Hydrothermal Effect on Mechanical Properties of Nephila pilipes Spidroin
Abstract
1. Introduction
2. Materials and Methods
2.1. Silk Collection and Preparation of Silk Films
2.2. Characterization of Mechanical Properties of Spider Silk Fibers
2.3. Circular Dichroism
2.4. FT-IR Analysis
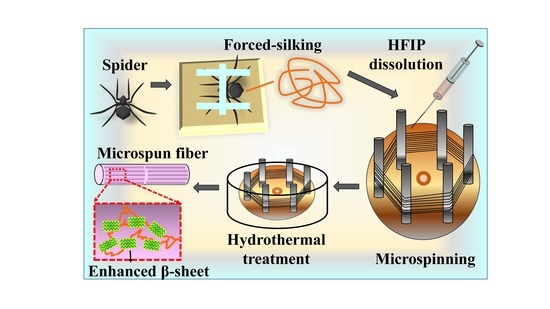
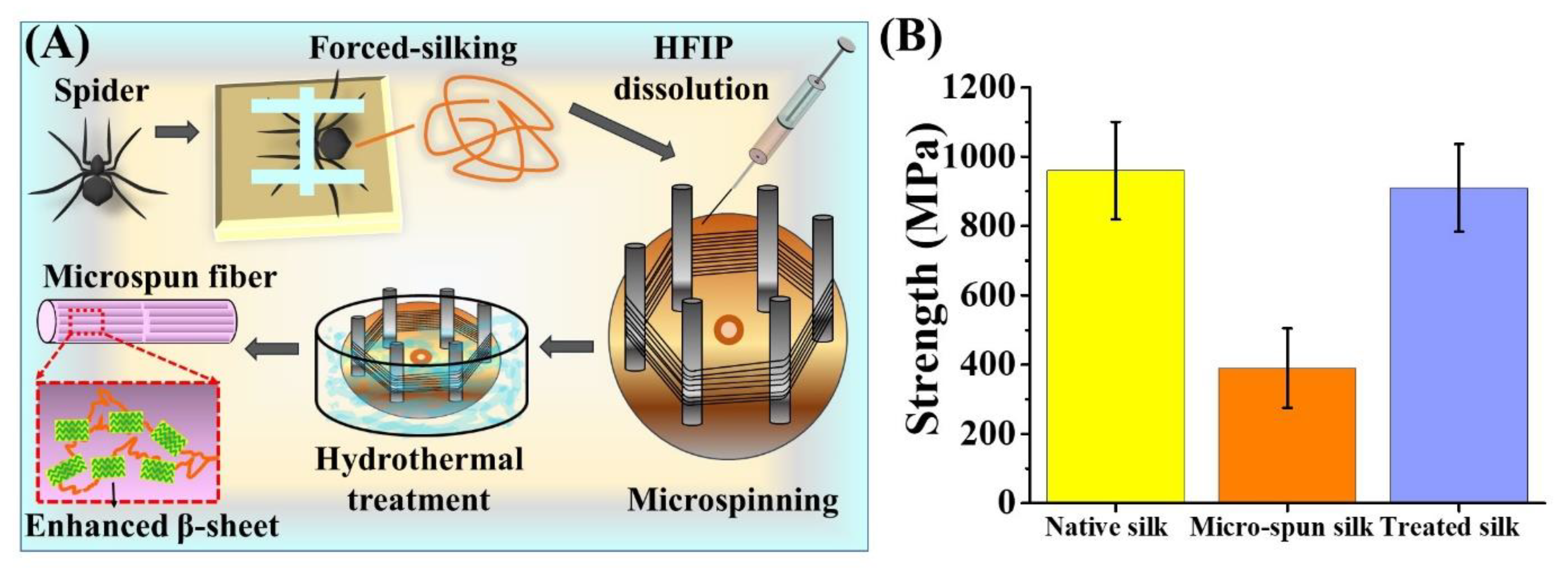
2.5. Reconstitution of Spider Silk and Microspinning
2.6. Statistical Analysis
3. Results and Discussion
3.1. Analysis of Secondary Structures of Silk Fibroin by Circular Dichroism
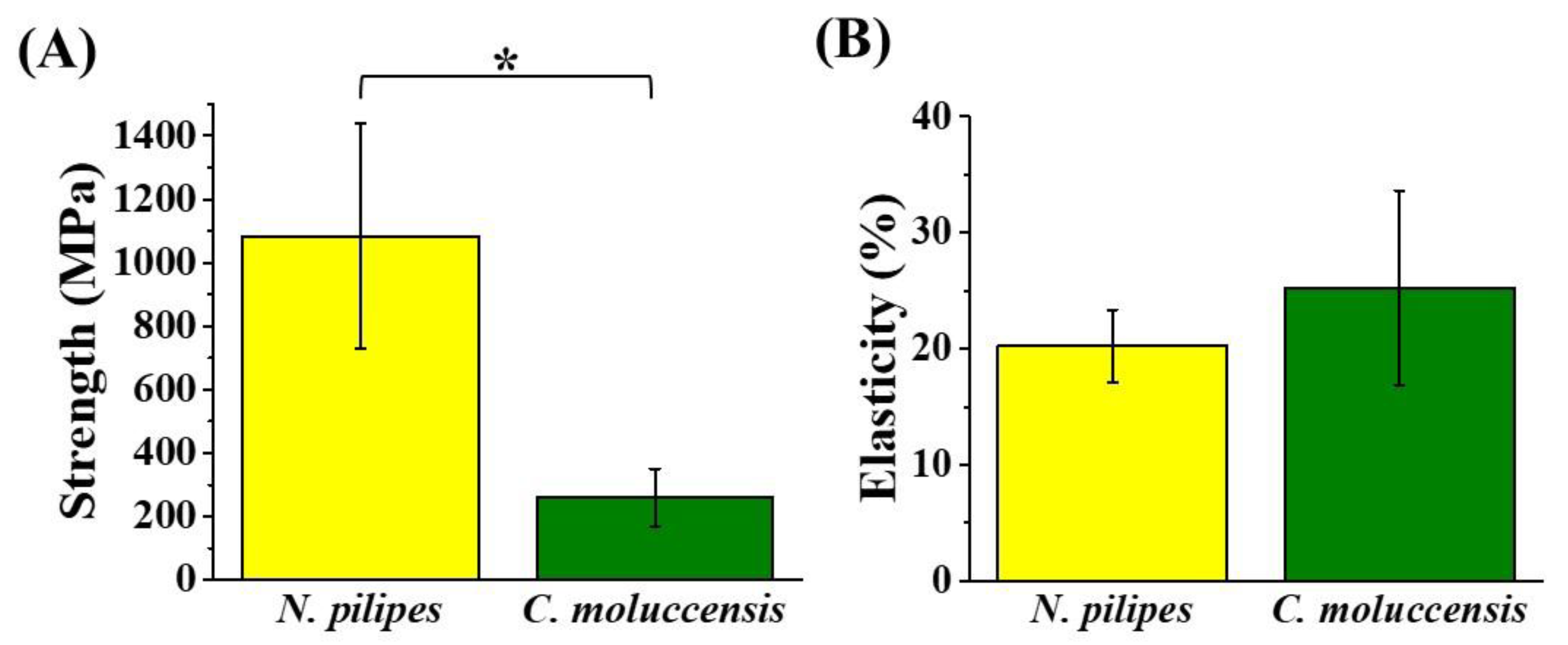
3.2. Mechanical Strength of Dragline Silk Fibers
3.3. Effect of Thermal Treatment on Spider Silk Samples
3.4. FT-IR Analysis of Hydrothermally Treated Silk Samples
3.5. Mechanical Property of Hydrothermally-Treated Regenerated Spider Silk Fibers
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Omenetto, F.G.; Kaplan, D.L. New opportunities for an ancient material. Science 2010, 329, 528–531. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.V. Spider silk: Ancient ideas for new biomaterials. Chem. Rev. 2006, 106, 3762–3774. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.-T.; Yen, C.-K.; Hsieh, M.-C.; Wang, S.-Y.; Chien, C.-H.; Huang, J.C.-C.; Lin, L.; Shiue, Y.-L.; Kuo, S.-W. Energy Harvesters Incorporating Silk from the Taiwan-Native Spider Nephila pilipes. ACS Appl. Energy Mater. 2018, 1, 5627–5635. [Google Scholar] [CrossRef]
- Keten, S.; Buehler, M.J. Nanostructure and molecular mechanics of spider dragline silk protein assemblies. J. R. Soc. Interface 2010, 7, 1709–1721. [Google Scholar] [CrossRef]
- Van Beek, J.D.; Hess, S.; Vollrath, F.; Meier, B.H. The molecular structure of spider dragline silk: Folding and orientation of the protein backbone. Proc. Natl. Acad. Sci. USA 2002, 99, 10266. [Google Scholar] [CrossRef]
- Scheibel, T. Spider silks: Recombinant synthesis, assembly, spinning, and engineering of synthetic proteins. Microb. Cell Factories 2004, 3, 14. [Google Scholar] [CrossRef] [PubMed]
- Altman, G.H.; Diaz, F.; Jakuba, C.; Calabro, T.; Horan, R.L.; Chen, J.; Lu, H.; Richmond, J.; Kaplan, D.L. Silk-based biomaterials. Biomaterials 2003, 24, 401–416. [Google Scholar] [CrossRef]
- Tokareva, O.; Michalczechen-Lacerda, V.A.; Rech, E.L.; Kaplan, D.L. Recombinant DNA production of spider silk proteins. Microb. Biotechnol. 2013, 6, 651–663. [Google Scholar] [CrossRef]
