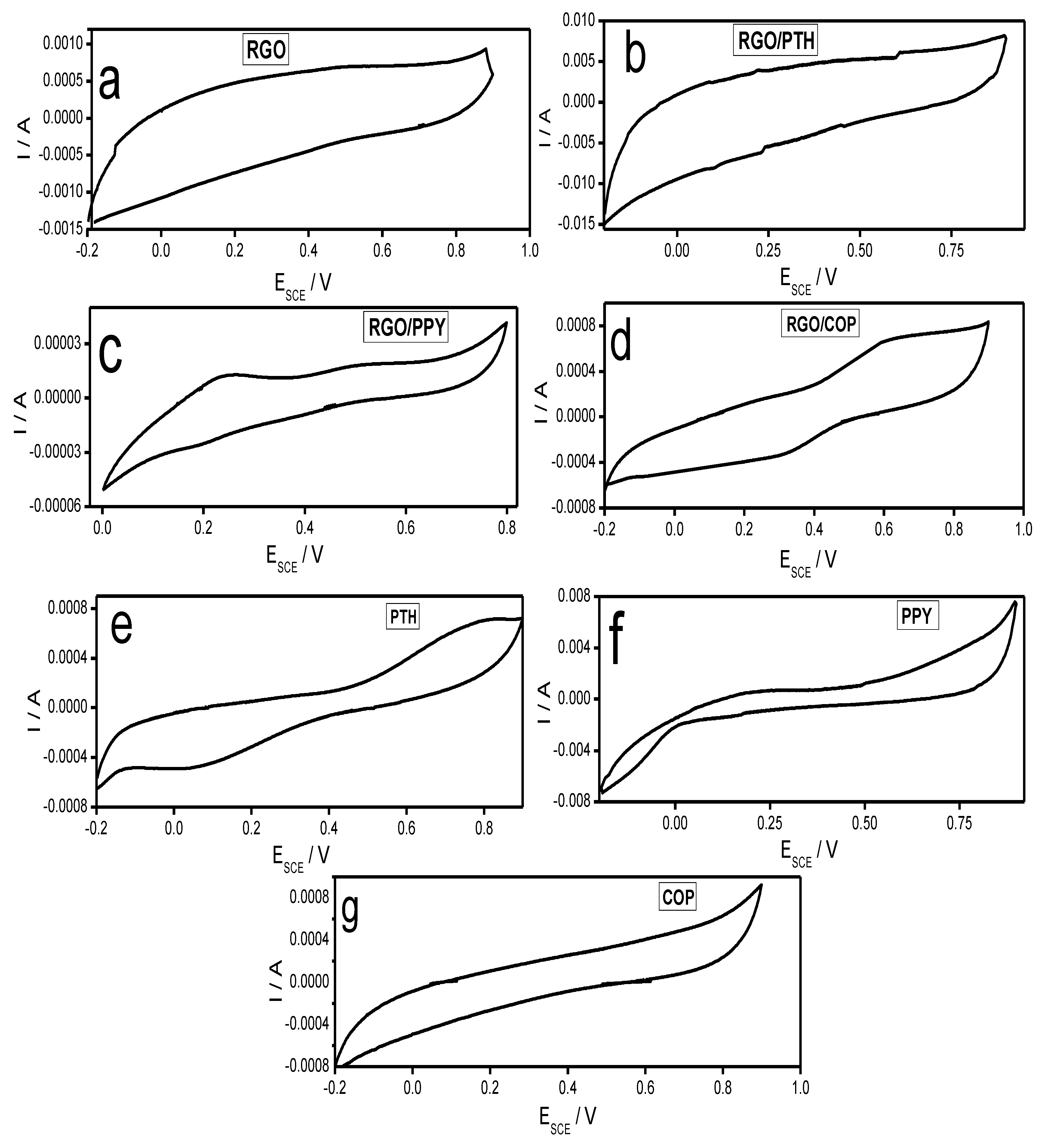
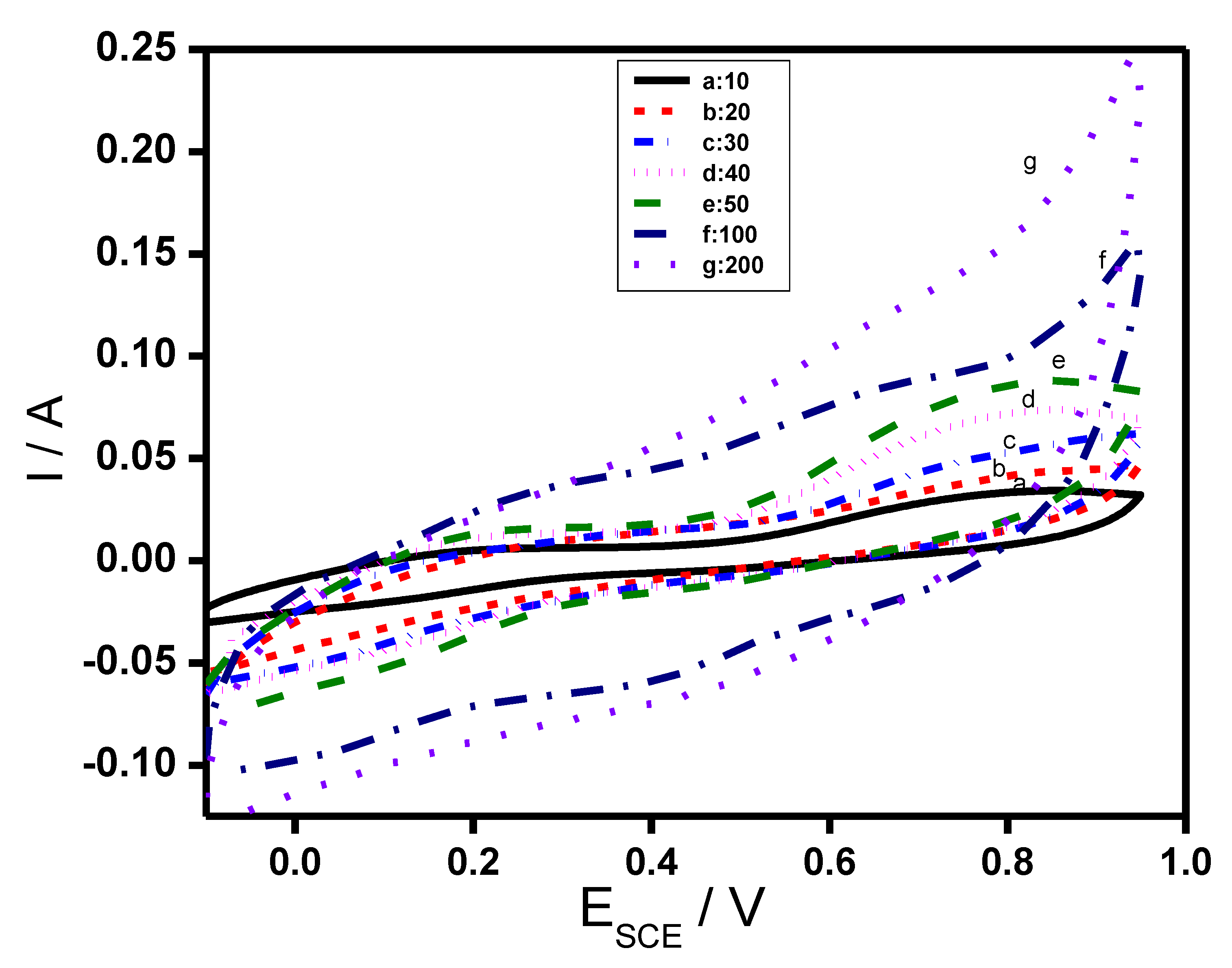
3.5.1. Cyclic Voltammetry
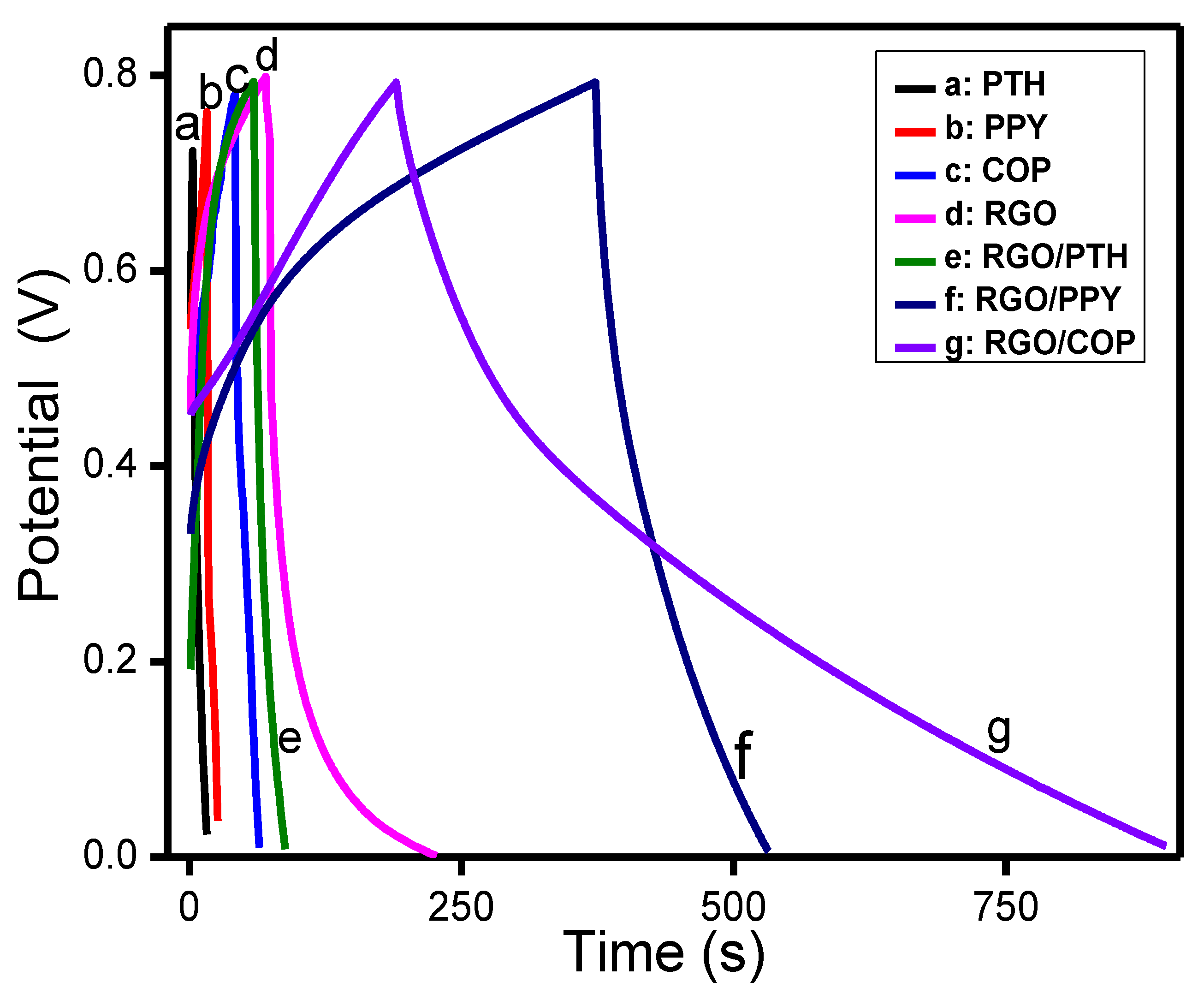
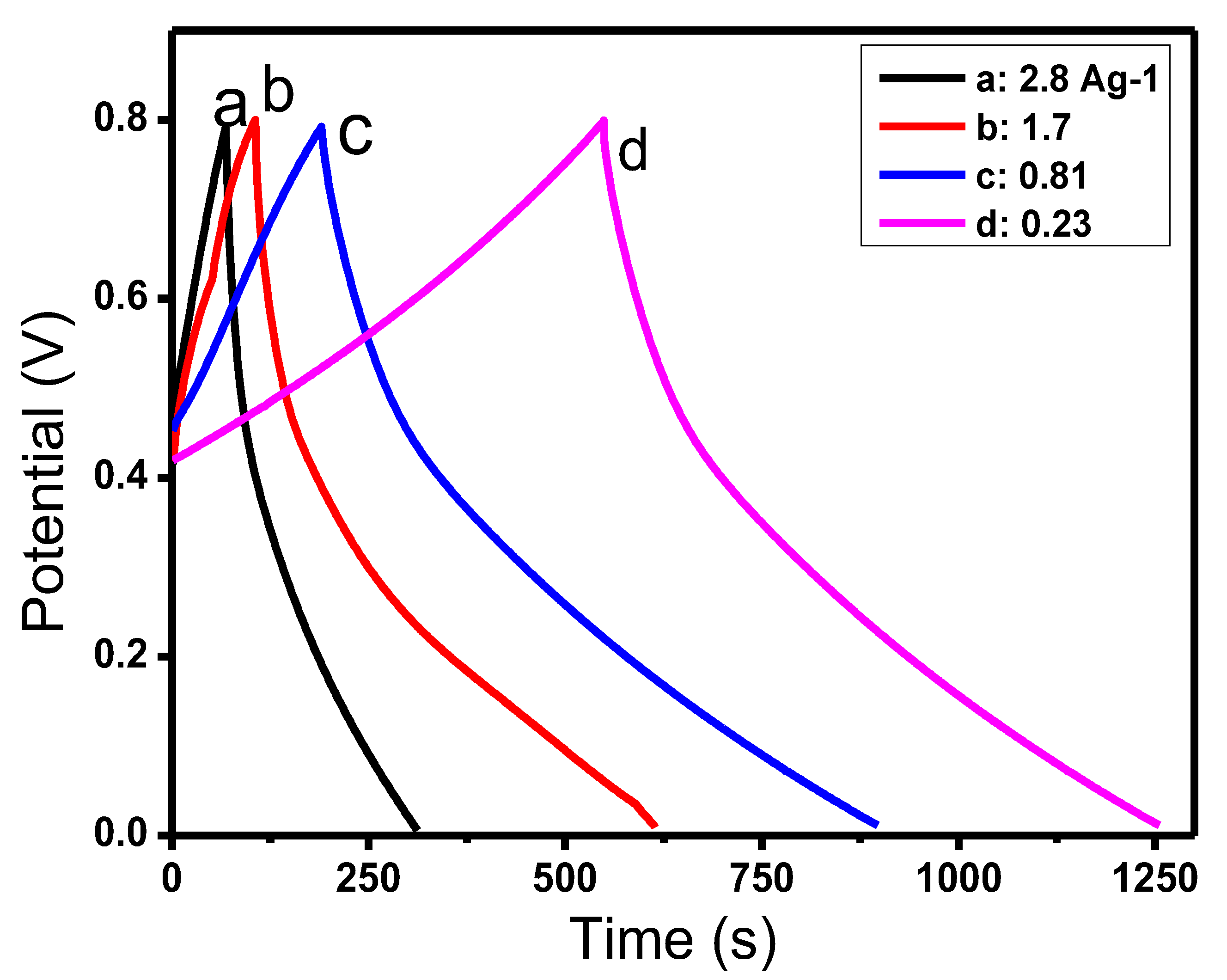
3.5.2. Galvanostatic Charge/Discharge Analysis
3.5.3. Electrostatic Impedance Spectroscopy
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kou, L.; Huang, T.; Zheng, B.; Han, Y.; Zhao, X.; Gopalsamy, K.; Sun, H.; Gao, C. Coaxial wet-spun yarn supercapacitors for high-energy density and safe wearable electronics. Nat. Commun. 2014, 5, 3754. [Google Scholar] [CrossRef] [PubMed]
- Anichini, C.; Czepa, W.; Pakulski, D.; Aliprandi, A.; Ciesielski, A.; Samorì, P. Chemical sensing with 2D materials. Chem. Soc. Rev. 2018, 47, 4860–4908. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Choi, T.K.; Lee, Y.B.; Cho, H.R.; Ghaffari, R.; Wang, L.; Choi, H.J.; Chung, T.D.; Lu, N.; Hyeon, T. A graphene-based electrochemical device with thermoresponsive microneedles for diabetes monitoring and therapy. Nat. Nanotechnol. 2016, 11, 566. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Liang, J.; Chen, Y. An overview of the applications of graphene-based materials in supercapacitors. Small 2012, 8, 1805–1834. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Tetard, L.; Zhai, L.; Thomas, J. Supercapacitor electrode materials: Nanostructures from 0 to 3 dimensions. Energy Environ. Sci. 2015, 8, 702–730. [Google Scholar] [CrossRef]
- Simon, P.; Gogotsi, Y.; Dunn, B. Where do batteries end and supercapacitors begin? Science 2014, 343, 1210–1211. [Google Scholar] [CrossRef]
- Béguin, F.; Presser, V.; Balducci, A.; Frackowiak, E. Carbons and electrolytes for advanced supercapacitors. Adv. Mater. 2014, 26, 2219–2251. [Google Scholar] [CrossRef]
- Jiang, H.; Lee, P.S.; Li, C. 3D carbon based nanostructures for advanced supercapacitors. Energy Environ. Sci. 2013, 6, 41–53. [Google Scholar] [CrossRef]
- Wu, D.; Li, Z.; Zhong, M.; Kowalewski, T.; Matyjaszewski, K. Templated Synthesis of Nitrogen-Enriched Nanoporous Carbon Materials from Porogenic Organic Precursors Prepared by ATRP. Angew. Chem. Int. Ed. 2014, 53, 3957–3960. [Google Scholar] [CrossRef]
- Winter, M.; Brodd, R.J. What are batteries, fuel cells, and supercapacitors? Chem. Rev. 2005, 105, 1021. [Google Scholar] [CrossRef]
