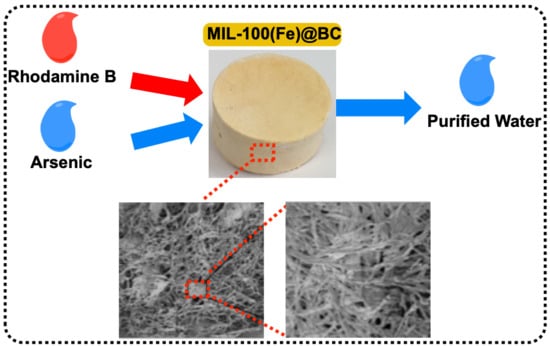
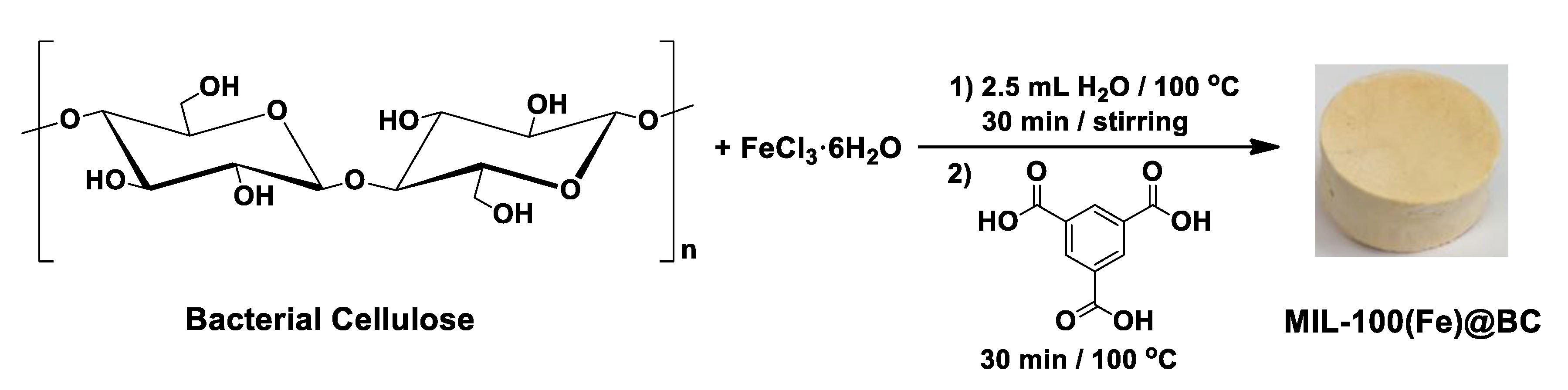
Green Synthesis of Metal-Organic Framework Bacterial Cellulose Nanocomposites for Separation Applications
Abstract
1. Introduction
2. Materials and Methods
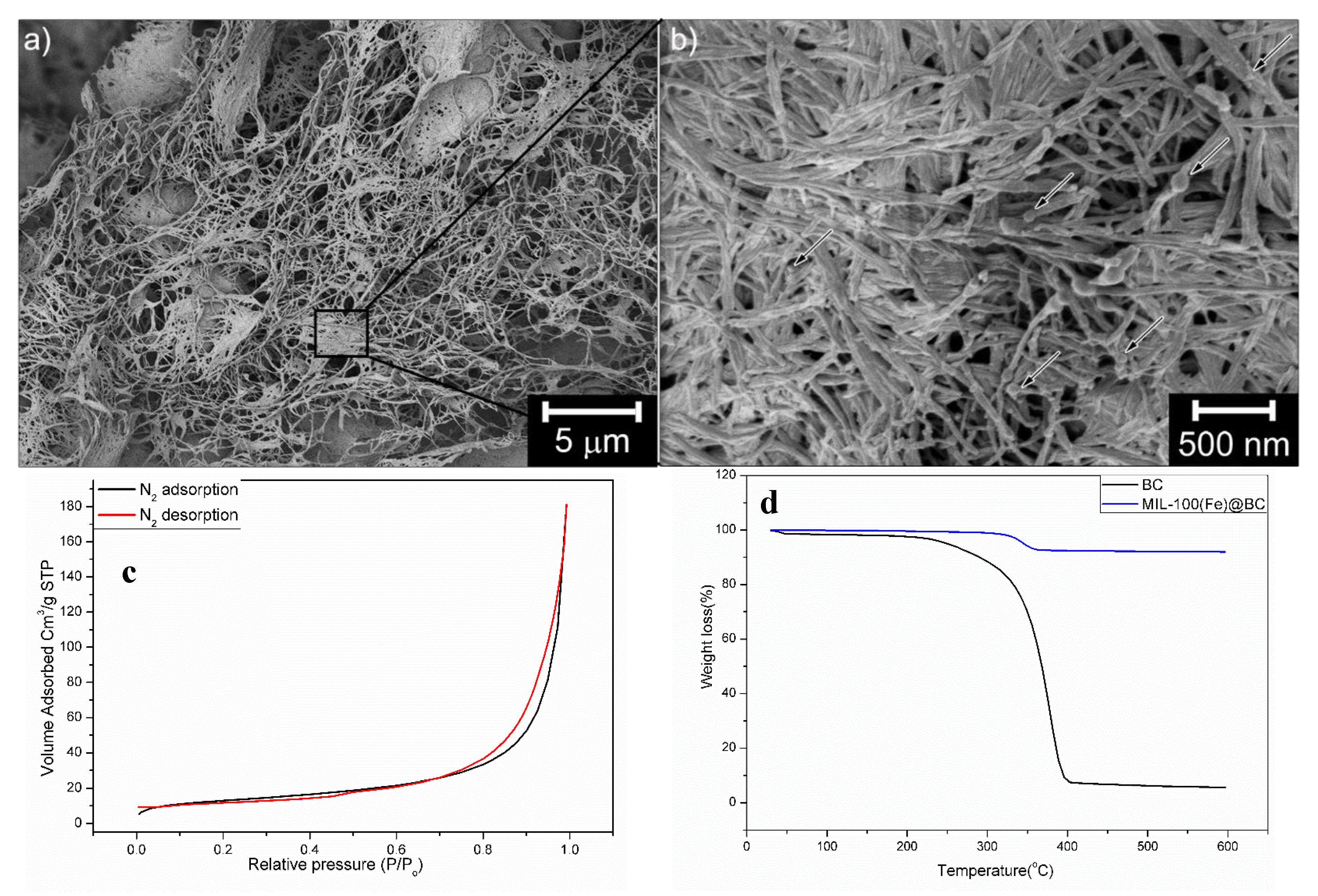
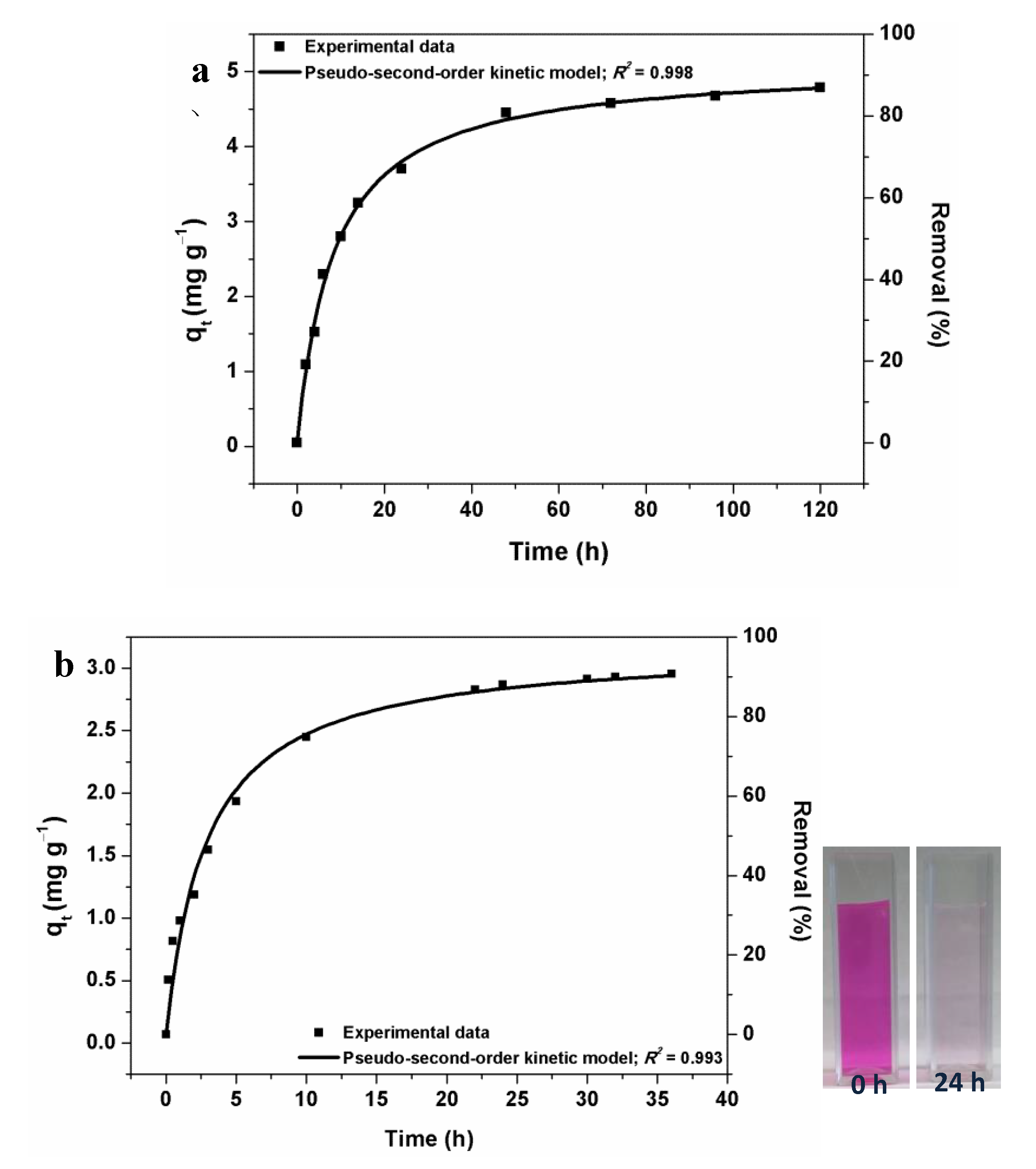
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Yin, F.; Deng, X.; Jin, Q.; Yuan, Y.; Zhao, C. The impacts of climate change and human activities on grassland productivity in Qinghai Province, China. Frontiers of Earth Science 2014, 8, 93–103. [Google Scholar] [CrossRef]
- Geisse, R.A.; Ngule, M.C.; Genna, T.D. Removal of lead ions from water using thiophene-functionalized metal–organic frameworks. Chem. Commun. 2020, 56, 237–240. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Tian, X.; Wang, Z.; Guan, Z.; Li, X.; Qiao, H.; Ke, H.; Luo, L.; Wei, Q. Multifunctional adsorbent based on metal-organic framework modified bacterial cellulose/chitosan composite aerogel for high efficient removal of heavy metal ion and organic pollutant. Chem. Eng. J. 2020, 383, 123127. [Google Scholar] [CrossRef]
- Huang, L.; He, M.; Chen, B.; Hu, B. Magnetic Zr-MOFs nanocomposites for rapid removal of heavy metal ions and dyes from water. Chemosphere 2018, 199, 435–444. [Google Scholar] [CrossRef]
- Chen, Q.; Luo, Z.; Hills, C.; Xue, G.; Tyrer, M. Precipitation of heavy metals from wastewater using simulated flue gas: Sequent additions of fly ash, lime and carbon dioxide. Water Res. 2009, 43, 2605–2614. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Feng, Y.; Hao, X.; Huang, W.; Feng, X. A novel potential-responsive ion exchange film system for heavy metal removal. J. Mater. Chem. A 2014, 2, 10263–10272. [Google Scholar] [CrossRef]
- Tofighy, A.M.; Mohammadi, T. Divalent heavy metal ions removal from contaminated water using positively charged membrane prepared from a new carbon nanomaterial and HPEI. Chem. Eng. J. 2020, 388, 124192. [Google Scholar] [CrossRef]