- Lin, T.-Y.; Masunaga, H.; Sato, R.; Malay, A.D.; Toyooka, K.; Hikima, T.; Numata, K. Liquid crystalline granules align in a hierarchical structure to produce spider dragline microfibrils. Biomacromolecules 2017, 18, 1350–1355. [Google Scholar] [CrossRef]
- Jin, H.-J.; Kaplan, D.L. Mechanism of silk processing in insects and spiders. Nature 2003, 424, 1057. [Google Scholar] [CrossRef]
- Parent, L.R.; Onofrei, D.; Xu, D.; Stengel, D.; Roehling, J.D.; Addison, J.B.; Forman, C.; Amin, S.A.; Cherry, B.R.; Yarger, J.L.; et al. Hierarchical spidroin micellar nanoparticles as the fundamental precursors of spider silks. Proc. Natl. Acad. Sci. USA 2018, 115, 11507–11512. [Google Scholar] [CrossRef] [PubMed]
- Kronqvist, N.; Sarr, M.; Lindqvist, A.; Nordling, K.; Otikovs, M.; Venturi, L.; Pioselli, B.; Purhonen, P.; Landreh, M.; Biverstål, H.; et al. Efficient protein production inspired by how spiders make silk. Nat. Commun. 2017, 8, 15504. [Google Scholar] [CrossRef] [PubMed]
- Vollrath, F.; Knight, D.P. Liquid crystalline spinning of spider silk. Nature 2001, 410, 541. [Google Scholar] [CrossRef] [PubMed]
- Kronqvist, N.; Otikovs, M.; Chmyrov, V.; Chen, G.; Andersson, M.; Nordling, K.; Landreh, M.; Sarr, M.; Jörnvall, H.; Wennmalm, S. Sequential pH-driven dimerization and stabilization of the N-terminal domain enables rapid spider silk formation. Nat. Commun. 2014, 5, 3254. [Google Scholar] [CrossRef] [PubMed]
- Andersson, M.; Chen, G.; Otikovs, M.; Landreh, M.; Nordling, K.; Kronqvist, N.; Westermark, P.; Jörnvall, H.; Knight, S.; Ridderstråle, Y. Carbonic anhydrase generates CO2 and H+ that drive spider silk formation via opposite effects on the terminal domains. PLoS Biol. 2014, 12, e1001921. [Google Scholar] [CrossRef] [PubMed]
- Foo, C.W.P.; Bini, E.; Hensman, J.; Knight, D.P.; Lewis, R.V.; Kaplan, D.L. Role of pH and charge on silk protein assembly in insects and spiders. Appl. Phys. A 2006, 82, 223–233. [Google Scholar] [CrossRef]
- Zhang, X.; Pan, Z. Microstructure Transitions and Dry-Wet Spinnability of Silk Fibroin Protein from Waste Silk Quilt. Polymers 2019, 11, 1622. [Google Scholar] [CrossRef]
- Vollrath, F.; Porter, D. Silks as ancient models for modern polymers. Polymer 2009, 50, 5623–5632. [Google Scholar] [CrossRef]