- El-Kady, M.F.; Kaner, R.B. Scalable fabrication of high-power graphene micro-supercapacitors for flexible and on-chip energy storage. Nat. Commun. 2013, 4, 1475. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Singh, N.; Song, L.; Liu, Z.; Reddy, A.L.M.; Ci, L.; Vajtai, R.; Zhang, Q.; Wei, B.; Ajayan, P.M. Direct laser writing of micro-supercapacitors on hydrated graphite oxide films. Nat. Nanotechnol. 2011, 6, 496. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.J.; Balakrishnan, K.; Huang, J.; Meunier, V.; Sumpter, B.G.; Srivastava, A.; Conway, M.; Reddy, A.L.M.; Yu, J.; Vajtai, R. Ultrathin planar graphene supercapacitors. Nano Lett. 2011, 11, 1423–1427. [Google Scholar] [CrossRef] [PubMed]
- Niu, Z.; Zhang, L.; Liu, L.; Zhu, B.; Dong, H.; Chen, X. All-solid-state flexible ultrathin micro-supercapacitors based on graphene. Adv. Mater. 2013, 25, 4035–4042. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.W.; Feng, Y.Q.; Yan, X.B.; Chen, J.T.; Xue, Q.J. Superior micro-supercapacitors based on graphene quantum dots. Adv. Funct. Mater. 2013, 23, 4111–4122. [Google Scholar] [CrossRef]
- Liu, W.; Yan, X.; Chen, J.; Feng, Y.; Xue, Q. Novel and high-performance asymmetric micro-supercapacitors based on graphene quantum dots and polyaniline nanofibers. Nanoscale 2013, 5, 6053–6062. [Google Scholar] [CrossRef]
- Lukatskaya, M.R.; Mashtalir, O.; Ren, C.E.; Dall’Agnese, Y.; Rozier, P.; Taberna, P.L.; Naguib, M.; Simon, P.; Barsoum, M.W.; Gogotsi, Y. Cation intercalation and high volumetric capacitance of two-dimensional titanium carbide. Science 2013, 341, 1502–1505. [Google Scholar] [CrossRef]
- Jeong, H.M.; Lee, J.W.; Shin, W.H.; Choi, Y.J.; Shin, H.J.; Kang, J.K.; Choi, J.W. Nitrogen-doped graphene for high-performance ultracapacitors and the importance of nitrogen-doped sites at basal planes. Nano Lett. 2011, 11, 2472–2477. [Google Scholar] [CrossRef]
- Zhao, L.; Fan, L.Z.; Zhou, M.Q.; Guan, H.; Qiao, S.; Antonietti, M.; Titirici, M.M. Nitrogen-containing hydrothermal carbons with superior performance in supercapacitors. Adv. Mater. 2010, 22, 5202–5206. [Google Scholar] [CrossRef]
- Georgakilas, V.; Otyepka, M.; Bourlinos, A.B.; Chandra, V.; Kim, N.; Kemp, K.C.; Hobza, P.; Zboril, R.; Kim, K.S. Functionalization of graphene: Covalent and non-covalent approaches, derivatives and applications. Chem. Rev. 2012, 112, 6156–6214. [Google Scholar] [CrossRef]
- Xue, M.; Li, F.; Zhu, J.; Song, H.; Zhang, M.; Cao, T. Structure-based enhanced capacitance: In situ growth of highly ordered polyaniline nanorods on reduced graphene oxide patterns. Adv. Funct. Mater. 2012, 22, 1284–1290. [Google Scholar] [CrossRef]
