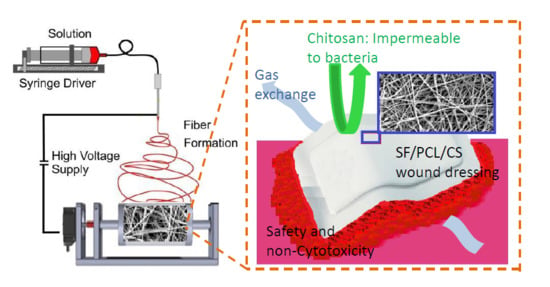
Characterization, Biocompatibility, and Optimization of Electrospun SF/PCL/CS Composite Nanofibers
Abstract
1. Introduction
2. Experimental Method
2.1. Preparation of Regenerated Silk Fibroin (SF)
2.2. Preparation of the Electrospinning Solutions and Electrospinning
2.3. Taguchi DOE Parameter Setting
2.4. Characterization of Nanofiber Scaffolds
3. Results and Discussion
3.1. Optimum Combination of Factors for the Application of Dressings
3.1.1. Taguchi Method to Optimize Dressings
3.1.2. Porosity and Water Uptake Abilities
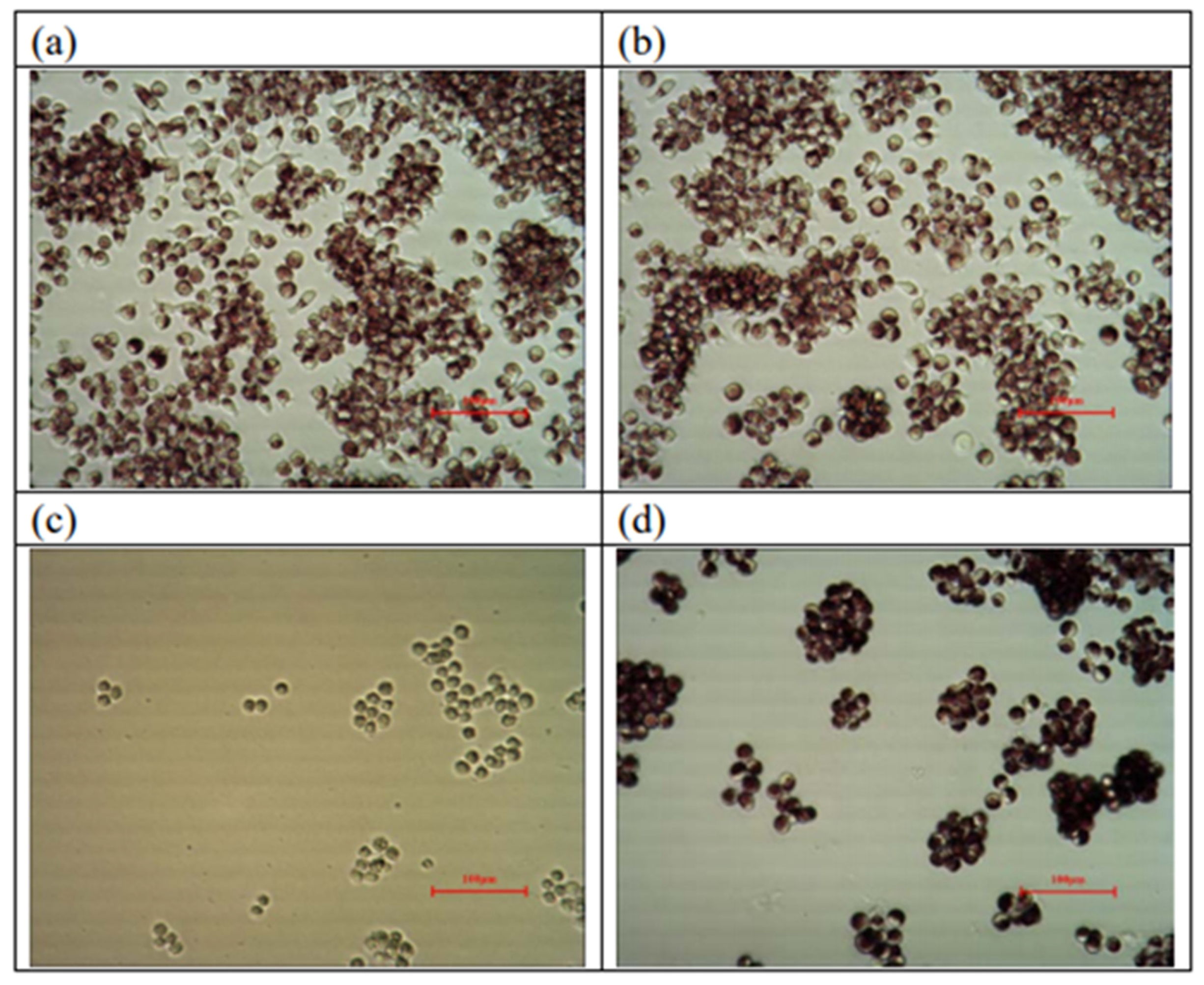
3.1.3. Cytotoxicity Tests
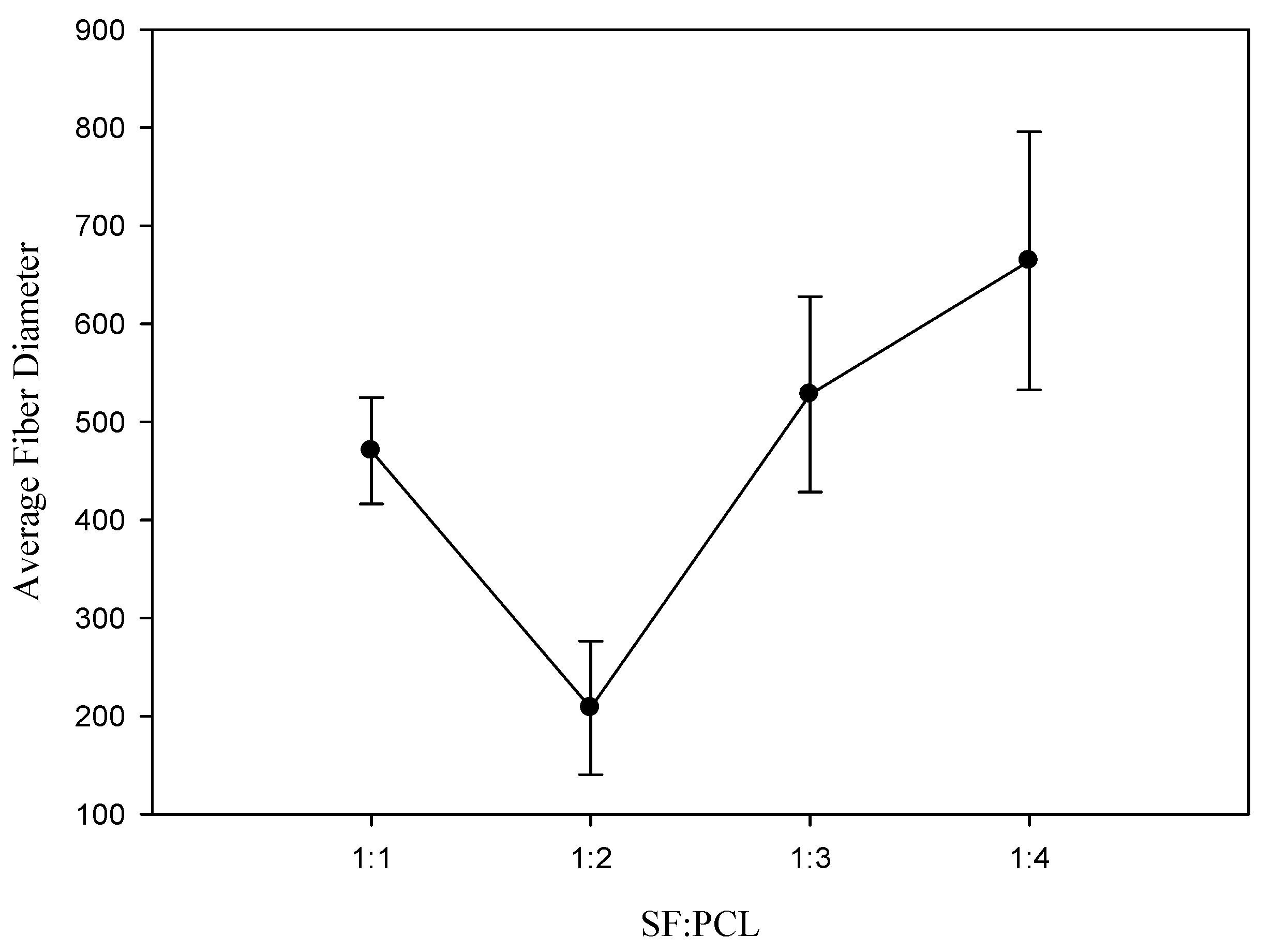
3.2. Effect of the Ratio of Silk Fibroin to Polycaprolactone on Fiber Diameter
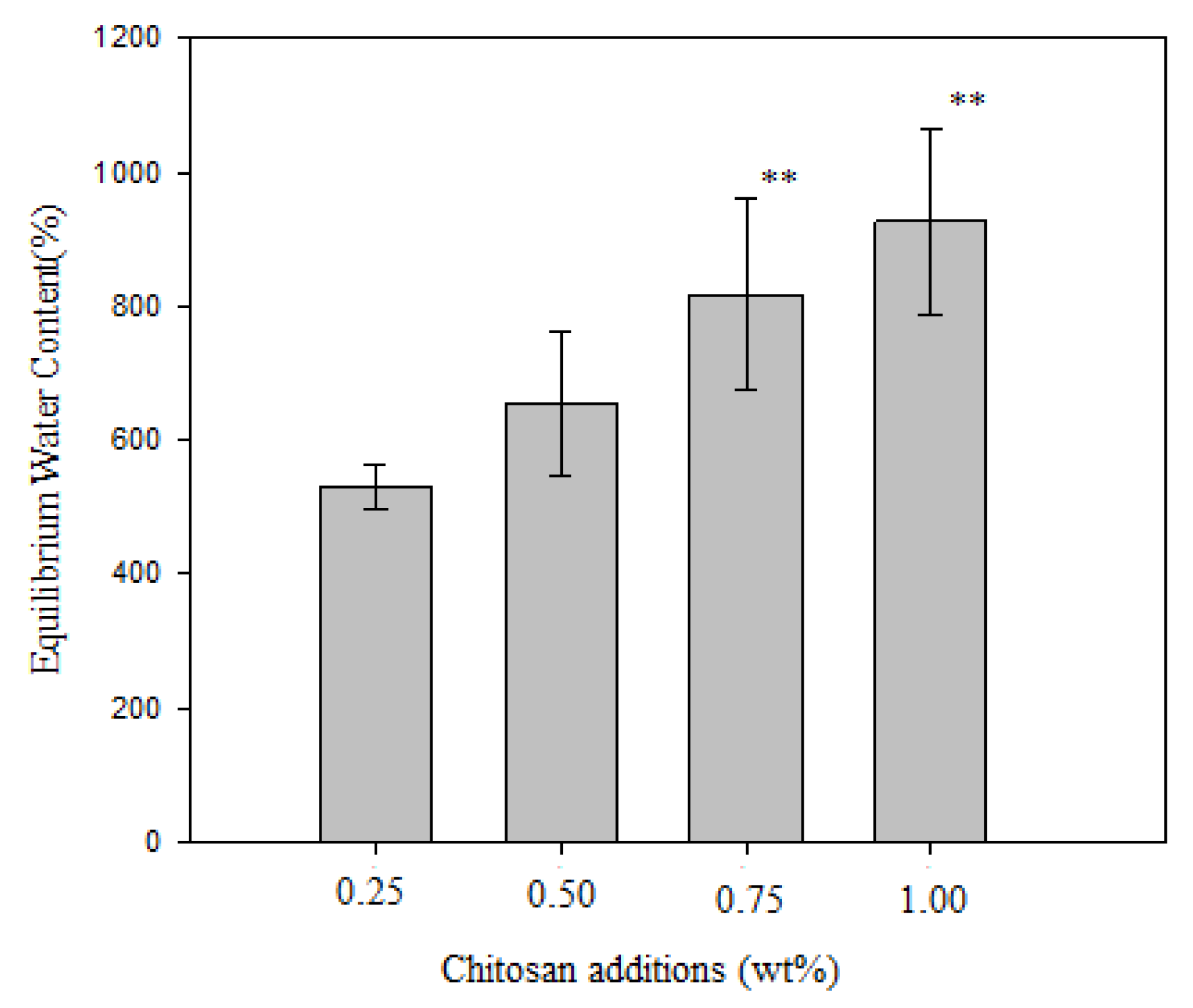
3.3. Effect of Chitosan Addition on Fiber Diameter, Antimicrobial Activity, and Equilibrium Water Content
3.4. Effect of Flow Rate on Fiber Diameter
3.5. Effect of Applied Voltage on Fiber Diameter
4. Conclusion
Author Contributions
Funding
Conflicts of Interest
References
- Ayutsede, J.; Gandhi, M.; Sukigara, S.; Micklus, M.; Chen, H.-E.; Ko, F. Regeneration of Bombyx mori silk by electrospinning. Part 3: Characterization of electrospun nonwoven mat. Polymer 2005, 46, 1625–1634. [Google Scholar] [CrossRef]
- Yao, Y.; Wang, J.; Cui, Y.; Xu, R.; Wang, Z.; Zhang, J.; Wang, K.; Li, Y.; Zhao, Q.; Kong, D. Effect of sustained heparin release from PCL/chitosan hybrid small-diameter vascular grafts on anti-thrombogenic property and endothelialization. Acta Biomater. 2014, 10, 2739–2749. [Google Scholar] [CrossRef]
- Dias, J.R.; Baptista-Silva, S.; Sousa, A.; Oliveira, A.L.; Bártolo, P.J.; Granja, P.L. Biomechanical performance of hybrid electrospun structures for skin regeneration. Mater. Sci. Eng. C 2018, 93, 816–827. [Google Scholar] [CrossRef]