- Yazawa, K.; Ishida, K.; Masunaga, H.; Hikima, T.; Numata, K. Influence of Water Content on the β-Sheet Formation, Thermal Stability, Water Removal, and Mechanical Properties of Silk Materials. Biomacromolecules 2016, 17, 1057–1066. [Google Scholar] [CrossRef]
- Vollrath, F.; Madsen, B.; Shao, Z. The effect of spinning conditions on the mechanics of a spider’s dragline silk. Proc. R. Soc. Lond. Ser. B Biol. Sci. 2001, 268, 2339–2346. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, X.; Shao, Z.; Zhou, P.; Porter, D.; Knight, D.P.; Vollrath, F. Toughness of spider silk at high and low temperatures. Adv. Mater. 2005, 17, 84–88. [Google Scholar] [CrossRef]
- Sheu, H.-S.; Phyu, K.W.; Jean, Y.-C.; Chiang, Y.-P.; Tso, I.-M.; Wu, H.-C.; Yang, J.-C.; Ferng, S.-L. Lattice deformation and thermal stability of crystals in spider silk. Int. J. Biol. Macromol. 2004, 34, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Tsukada, M.; Freddi, G.; Nagura, M.; Ishikawa, H.; Kasai, N. Structural changes of silk fibers induced by heat treatment. J. Appl. Polym. Sci. 1992, 46, 1945–1953. [Google Scholar] [CrossRef]
- Guan, J.; Porter, D.; Vollrath, F. Thermally induced changes in dynamic mechanical properties of native silks. Biomacromolecules 2013, 14, 930–937. [Google Scholar] [CrossRef]
- Teulé, F.; Cooper, A.R.; Furin, W.A.; Bittencourt, D.; Rech, E.L.; Brooks, A.; Lewis, R.V. A protocol for the production of recombinant spider silk-like proteins for artificial fiber spinning. Nat. Protoc. 2009, 4, 341–355. [Google Scholar] [CrossRef]
- Hu, X.; Vasanthavada, K.; Kohler, K.; McNary, S.; Moore, A.; Vierra, C. Molecular mechanisms of spider silk. Cell. Mol. Life Sci. 2006, 63, 1986–1999. [Google Scholar] [CrossRef]
- López Barreiro, D.; Yeo, J.; Tarakanova, A.; Martin-Martinez, F.J.; Buehler, M.J. Multiscale Modeling of Silk and Silk-Based Biomaterials—A Review. Macromol. Biosci. 2019, 19, 1800253. [Google Scholar] [CrossRef]
- Tokareva, O.; Jacobsen, M.; Buehler, M.; Wong, J.; Kaplan, D.L. Structure–function–property–design interplay in biopolymers: Spider silk. Acta Biomater. 2014, 10, 1612–1626. [Google Scholar] [CrossRef]
- Yang, Y.; Greco, G.; Maniglio, D.; Mazzolai, B.; Migliaresi, C.; Pugno, N.; Motta, A. Spider (Linothele megatheloides) and silkworm (Bombyx mori) silks: Comparative physical and biological evaluation. Mater. Sci. Eng. C 2020, 107, 110197. [Google Scholar] [CrossRef]