- Iroh, J.O.; Wood, G.A. Control of carbon fiber-polypyrrole interphases by aqueous electrochemical process. Compos. Part B Eng. 1998, 29, 181–188. [Google Scholar] [CrossRef]
- Moussa, M.; El-Kady, M.F.; Zhao, Z.; Majewski, P.; Ma, J. Recent progress and performance evaluation for polyaniline/graphene nanocomposites as supercapacitor electrodes. Nanotechnology 2016, 27, 442001. [Google Scholar] [CrossRef] [PubMed]
- William, S.; Hummers, J.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar]
- Park, S.; An, J.; Potts, J.R.; Velamakanni, A.; Murali, S.; Ruoff, R.S. Hydrazine-reduction of graphite-and graphene oxide. Carbon 2011, 49, 3019–3023. [Google Scholar] [CrossRef]
- Shahriary, L.; Athawale, A.A. Graphene oxide synthesized by using modified hummers approach. Int. J. Renew. Energy Environ. Eng. 2014, 2, 58–63. [Google Scholar]
- Park, S.; An, J.; Jung, I.; Piner, R.D.; An, S.J.; Li, X.; Velamakanni, A.; Ruoff, R.S. Colloidal suspensions of highly reduced graphene oxide in a wide variety of organic solvents. Nano Lett. 2009, 9, 1593–1597. [Google Scholar] [CrossRef]
- Shiigi, H.; Kishimoto, M.; Yakabe, H.; Deore, B.; Nagaoka, T. Characterization of overoxidized polypyrrole colloids imprinted with L-lactate and their application to enantioseparation of amino acids. Anal. Sci. 2002, 18, 41–44. [Google Scholar] [CrossRef]
- Wang, Z.L.; Kong, X.Y.; Ding, Y.; Gao, P.; Hughes, W.L.; Yang, R.; Zhang, Y. Semiconducting and piezoelectric oxide nanostructures induced by polar surfaces. Adv. Funct. Mater. 2004, 14, 943–956. [Google Scholar] [CrossRef]
- Emadzadeh, D.; Lau, W.J.; Matsuura, T.; Rahbari-Sisakht, M.; Ismail, A.F. A novel thin film composite forward osmosis membrane prepared from PSf–TiO2 nanocomposite substrate for water desalination. Chem. Eng. J. 2014, 237, 70–80. [Google Scholar] [CrossRef]
- Wu, T.M.; Lin, S.H. Synthesis, characterization, and electrical properties of polypyrrole/multiwalled carbon nanotube composites. J. Polym. Sci. Part A Polym. Chem. 2006, 44, 6449–6457. [Google Scholar] [CrossRef]
- McAllister, M.J.; Li, J.-L.; Adamson, D.H.; Schniepp, H.C.; Abdala, A.A.; Liu, J.; Herrera-Alonso, M.; Milius, D.L.; Car, R.; Prud’homme, R.K. Single sheet functionalized graphene by oxidation and thermal expansion of graphite. Chem. Mater. 2007, 19, 4396–4404. [Google Scholar] [CrossRef]