- Avsar, G.; Agirbasli, D.; Agirbasli, M.A.; Gunduz, O.; Oner, E.T. Levan based fibrous scaffolds electrospun via co-axial and single-needle techniques for tissue engineering applications. Carbohydr. Polym. 2018, 193, 316–325. [Google Scholar] [CrossRef] [PubMed]
- Surucu, S.; Sasmazel, H.T. Development of core-shell coaxially electrospun composite PCL/chitosan scaffolds. Int. J. Biol. Macromol. 2016, 92, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Pakravan, M.; Heuzey, M.-C.; Ajji, A. A fundamental study of chitosan/PEO electrospinning. Polymer 2011, 52, 4813–4824. [Google Scholar] [CrossRef]
- Narayanan, G.; Gupta, B.S.; Tonelli, A.E. Enhanced mechanical properties of poly(ε-caprolactone) nanofibers produced by the addition of non-stoichiometric inclusion complexes of poly(ε-caprolactone) and α-cyclodextrin. Polymer 2015, 73, 321–330. [Google Scholar] [CrossRef]
- Yang, X.; Fan, L.; Ma, L.; Wang, Y.; Lin, S.; Yu, F.; Pan, X.; Luo, G.; Zhang, D.; Wang, H. Green electrospun Manuka honey/silk fibroin fibrous matrices as potential wound dressing. Mater. Des. 2017, 119, 76–84. [Google Scholar] [CrossRef]
- Lin, L.; Zhu, Y.; Li, C.; Liu, L.; Surendhiran, D.; Cui, H. Antibacterial activity of PEO nanofibers incorporating polysaccharide from dandelion and its derivative. Carbohydr. Polym. 2018, 198, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, R.; Tariq, M.; Ali, I.; Asghar, R.; Khanam, P.N.; Augustine, R.; Hasan, A. Novel electrospun chitosan/polyvinyl alcohol/zinc oxide nanofibrous mats with antibacterial and antioxidant properties for diabetic wound healing. Int. J. Biol. Macromol. 2018, 120, 385–393. [Google Scholar] [CrossRef]