- Selden, P.A.; Shih, C.; Ren, D. A golden orb-weaver spider (Araneae: Nephilidae: Nephila) from the Middle Jurassic of China. Biol. Lett. 2011, 7, 775–778. [Google Scholar] [CrossRef]
- Wilson, D.; Valluzzi, R.; Kaplan, D. Conformational transitions in model silk peptides. Biophys. J. 2000, 78, 2690–2701. [Google Scholar] [CrossRef]
- Becker, N.; Oroudjev, E.; Mutz, S.; Cleveland, J.P.; Hansma, P.K.; Hayashi, C.Y.; Makarov, D.E.; Hansma, H.G. Molecular nanosprings in spider capture-silk threads. Nat. Mater. 2003, 2, 278. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, C.Y.; Shipley, N.H.; Lewis, R.V. Hypotheses that correlate the sequence, structure, and mechanical properties of spider silk proteins. Int. J. Biol. Macromol. 1999, 24, 271–275. [Google Scholar] [CrossRef]
- Hayashi, C.Y.; Lewis, R.V. Evidence from flagelliform silk cDNA for the structural basis of elasticity and modular nature of spider silks. J. Mol. Biol. 1998, 275, 773–784. [Google Scholar] [CrossRef]
- Römer, L.; Scheibel, T. The elaborate structure of spider silk: Structure and function of a natural high performance fiber. Prion 2008, 2, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Yao, J.; Masuda, H.; Kishore, R.; Asakura, T. Structural characterization and artificial fiber formation of Bombyx mori silk fibroin in hexafluoro-iso-propanol solvent system. Biopolym. Orig. Res. Biomol. 2003, 69, 253–259. [Google Scholar]
- Barth, A. Infrared spectroscopy of proteins. Biochim. Biophys. Acta-Bioenerg. 2007, 1767, 1073–1101. [Google Scholar] [CrossRef]
- Kong, J.; Yu, S. Fourier transform infrared spectroscopic analysis of protein secondary structures. Acta Biochim. Biophys. Sin. 2007, 39, 549–559. [Google Scholar] [CrossRef]
- Dong, A.; Huang, P.; Caughey, W.S. Redox-dependent changes in. beta.-extended chain and turn structures of cytochrome c in water solution determined by second derivative amide I infrared spectra. Biochemistry 1992, 31, 182–189. [Google Scholar] [CrossRef]
- Huang, W.; Krishnaji, S.; Hu, X.; Kaplan, D.; Cebe, P. Heat capacity of spider silk-like block copolymers. Macromolecules 2011, 44, 5299–5309. [Google Scholar] [CrossRef]
- Tamm, L.K.; Tatulian, S.A. Infrared spectroscopy of proteins and peptides in lipid bilayers. Q. Rev. Biophys. 1997, 30, 365–429. [Google Scholar] [CrossRef] [PubMed]