- Senthilkumar, B.; Thenamirtham, P.; Selvan, R.K. Structural and electrochemical properties of polythiophene. Appl. Surf. Sci. 2011, 257, 9063–9067. [Google Scholar] [CrossRef]
- Kong, F.; Wang, Y.; Zhang, J.; Xia, H.; Zhu, B.; Wang, Y.; Wang, S.; Wu, S. The preparation and gas sensitivity study of polythiophene/SnO2 composites. Mater. Sci. Eng. B 2008, 150, 6–11. [Google Scholar] [CrossRef]
- Khatamian, M.; Divband, B.; Jodaei, A. Degradation of 4-nitrophenol (4-NP) using ZnO nanoparticles supported on zeolites and modeling of experimental results by artificial neural networks. Mater. Chem. Phys. 2012, 134, 31–37. [Google Scholar] [CrossRef]
- Gómez-Navarro, C.; Weitz, R.T.; Bittner, A.M.; Scolari, M.; Mews, A.; Burghard, M.; Kern, K. Electronic Transport Properties of Individual Chemically Reduced Graphene Oxide Sheets. Nano Lett. 2007, 7, 3499–3503. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Babaei, F.; Fakhri, P.; Jaleh, B. Synthesis, characterization, structural, optical properties and catalytic activity of reduced graphene oxide/copper nanocomposites. RSC Adv. 2015, 5, 10782–10789. [Google Scholar] [CrossRef]
- Ouyang, J.; Li, Y. Great improvement of polypyrrole films prepared electrochemically from aqueous solutions by adding nonaphenol polyethyleneoxy (10) ether. Polymer 1997, 38, 3997–3999. [Google Scholar] [CrossRef]
- Wu, F.; Chen, J.; Chen, R.; Wu, S.; Li, L.; Chen, S.; Zhao, T. Sulfur/polythiophene with a core/shell structure: Synthesis and electrochemical properties of the cathode for rechargeable lithium batteries. J. Phys. Chem. C 2011, 115, 6057–6063. [Google Scholar] [CrossRef]
- Partch, R.; Gangolli, S.; Matijević, E.; Cal, W.; Arajs, S. Conducting polymer composites: I. Surface-induced polymerization of pyrrole on iron (III) and cerium (IV) oxide particles. J. Coll. Interface Sci. 1991, 144, 27–35. [Google Scholar] [CrossRef]
- Ju, H.-M.; Choi, S.-H.; Huh, S.-H. X-ray diffraction patterns of thermally-reduced graphenes. J. Korean Phys. Soc. 2010, 57, 1649–1652. [Google Scholar]
- Tang, L.; Wang, Y.; Li, Y.; Feng, H.; Lu, J.; Li, J. Preparation, structure, and electrochemical properties of reduced graphene sheet films. Adv. Funct. Mater. 2009, 19, 2782–2789. [Google Scholar] [CrossRef]