- Zhang, D.; Karki, A.B.; Rutman, D.; Young, D.P.; Wang, A.; Cocke, D.; Ho, T.H.; Guo, Z. Electrospun polyacrylonitrile nanocomposite fibers reinforced with Fe3O4 nanoparticles: Fabrication and property analysis. Polymer 2009, 50, 4189–4198. [Google Scholar] [CrossRef]
- Zhu, J.; Wei, S.; Patil, R.; Rutman, D.; Kucknoor, A.S.; Wang, A.; Guo, Z. Ionic liquid assisted electrospinning of quantum dots/elastomer composite nanofibers. Polymer 2011, 52, 1954–1962. [Google Scholar] [CrossRef]
- Aslan, T.; Arslan, S.; Eyvaz, M.; Güçlü, S.; Yüksel, E.; Koyuncu, İ. A novel nanofiber microfiltration membrane: Fabrication and characterization of tubular electrospun nanofiber (TuEN) membrane. J. Membr. Sci. 2016, 520, 616–629. [Google Scholar] [CrossRef]
- Jiang, L.; Tu, H.; Lu, Y.; Wu, Y.; Tian, J.; Shi, X.; Wang, Q.; Zhan, Y.; Huang, Z.; Deng, H. Spherical and rodlike inorganic nanoparticle regulated the orientation of carbon nanotubes in polymer nanofibers. Chem. Phys. Lett. 2016, 650, 82–87. [Google Scholar] [CrossRef]
- Yao, Y.; Wei, H.; Wang, J.; Lu, H.; Leng, J.; Hui, D. Fabrication of hybrid membrane of electrospun polycaprolactone and polyethylene oxide with shape memory property. Compos. B. Eng. 2015, 83, 264–269. [Google Scholar] [CrossRef]
- Rao, M.; Geng, X.; Liao, Y.; Hu, S.; Li, W. Preparation and performance of gel polymer electrolyte based on electrospun polymer membrane and ionic liquid for lithium ion battery. J. Membr. Sci. 2012, 399–400, 37–42. [Google Scholar] [CrossRef]
- Narayanan, G.; Tekbudak, M.Y.; Caydamli, Y.; Dong, J.; Krause, W.E. Accuracy of electrospun fiber diameters: The importance of sampling and person-to-person variation. Polym Test. 2017, 61, 240–248. [Google Scholar] [CrossRef]