- Li, H.; Eddaoudi, M.; O’Keeffe, M.; Yaghi, O.M. Design and synthesis of an exceptionally stable and highly porous metal-organic framework. Nature 1999, 402, 276. [Google Scholar] [CrossRef]
- David, F.; Sonia, A.; Catherine, P. Metal-Organic frameworks: Opportunities for catalysis. Angew. Chem. Int. Ed. 2009, 48, 7502–7513. [Google Scholar]
- Lee, J.; Farha, O.K.; Roberts, J.; Scheidt, K.A.; Nguyen, S.T.; Hupp, J.T. Metal-Organic framework materials as catalysts. Chem. Soc. Rev. 2009, 38, 1450–1459. [Google Scholar] [CrossRef]
- Zhu, L.; Liu, X.-Q.; Jiang, H.-L.; Sun, L.-B. Metal-Organic frameworks for heterogeneous basic catalysis. Chem. Rev. 2017, 117, 8129–8176. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.-X.; Yang, Y.-W. Metal-Organic framework (MOF)-based drug/Cargo delivery and cancer therapy. Adv. Mater. 2017, 29, 1606134. [Google Scholar] [CrossRef] [PubMed]
- Alkordi, M.H.; Belmabkhout, Y.; Cairns, A.; Eddaoudi, M. Metal–organic frameworks for H2 and CH4 storage: Insights on the pore geometry–sorption energetics relationship. IUCrJ 2017, 4, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Zeng, Z.; Vulpe, D.; Casco, M.E.; Divitini, G.; Midgley, P.A.; Silvestre-Albero, J.; Tan, J.-C.; Moghadam, P.Z.; Fairen-Jimenez, D. A sol-gel monolithic metal-organic framework with enhanced methane uptake. Nat. Mater. 2017, 17, 174. [Google Scholar] [CrossRef]
- Zheng, H.; Zhang, Y.; Liu, L.; Wan, W.; Guo, P.; Nyström, A.M.; Zou, X. One-pot synthesis of metal–organic frameworks with encapsulated target molecules and their applications for controlled drug delivery. J. Am. Chem. Soc. 2016, 138, 962–968. [Google Scholar] [CrossRef]
- Chen, B.; Yang, Z.; Zhu, Y.; Xia, Y. Zeolitic imidazolate framework materials: Recent progress in synthesis and applications. J. Mater. Chem. A 2014, 2, 16811–16831. [Google Scholar] [CrossRef]
- Li, J.-R.; Sculley, J.; Zhou, H.-C. Metal-organic frameworks for separations. Chem. Rev. 2012, 112, 869–932. [Google Scholar] [CrossRef]