- Gong, F.; Xu, X.; Zhou, G.; Wang, Z.-S. Enhanced charge transportation in a polypyrrole counter electrode via incorporation of reduced graphene oxide sheets for dye-sensitized solar cells. Phys. Chem. Chem. Phys. 2013, 15, 546–552. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Kim, H.; Cho, M.S.; Choi, J.; Lee, Y. Fabrication of polypyrrole (PPy)/carbon nanotube (CNT) composite electrode on ceramic fabric for supercapacitor applications. Electrochim. Acta 2011, 56, 7460–7466. [Google Scholar] [CrossRef]
- Chang, H.-H.; Chang, C.-K.; Tsai, Y.-C.; Liao, C.-S. Electrochemically synthesized graphene/polypyrrole composites and their use in supercapacitor. Carbon 2012, 50, 2331–2336. [Google Scholar] [CrossRef]
- Chen, L.; Tang, Y.; Wang, K.; Liu, C.; Luo, S. Direct electrodeposition of reduced graphene oxide on glassy carbon electrode and its electrochemical application. Electrochem. Commun. 2011, 13, 133–137. [Google Scholar]
- Wu, Z.; Huang, X.-L.; Wang, Z.-L.; Xu, J.-J.; Wang, H.-G.; Zhang, X.-B. Direct electrodeposition of reduced graphene oxide on glassy carbon electrode and its electrochemical application. Sci. Rep. 2014, 4, 3669. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, J.; Hu, Y.; Cheng, H.; Hu, C.; Jiang, C.; Jiang, L.; Cao, A.; Qu, L. Highly compression-tolerant supercapacitor based on polypyrrole-mediated graphene foam electrodes. Adv. Mater. 2013, 25, 591–595. [Google Scholar] [CrossRef]
- Khomenko, V.; Frackowiak, E.; Beguin, F. Determination of the specific capacitance of conducting polymer/nanotubes composite electrodes using different cell configurations. Electrochim. Acta 2005, 50, 2499–2506. [Google Scholar] [CrossRef]
- Wang, D.-W.; Li, F.; Zhao, J.; Ren, W.; Chen, Z.-G.; Tan, J.; Wu, Z.-S.; Gentle, I.; Lu, G.Q.; Cheng, H.-M. Fabrication of Graphene/Polyaniline Composite Paper viaIn situ Anodic Electropolymerization for High-Performance Flexible Electrode. ACS Nano 2009, 3, 1745–1752. [Google Scholar] [CrossRef]
- Shah, A.u.H.A.; Khan, M.O.; Bilal, S.; Rahman, G.; Hoang, H.V. Electrochemical co-deposition and characterization of polyaniline and manganese oxide nanofibrous composites for energy storage properties. Adv. Polym. Technol. 2018, 37, 2230–2237. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Ma, G.; Wang, Z.; Liu, K.; Liu, H. Ethylene glycol reduced graphene oxide/polypyrrole composite for supercapacitor. Electrochim. Acta 2013, 88, 519–525. [Google Scholar] [CrossRef]
- Liu, J.; An, J.; Ma, Y.; Li, M.; Ma, R. Synthesis of a graphene-polypyrrole nanotube composite and its application in supercapacitor electrode. J. Electrochem. Soc. 2012, 159, A828–A833. [Google Scholar] [CrossRef]