- Yu, W.-J.; Xu, S.-M.; Zhang, L.; Fu, Q. Morphology and mechanical properties of immiscible polyethylene/polyamide12 blends prepared by high shear processing. Chinese J. Polym. Sci. 2017, 35, 1132–1142. [Google Scholar] [CrossRef]
- Park, Y.R.; Ju, H.W.; Lee, J.M.; Kim, D.-K.; Lee, O.J.; Moon, B.M.; Park, H.J.; Jeong, J.Y.; Yeon, Y.K.; Park, C.H. Three-dimensional electrospun silk-fibroin nanofiber for skin tissue engineering. Int. J. Biol. Macromol. 2016, 93, 1567–1574. [Google Scholar] [CrossRef]
- Aznar-Cervantes, S.D.; Daniel, V.C.; Luis, M.O.; Cenis, J.L.; Abel, L.P.A. Influence of the protocol used for fibroin extraction on the mechanical properties and fiber sizes of electrospun silk mats. Mater. Sci. Eng. C 2013, 33, 1945–1950. [Google Scholar] [CrossRef]
- Calamak, S.; Aksoy, E.A.; Ertas, N.; Erdogdu, C.; Sagıroglu, M.; Ulubayram, K. Ag/silk fibroin nanofibers: Effect of fibroin morphology on Ag+ release and antibacterial activity. Eur. Polym. J. 2015, 67, 99–112. [Google Scholar] [CrossRef]
- Vaz, J.M.; Taketa, T.B.; Hernandez-Montelongo, J.; Chevallier, P.; Cotta, M.A.; Mantovani, D.; Beppu, M.M. Antibacterial properties of chitosan-based coatings are affected by spacer-length and molecular weight. Appl. Surf. 2018, 445, 478–487. [Google Scholar] [CrossRef]
- Albetran, H.; Dong, Y.; Low, I.M. Characterization and optimization of electrospun TiO2/PVP nanofibers using Taguchi design of experiment method. J. Asian Ceram. Soc. 2015, 3, 292–300. [Google Scholar] [CrossRef]
- Senthil, T.; Anandhan, S. Electrospinning of non-woven poly(styrene-co-acrylonitrile) nanofibrous webs for corrosive chemical filtration: Process evaluation and optimization by Taguchi and multiple regression analyses. J. Electrostat. 2015, 73, 43–55. [Google Scholar] [CrossRef]