- Belton, D.J.; Plowright, R.; Kaplan, D.L.; Perry, C.C. A robust spectroscopic method for the determination of protein conformational composition–Application to the annealing of silk. Acta Biomater. 2018, 73, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Shmelev, K.; Sun, L.; Gil, E.-S.; Park, S.-H.; Cebe, P.; Kaplan, D.L. Regulation of silk material structure by temperature-controlled water vapor annealing. Biomacromolecules 2011, 12, 1686–1696. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, A.; Matsuda, H.; Tasei, Y.; Asakura, T. Effect of Water on the Structure and Dynamics of Regenerated [3-13C] Ser,[3-13C], and [3-13C] Ala-Bombyx mori Silk Fibroin Studied with 13C Solid-State Nuclear Magnetic Resonance. Biomacromolecules 2018, 19, 563–575. [Google Scholar] [CrossRef]
- Wu, H.-C.; Wu, S.-R.; Yang, T.; Yang, J.-C. A facile measurement for monitoring dragline silk dope concentration in Nephila pilipes upon spinning. Materials 2018, 11, 1951. [Google Scholar] [CrossRef]


| Conformation | Liquid Silk of N. pilipes (%) | Dragline Silk of N. pilipes (%) | Dragline Silk of C. moluccensis (%) |
|---|---|---|---|
| α Helix | 19.8 | 20.7 | 30.0 |
| Antiparallel β-sheet | 27.4 | 28.4 | 21.9 |
| Parallel β-sheet | 8.6 | 8.1 | 7.1 |
| β turn | 18.3 | 18.8 | 20.3 |
| Random coil | 16.0 | 24.1 | 20.7 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, H.-C.; Pandey, A.; Chang, L.-Y.; Hsu, C.-Y.; Yang, T.C.-K.; Tso, I.-M.; Sheu, H.-S.; Yang, J.-C. Hydrothermal Effect on Mechanical Properties of Nephila pilipes Spidroin. Polymers 2020, 12, 1013. https://doi.org/10.3390/polym12051013
Wu H-C, Pandey A, Chang L-Y, Hsu C-Y, Yang TC-K, Tso I-M, Sheu H-S, Yang J-C. Hydrothermal Effect on Mechanical Properties of Nephila pilipes Spidroin. Polymers. 2020; 12(5):1013. https://doi.org/10.3390/polym12051013
Chicago/Turabian StyleWu, Hsuan-Chen, Aditi Pandey, Liang-Yu Chang, Chieh-Yun Hsu, Thomas Chung-Kuang Yang, I-Min Tso, Hwo-Shuenn Sheu, and Jen-Chang Yang. 2020. "Hydrothermal Effect on Mechanical Properties of Nephila pilipes Spidroin" Polymers 12, no. 5: 1013. https://doi.org/10.3390/polym12051013
APA StyleWu, H.-C., Pandey, A., Chang, L.-Y., Hsu, C.-Y., Yang, T. C.-K., Tso, I.-M., Sheu, H.-S., & Yang, J.-C. (2020). Hydrothermal Effect on Mechanical Properties of Nephila pilipes Spidroin. Polymers, 12(5), 1013. https://doi.org/10.3390/polym12051013