- Fan, L.-Q.; Liu, G.-J.; Wu, J.-H.; Liu, L.; Lin, J.-M.; Wei, Y.-L. Asymmetric supercapacitor based on graphene oxide/polypyrrole composite and activated carbon electrodes. Electrochim. Acta 2014, 137, 26–33. [Google Scholar] [CrossRef]
- Liu, G.; Shi, Y.; Wang, L.; Song, Y.; Gao, S.; Liu, D.; Fan, L. Reduced graphene oxide/polypyrrole composite: An advanced electrode for high-performance symmetric/asymmetric supercapacitor. Carbon Lett. 2019. [Google Scholar] [CrossRef]
- Barakzehi, M.; Montazer, M.; Sharif, F.; Norby, T.; Chatzitakis, A. A textile-based wearable supercapacitor using reduced graphene oxide/polypyrrole composite. Electrochim. Acta 2019, 305, 187–196. [Google Scholar] [CrossRef]
- Ma, C.; Cao, W.-T.; Xin, W.; Bian, J.; Ma, M.-G. Flexible and free-standing reduced graphene oxide and polypyrrole coated air-laid paper-based supercapacitor electrodes. Ind. Eng. Chem. Res. 2019, 58, 12018–12027. [Google Scholar] [CrossRef]
- Ibariez, M.F.; Morales-Verdejo, C.; Camarada, M. Interaction of Graphene Quantum Dots with Oligothiophene: A Comprehensive Theoretical Study. Int. J. Electrochem. Sci. 2017, 12, 11546–11555. [Google Scholar]
- Alvi, F.; Ram, M.K.; Basnayaka, P.A.; Stefanakos, E.; Goswami, Y.; Kumar, A. Graphene/polyethylenedioxythiophene conducting polymer nanocomposite based supercapacitor. Electrochim. Acta 2011, 56, 9406–9412. [Google Scholar] [CrossRef]
- Zhou, H.; Zhai, H.-J.; Han, G. Superior performance of highly flexible solid-state supercapacitor based on the ternary composites of graphene oxide supported poly(3,4-ethylenedioxythiophene)-carbon nanotubes. J. Power Sources 2016, 323, 125–133. [Google Scholar] [CrossRef]
- Zhou, H.; Han, G. One-step fabrication of heterogeneous conducting polymers-coated graphene oxide/carbon nanotubes composite films for high-performance supercapacitors. Electrochim. Acta 2016, 192, 448–455. [Google Scholar] [CrossRef]
- Alabadi, A.; Razzaque, S.; Dong, Z.; Wang, W.; Tan, B. Graphene oxide-polythiophene derivative hybrid nanosheet for enhancing performance of supercapacitor. J. Power Sources 2016, 306, 241–247. [Google Scholar] [CrossRef]
- Dhibar, S.; Bhattacharya, P.; Ghosh, D.; Hatui, G.; Das, C.K. Graphene–single-walled carbon nanotubes–poly(3-methylthiophene) ternary nanocomposite for supercapacitor electrode materials. Ind. Eng. Chem. Res. 2014, 53, 13030–13045. [Google Scholar] [CrossRef]