- Ryan, M.P.; Rea, M.C.; Hill, C.; Ross, R.P. An application in cheddar cheese manufacture for a strain of Lactococcus lactis producing a novel broad-spectrum bacteriocin, lacticin 3147. Appl. Environ. Microbiol. 1996, 62, 612–619. [Google Scholar] [CrossRef]
- Duffy, G.; Whiting, R.C.; Sheridan, J.J. The effect of a competitive microflora, pH and temperature on the growth kinetics of Escherichia coli O157:H7. Food Microbiol. 1999, 16, 299–307. [Google Scholar] [CrossRef]
- Rosengren, A.; Faxius, L.; Tanaka, N.; Watanabe, M.; Bjursten, L.M. Comparison of implantation and cytotoxicity testing for initially toxic biomaterials. J. Biomed. Mater. Res. A 2005, 75, 115–122. [Google Scholar] [CrossRef]
- Izquierdo, R.; Garcia-Giralt, N.; Rodriguez, M.T.; Cáceres, E.; García, S.J.; Ribelles, J.L.G.; Joan, M.M.; Monllau, C.; Suay, J. Biodegradable PCL scaffolds with an interconnected spherical network for tissue engineering. J. Biomed. Mater. Res. A 2008, 85, 25–35. [Google Scholar] [CrossRef]
- Mehrabani, M.G.; Karimian, R.; Rakhshaei, R.; Pakdel, F.; Eslami, H.; Fakhrzadeh, V.; Rahimi, M.; Salehi, R.; Kafil, H.S. Chitin/silk fibroin/TiO2 bio-nanocomposite as a biocompatible wound dressing bandage with strong antimicrobial activity. Int. J. Biol. Macromol. 2018, 116, 966–976. [Google Scholar] [CrossRef]
- Zhou, J.; Cao, C.; Ma, X. A novel three-dimensional tubular scaffold prepared from silk fibroin by electrospinning. Int. J. Biol. Macromol. 2009, 45, 504–510. [Google Scholar] [CrossRef]
- Qi, R.; Shen, M.; Cao, X.; Guo, R.; Tian, X.; Yu, J.; Shi, X. Exploring the dark side of MTT viability assay of cells cultured onto electrospun PLGA-based composite nanofibrous scaffolding materials. Analyst 2011, 14, e1800403. [Google Scholar] [CrossRef] [PubMed]