- Qiu, S.; Xue, M.; Zhu, G. Metal–organic framework membranes: From synthesis to separation application. Chem. Soc. Rev. 2014, 43, 6116–6140. [Google Scholar] [CrossRef]
- Abdel-Magied, A.F.; Abdelhamid, H.N.; Ashour, R.M.; Zou, X.; Forsberg, K. Hierarchical porous zeolitic imidazolate frameworks nanoparticles for efficient adsorption of rare-earth elements. Microporous Mesoporous Mater. 2019, 278, 175–184. [Google Scholar] [CrossRef]
- Almeida Paz, F.A.; Klinowski, J.; Vilela SM, F.; Tomé JP, C.; Cavaleiro JA, S.; Rocha, J. Ligand design for functional metal–organic frameworks. Chem. Soc. Rev. 2012, 41, 1088–1110. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, X.; Zhang, S.; Li, S.; Cao, S.; Pei, X.; Zhou, J.; Feng, X.; Wang, B. Shaping of metal–organic frameworks: From fluid to shaped bodies and robust foams. J. Am. Chem. Soc. 2016, 138, 10810–10813. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhang, J.; Zheng, L.; Zhang, J.; Sang, X.; Kang, X.; Zhang, B.; Luo, T.; Tan, X.; Han, B. Metal-organic framework for emulsifying carbon dioxide and water. Angew. Chem. Int. Ed. 2016, 55, 11372–11376. [Google Scholar] [CrossRef] [PubMed]
- Bétard, A.; Fischer, R.A. Metal—organic framework thin films: From fundamentals to applications. Chem. Rev. 2012, 112, 1055–1083. [Google Scholar] [CrossRef] [PubMed]
- Denny, M.S.; Cohen, S.M. In situ modification of metal—organic frameworks in mixed-matrix membranes. Angew. Chem. Int. Ed. 2015, 54, 9029–9032. [Google Scholar] [CrossRef]
- Moon, R.J.; Martini, A.; Nairn, J.; Simonsen, J.; Youngblood, J. Cellulose nanomaterials review: Structure, properties and nanocomposites. Chem. Soc. Rev. 2011, 40, 3941–3994. [Google Scholar] [CrossRef]
- Olsson, R.T.; Azizi Samir MA, S.; Salazar-Alvarez, G.; Belova, L.; Ström, V.; Berglund, L.A.; Ikkala, O.; Nogués, J.; Gedde, U.W. Making flexible magnetic aerogels and stiff magnetic nanopaper using cellulose nanofibrils as templates. Nat. Nanotech. 2010, 5, 584. [Google Scholar] [CrossRef]