- Bhattacharya, P.; Das, C. Poly(3-methylthiophene)/Graphene Composite: In-situ Synthesis and Its Electrochemical Characterization. J. Nanosci. Nanotechnol. 2012, 12, 7173–7180. [Google Scholar] [CrossRef]
- Sivaraman, P.; Thakur, A.P.; Shashidhara, K. Poly(3-methyl thiophene)-graphene nanocomposites for asymmetric supercapacitors. Synth. Met. 2020, 259, 116255. [Google Scholar] [CrossRef]
- Yan, J.; Wei, T.; Shao, B.; Fan, Z.; Qian, W.; Zhang, M.; Wei, F. Fast and reversible surface redox reaction of graphene–MnO2 composites as supercapacitor electrodes. Carbon 2010, 48, 487–493. [Google Scholar] [CrossRef]
- Bao, S.-J.; Li, C.M.; Guo, C.-X.; Qiao, Y. Biomolecule-assisted synthesis of cobalt sulfide nanowires for application in supercapacitors. J. Power Sources 2008, 180, 676–681. [Google Scholar] [CrossRef]
- Lu, Q.; Zhou, Y. Synthesis of mesoporous polythiophene/MnO2 nanocomposite and its enhanced pseudocapacitive properties. J. Power Sources 2011, 196, 4088–4094. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, C.; Xu, C.; Zhu, Z.; Chen, C. Hollow polypyrrole nanosphere embedded in nitrogen-doped graphene layers to obtain a three-dimensional nanostructure as electrode material for electrochemical supercapacitor. Ionics 2017, 23, 147–156. [Google Scholar] [CrossRef]
- Gupta, S.; Smith, T.; Banaszak, A.; Boeckl, J. Graphene quantum dots electrochemistry and sensitive electrocatalytic glucose sensor development. Nanomaterials 2017, 7, 301. [Google Scholar] [CrossRef]
- Conway, B.; Pell, W. Double-layer and pseudocapacitance types of electrochemical capacitors and their applications to the development of hybrid devices. J. Solid State Electrochem. 2003, 7, 637–644. [Google Scholar] [CrossRef]
- Tüken, T.; Yazıcı, B.; Erbil, M. White-coat hypertension contributes to the presence of carotid arteriosclerosis. Prog. Organ. Coat. 2004, 50, 115–122. [Google Scholar] [CrossRef]
- Gupta, S.; Price, C. Investigating graphene/conducting polymer hybrid layered composites as pseudocapacitors: Interplay of heterogeneous electron transfer, electric double layers and mechanical stability. AIP Adv. 2015, 5, 107–113. [Google Scholar] [CrossRef]
- Gupta, S.; Heintzman, E.; Price, C. Electrostatic layer-by-layer self-assembled graphene/multi-walled carbon nanotubes hybrid multilayers as efficient ‘all carbon’ supercapacitors. J. Nanosci. Nanotechnol. 2016, 16, 4771–4782. [Google Scholar] [CrossRef] [PubMed]


| Electrode Material | Specific Capacitance | Current Density/Scan Rate | Reference |