- Sutasinpromprae, J.; Jitjaicham, S.; Nithitanakul, M.; Meechaisue, C.; Supaphol, P. Preparation and characterization of ultrafine electrospun polyacrylonitrile fibers and their subsequent pyrolysis to carbon fibers. Polym. Int. 2006, 55, 825–833. [Google Scholar] [CrossRef]
- Jacobs, V.; Anandjiwala, R.D.; Maaza, M. The influence of electrospinning parameters on the structural morphology and diameter of electrospun nanofibers. J. Appl. Polym. Sci. 2010, 115, 3130–3136. [Google Scholar] [CrossRef]
- Jia, Y.-T.; Gong, J.; Gu, X.-H.; Kim, H.-Y.; Dong, J.; Shen, X.-Y. Fabrication and characterization of poly (vinyl alcohol)/chitosan blend nanofibers produced by electrospinning method. Carbohydr. Polym. 2007, 67, 403–409. [Google Scholar] [CrossRef]
- Vrieze, S.D.; Westbroek, P.; Camp, T.V.; Clerck, K.D. Solvent system for steady state electrospinning of polyamide 6.6. J. Appl. Polym. Sci. 2010, 115, 837–842. [Google Scholar] [CrossRef]
- Da Silva, L.P.; De Britto, D.; Seleghim, M.H.R.; Assis, O.B.G. In vitro activity of water-soluble quaternary chitosan chloride salt against E coli. World J. Microbiol. Biotechnol. 2010, 26, 2089–2092. [Google Scholar] [CrossRef]
- No, H.K.; Park, N.Y.; Lee, S.H.; Meyers, S.P. Antibacterial activity of chitosans and chitosan oligomers with different molecular weights. Int. J. Food Microbiol. 2002, 75, 65–72. [Google Scholar] [CrossRef]
- Li, S.; Li, L.; Guo, C.; Qin, H.; Yu, X. A promising wound dressing material with excellent cytocompatibility and proangiogenesis action for wound healing: Strontium loaded Silk fibroin/Sodium alginate (SF/SA) blend films. Int. J. Biol. Macromol. 2017, 104, 969–978. [Google Scholar] [CrossRef]
- Someswararao, M.V.; Dubey, R.S.; Subbarao, P.S.V.; Singh, S. Electrospinning process parameters dependent investigation of TiO2 nanofibers. Results Phys. 2018, 11, 223–231. [Google Scholar] [CrossRef]