- Lee, K.Y. Nanocellulose and Sustainability: Production, Properties, Applications, and Case Studies; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Matsumoto, M.; Kitaoka, T. Ultraselective gas separation by nanoporous metal-organic frameworks embedded in gas-barrier nanocellulose films. Adv. Mater. 2016, 28, 1765–1769. [Google Scholar] [CrossRef]
- Zhu, H.; Yang, X.; Cranston, E.D.; Zhu, S. Flexible and porous nanocellulose aerogels with high loadings of metal–organic-framework particles for separations applications. Adv. Mater. 2016, 28, 7652–7657. [Google Scholar] [CrossRef]
- Au-Duong, A.-N.; Lee, C.-K. Flexible metal–organic framework-bacterial cellulose nanocomposite for iodine capture. Cryst. Growth Des. 2018, 18, 356–363. [Google Scholar] [CrossRef]
- Férey, G.; Serre, C.; Mellot-Draznieks, C.; Millange, F.; Surblé, S.; Dutour, J.; Margiolaki, I. A hybrid solid with giant pores prepared by a combination of targeted chemistry, simulation, and powder diffraction. Angew. Chem. Int. Ed. 2004, 43, 6296–6301. [Google Scholar] [CrossRef]
- Roman, M.; Winter, W.T. Effect of sulfate groups from sulfuric acid hydrolysis on the thermal degradation behavior of bacterial cellulose. Biomacromolecules 2004, 5, 1671–1677. [Google Scholar] [CrossRef] [PubMed]
- Cravillon, J.; Nayuk, R.; Springer, S.; Feldhoff, A.; Huber, K.; Wiebcke, M. Controlling Zeolitic imidazolate framework nano- and microcrystal formation: Insight into crystal growth by time-resolved in situ static light scattering. Chem. Mater. 2011, 23, 2130–2141. [Google Scholar] [CrossRef]
- Pettinari, C.; Marchetti, F.; Mosca, N.; Tosi, G.; Drozdov, A. Applications of metal-organic frameworks. Polym. Int. 2017, 66, 731–744. [Google Scholar] [CrossRef]
- Georgiou, Y.; Perman, J.A.; Bourlinos, A.B.; Deligiannakis, Y. Highly efficient arsenite [As(III)] adsorption by an [MIL-100(Fe)] metal–organic framework: Structural and mechanistic insights. J. Phys. Chem. C 2018, 122, 4859–4869. [Google Scholar] [CrossRef]