|---|---|---|---|
| EG-RGO/PPy | 240 F g−1 | 5 A g−1 | [52] |
| RGO/PPy | 324 F g−1 | 1.5 A g−1 | [53] |
| GO/PPy | 332.6 F g−1 | 0.25 A g−1 | [54] |
| rGO/PPy | 389.3 F g−1 | 1.0 A g−1 | [55] |
| rGO/PPy | 5.5 F cm−3 | 1.6 mA cm−2 | [56] |
| rGO/PPy | 1685 mFcm−2 | 2 mA cm−2 | [57] |
| PTh/rGO | 318 F g−1 | 0.5 A g−1 | [58] |
| G- PEDOT | 374 Fg−1 | 0.01 Ag−1 | [59] |
| GO/PEDOT | 52.7 Fg−1 | 10 mVs−1 | [60] |
| GO/PEDOT | 64.8 mFcm−2 | 10 mVs−1 | [61] |
| GO-PT derivative | 296 Fg−1 | 0.3 Ag−1 | [62] |
| GR-P3MT | 332 Fg−1 | 0.5 Ag−1 | [63] |
| GR-P3MT | 240 F g−1 | 10mVs−1 | [64] |
| GNPs-P3MT | 215.5 F g−1 | 0.5 Ag−1 | [65] |
| RGO/COP | 417 F g−1 | 0.81 Ag−1 | present work |
| Sample | Rs | Rct | Zw | CPE | Cf | n |
|---|---|---|---|---|---|---|
| Ω | Ω | S*sˆ(1/2) | S*sˆa | F | ||
| PPY | 1.86 ± 0.03 | 2.05 ± 0.07 | 0.00022 | 0.00132 | 192 | 0.86 |
| PTH | 4.06 ± 0.57 | 9.9 ± 0.61 | 0.0003 | 0.083 | 97 | 0.68 |
| COP | 1.33 ± 0.03 | 0.597 ± 0.01 | 0.007 | 0.00013 | 173 | 0.40 |
| RGO | 3.56 ± 0.41 | 0.861 ± 0.02 | 0.0036 | 0.00053 | 139 | 0.67 |
| RGO/PTH | 3.54 ± 0.6 | 7.68 ± 0.63 | 0.00045 | 0.0015 | 152 | 0.58 |
| RGO/PPY | 4.02 ± 0.36 | 6.86 ± 0.51 | 0.00247 | 0.649 | 132.9 | 0.87 |
| RGO/COP | 2.46 ± 0.06 | 4.34 ± 0.52 | 0.0022 | 0.0009 | 460 | 0.79 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shah, A.u.H.A.; Ullah, S.; Bilal, S.; Rahman, G.; Seema, H. Reduced Graphene Oxide/Poly(Pyrrole-co-Thiophene) Hybrid Composite Materials: Synthesis, Characterization, and Supercapacitive Properties. Polymers 2020, 12, 1110. https://doi.org/10.3390/polym12051110
Shah AuHA, Ullah S, Bilal S, Rahman G, Seema H. Reduced Graphene Oxide/Poly(Pyrrole-co-Thiophene) Hybrid Composite Materials: Synthesis, Characterization, and Supercapacitive Properties. Polymers. 2020; 12(5):1110. https://doi.org/10.3390/polym12051110
Chicago/Turabian StyleShah, Anwar ul Haq Ali, Sami Ullah, Salma Bilal, Gul Rahman, and Humaira Seema. 2020. "Reduced Graphene Oxide/Poly(Pyrrole-co-Thiophene) Hybrid Composite Materials: Synthesis, Characterization, and Supercapacitive Properties" Polymers 12, no. 5: 1110. https://doi.org/10.3390/polym12051110
APA StyleShah, A. u. H. A., Ullah, S., Bilal, S., Rahman, G., & Seema, H. (2020). Reduced Graphene Oxide/Poly(Pyrrole-co-Thiophene) Hybrid Composite Materials: Synthesis, Characterization, and Supercapacitive Properties. Polymers, 12(5), 1110. https://doi.org/10.3390/polym12051110