- Kostina, N.Y.; Blanquer, S.; Pop-Georgievski, O.; Rahimi, K.; Dittrich, B.; Höcherl, A.; Michálek, J.; Grijpma, D.W.; Rodriguez-Emmenegger, C. Zwitterionic functionalizable scaffolds with gyroid pore Architecture for tissue engineering. Macromol. Biosci. 2019, 19, e1800403. [Google Scholar] [CrossRef]
- Jin, H.-J.; Fridrikh, S.V.; Rutledge, G.C.; Kaplan, D.L. Electrospinning Bombyx mori silk with poly(ethylene oxide). Biomacromolecules 2002, 3, 1233–1239. [Google Scholar] [CrossRef] [PubMed]

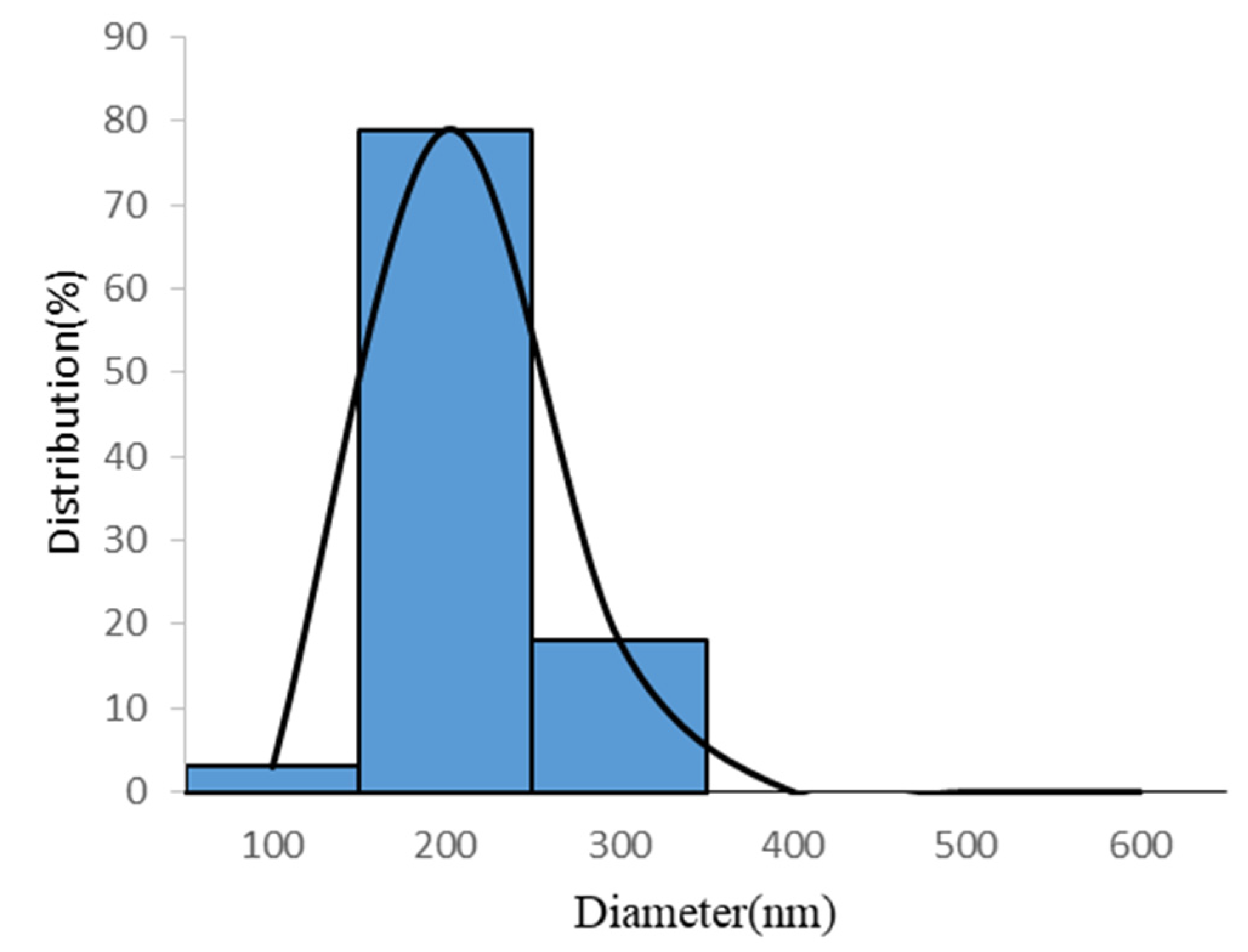





| Ratio of Silk Fibroin and Polycaprolactone | Chitosan Content (wt.%) | Flow Rate (mL/h) | Voltage (kV) | |
|---|---|---|---|---|
| Level 1 | 1:1 (5.00%:5.00%) | 0.50 | 0.2 | 15 |
| Level 2 | 1:2 (3.33%:6.67%) | 0.75 | 0.3 | 20 |
| Level 3 | 1:3 (2.50%:7.50%) | 1.00 | 0.4 | 25 |
| L9(34) | Ratio (SF: PCL) | Chitosan Addition (%) | Flow Rate (mL/h) | Voltage (kV) | S/N | Means of Diameter (nm) | Porosity (%) | WVTR (g m−2·24 h) |
|---|---|---|---|---|---|---|---|---|
| 1 | 1:1 | 0.50 | 0.2 | 15 | 12.65 | 232.55±60.28 | 88.01±4.32 | 4417.29±87.27 |
| 2 | 1:1 | 0.75 | 0.3 | 20 | 12.71 | 229.09±60.66 | 83.10±4.21 | 4362.97±91.67 |
| 3 | 1:1 | 1.00 | 0.4 | 25 | 13.61 | 208.39±55.81 | 91.96±5.16 | 4641.38±19.21 |
| 4 | 1:2 | 0.50 | 0.3 | 25 | 15.37 | 170.00±55.76 | 92.05±3.70 | 4768.71±85.04 |
| 5 | 1:2 | 0.75 | 0.4 | 15 | 11.92 | 253.42±65.37 | 91.43±5.50 | 4636.29±25.46 |
| 6 | 1:2 | 1.00 | 0.2 | 20 | 13.11 | 220.67±62.13 | 85.37±4.04 | 4432.89±45.84 |
| 7 | 1:3 | 0.50 | 0.4 | 20 | 10.86 | 285.71±78.08 | 92.60±4.10 | 4581.97±92.58 |
| 8 | 1:3 | 0.75 | 0.2 | 25 | 11.47 | 266.75±76.71 | 84.74±5.70 | 4330.71±5.09 |