- Mohammadi, M.; Hassani, A.J.; Mohamed, A.R.; Najafpour, G.D. Removal of Rhodamine B from aqueous solution using Palm shell-based activated carbon: Adsorption and kinetic studies. J. Chem. Eng. Data 2010, 55, 5777–5785. [Google Scholar] [CrossRef]
- Plazinski, W.; Dziuba, J.; Rudzinski, W. Modeling of sorption kinetics: The pseudo-second order equation and the sorbate intraparticle diffusivity. Adsorption 2013, 19, 1055–1064. [Google Scholar] [CrossRef]
- Haochi, L.; Xiaohui, R.; Ligang, C.J. Synthesis and characterization of magnetic metal–organic framework for the adsorptive removal of Rhodamine B from aqueous solution. Ind. Eng. Chem. 2016, 34, 278–285. [Google Scholar]
- Tonoy, C.; Lei, Z.; Junqing, Z.; Srijan, A. Removal of Arsenic(III) from aqueous solution using metal organic framework-graphene oxide nanocomposite. Nanomaterials 2018, 8, 1062. [Google Scholar]
- Samira, S.; Ghasem, A.; Faramarz, M.; Abdolreza, K.; Kazem, G.; Ehsan, N. Application of surfactant-modified montmorillonitefor As (III) removal from aqueous solutions: Kinetics and isotherm study. Desalin. Water Treat. 2018, 115, 236–248. [Google Scholar]
- Agnes, P.; Eliazer, B.N.; Augustine, E.O. Enhanced Arsenic (III) adsorption from aqueous solution by magnetic pine cone biomass. Mater. Chem. Phys. 2019, 222, 20–30. [Google Scholar]
- Jian, M.; Liu, B.; Zhang, G.; Liu, R.; Zhang, X. Adsorptive removal of arsenic from aqueous solution by zeolitic imidazolate framework-8 (ZIF-8) nanoparticles. Colloids Surf. Physicochem. Eng. Asp. 2015, 465, 67–76. [Google Scholar] [CrossRef]
- Huang, J.-H.; Huang, K.-L.; Liu, S.-Q.; Wang, A.-T.; Yan, C. Adsorption of Rhodamine B and methyl orange on a hypercrosslinked polymeric adsorbent in aqueous solution. Colloid Surf. A Physicochem. Eng. Asp. 2008, 330, 55. [Google Scholar] [CrossRef]
- Khan, T.A.; Sharma, S.; Ali, I. Adsorption of Rhodamine B dye from aqueous solution onto acid activated mango (Magnifera indica) leaf powder: Equilibrium, kinetic and thermodynamic studies. Toxicol. Environ. Health Sci. 2011, 3, 286–297. [Google Scholar]
- Zhang, J.; Li, F.; Sun, Q. Rapid and selective adsorption of cationic dyes by a unique metal-organic framework with decorated pore surface. Appl. Surf. Sci. 2018, 440, 1219–1226. [Google Scholar] [CrossRef]
| Adsorbents | qe (mg g−1) | Ref. |
|---|---|---|
| As(III) Removal | ||
| MIL-53(Al)-graphene oxide | 65.0 | [39] |
| Surfactant-modified montmorillonite | 1.48 | [40] |
| Magnetic pinecone biomass | 18.02 | [41] |
| Zn-MOF | 49.50 | [42] |
| MIL-100(Fe) | 120 | [35] |
| MIL-100(Fe)@BC | 4.81 | This work |
| Rhodamine B Removal | ||
| Hypercross-linked polymeric adsorbent | 2.1 | [43] |
| Mango leaf power | 3.31 | [44] |
| Zn-MOF | 3.750 | [45] |
| Fe3O4/MIL-100(Fe) | 28.36 | [38] |
| MIL-100(Fe)@BC | 2.77 | This work |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ashour, R.M.; Abdel-Magied, A.F.; Wu, Q.; Olsson, R.T.; Forsberg, K. Green Synthesis of Metal-Organic Framework Bacterial Cellulose Nanocomposites for Separation Applications. Polymers 2020, 12, 1104. https://doi.org/10.3390/polym12051104
Ashour RM, Abdel-Magied AF, Wu Q, Olsson RT, Forsberg K. Green Synthesis of Metal-Organic Framework Bacterial Cellulose Nanocomposites for Separation Applications. Polymers. 2020; 12(5):1104. https://doi.org/10.3390/polym12051104
Chicago/Turabian StyleAshour, Radwa M., Ahmed F. Abdel-Magied, Qiong Wu, Richard T. Olsson, and Kerstin Forsberg. 2020. "Green Synthesis of Metal-Organic Framework Bacterial Cellulose Nanocomposites for Separation Applications" Polymers 12, no. 5: 1104. https://doi.org/10.3390/polym12051104
APA StyleAshour, R. M., Abdel-Magied, A. F., Wu, Q., Olsson, R. T., & Forsberg, K. (2020). Green Synthesis of Metal-Organic Framework Bacterial Cellulose Nanocomposites for Separation Applications. Polymers, 12(5), 1104. https://doi.org/10.3390/polym12051104