| 9 | 1:3 | 1.00 | 0.3 | 15 | 12.68 | 231.94±63.64 | 82.03±1.45 | 4405.41±74.86 |
| Ratio of Silk Fibroin and Polycaprolactone | Chitosan Content | Flow Rate | Voltage | |
|---|---|---|---|---|
| 1 | 12.99 | 12.96 | 12.41 | 12.42 |
| 2 | 13.47 | 12.03 | 13.59 | 12.23 |
| 3 | 11.67 | 13.13 | 12.13 | 13.48 |
| ∆ | 1.80 | 1.10 | 1.46 | 1.26 |
| Factor influence order | 1 | 4 | 2 | 3 |
| Contribution (%) | 32.0 | 19.1 | 26.2 | 22.7 |
| Test Item | Absorbance (%) | Viability (%) |
|---|---|---|
| Blank | 0.504 ± 0.011 | 100 |
| Negative control | 0.502 ± 0.005 | 100 |
| Positive control | 0.064 ± 0.002 | 13 |
| Sample (SF/PCL/CS) | 0.288 ± 0.020 | 57 |
| Name of the Sample | Conc. (%) | Mean Diameter of Inhibition Zone (mm) | |
|---|---|---|---|
| Test Organisms | |||
| Staphylococcus Aureus | Escherichia Coli | ||
| Chitosan | 0.25 | 58 ± 4 | 65 ± 0 |
| 0.50 | 55 ± 1 | 63 ± 4 | |
| 0.75 | 51 ± 1 | 64 ± 0 | |
| 1.00 | 55 ± 1 | 63 ± 2 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.-W.; Lin, M.-F. Characterization, Biocompatibility, and Optimization of Electrospun SF/PCL/CS Composite Nanofibers. Polymers 2020, 12, 1439. https://doi.org/10.3390/polym12071439
Chen H-W, Lin M-F. Characterization, Biocompatibility, and Optimization of Electrospun SF/PCL/CS Composite Nanofibers. Polymers. 2020; 12(7):1439. https://doi.org/10.3390/polym12071439
Chicago/Turabian StyleChen, Hua-Wei, and Min-Feng Lin. 2020. "Characterization, Biocompatibility, and Optimization of Electrospun SF/PCL/CS Composite Nanofibers" Polymers 12, no. 7: 1439. https://doi.org/10.3390/polym12071439
APA StyleChen, H.-W., & Lin, M.-F. (2020). Characterization, Biocompatibility, and Optimization of Electrospun SF/PCL/CS Composite Nanofibers. Polymers, 12(7), 1439. https://doi.org/10.3390/polym12071439