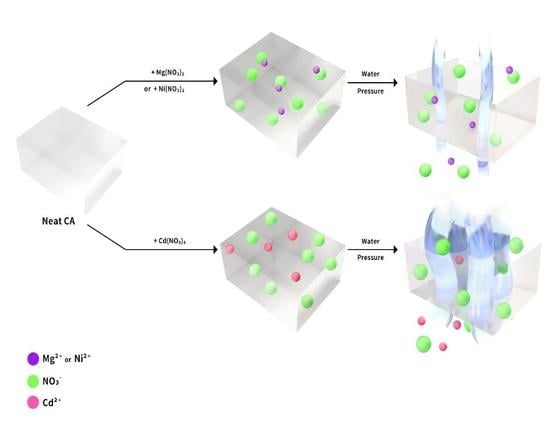
Effect of Ionic Radius in Metal Nitrate on Pore Generation of Cellulose Acetate in Polymer Nanocomposite
Abstract
1. Introduction
2. Experimental
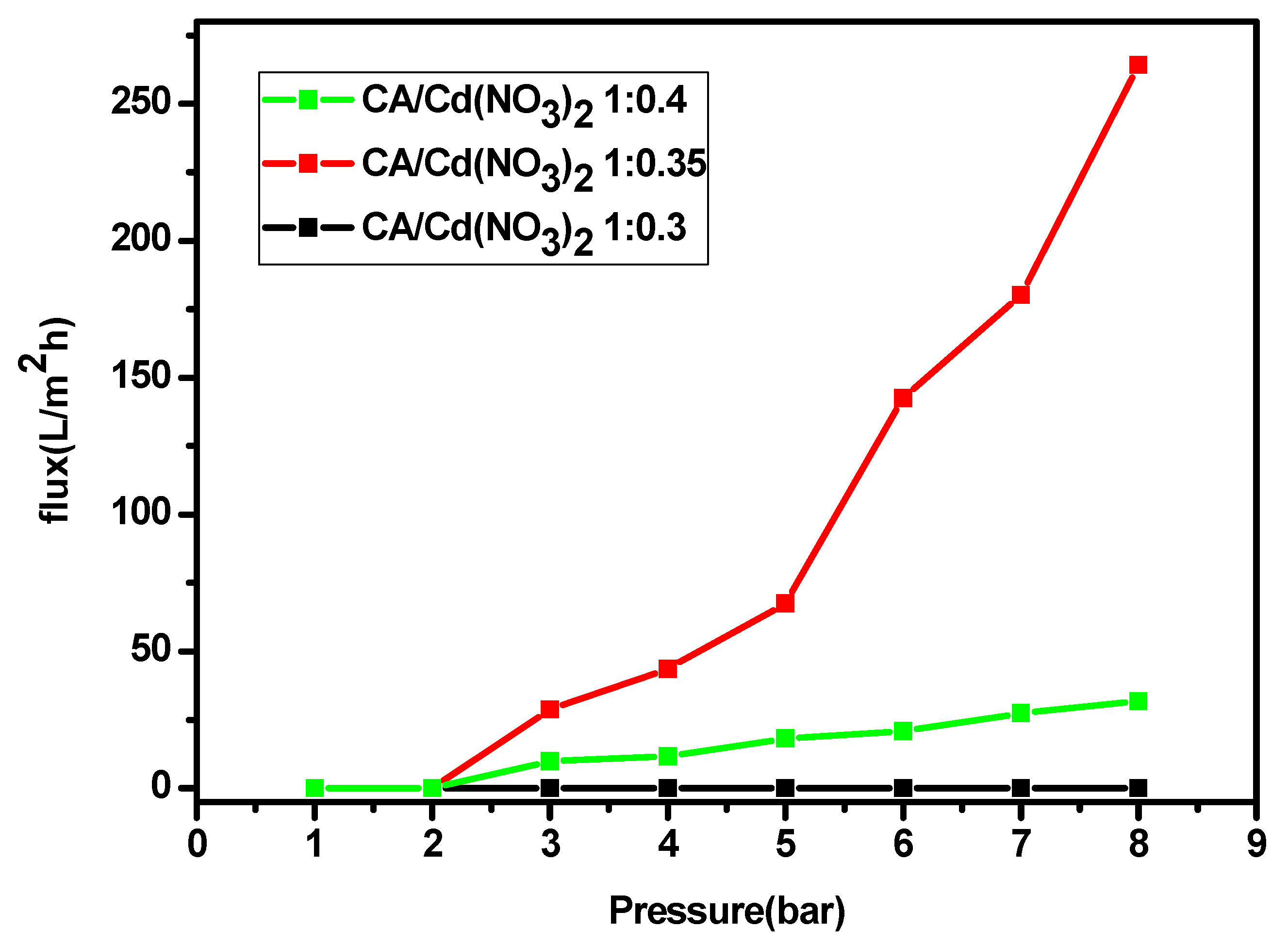
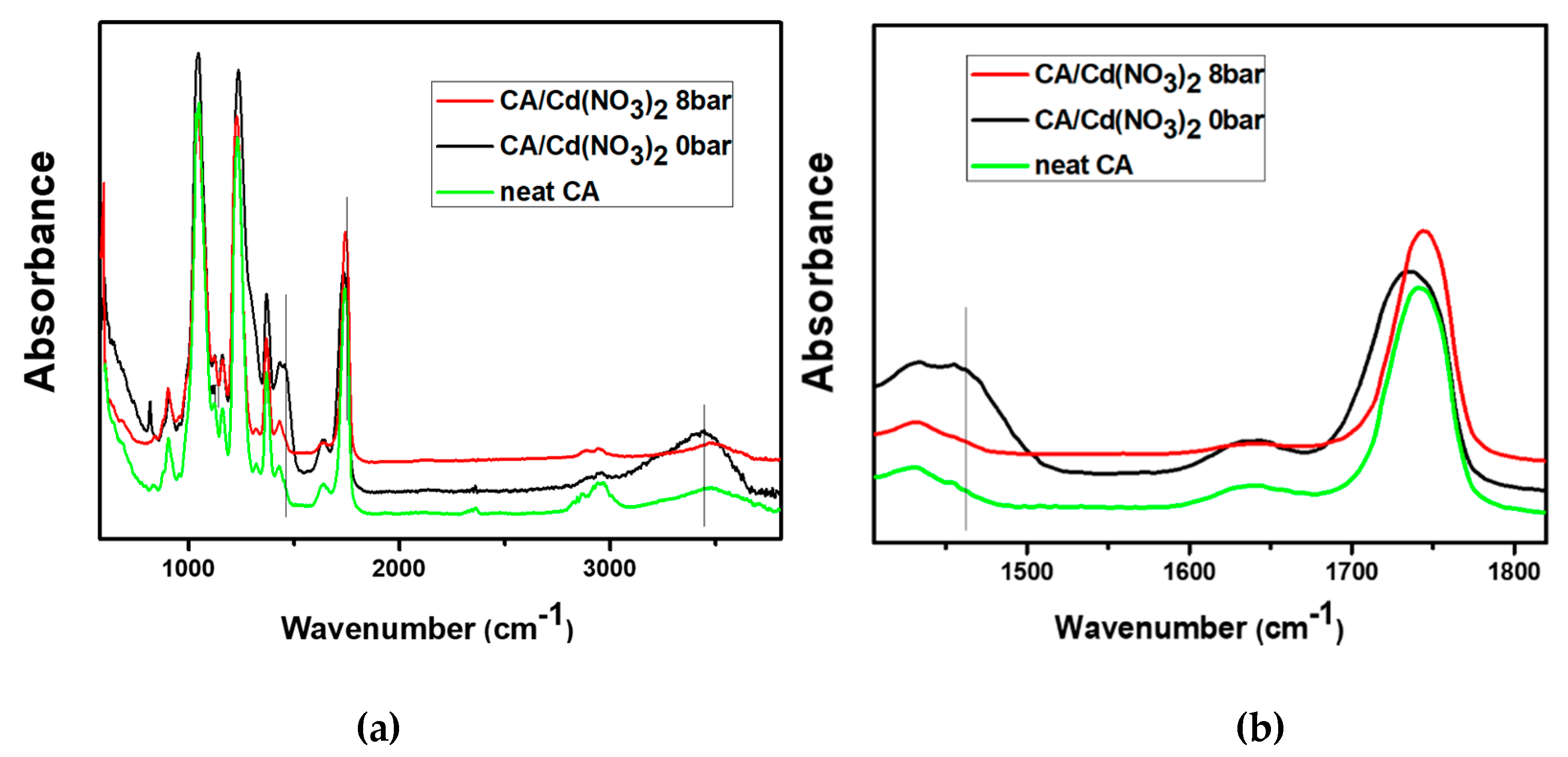
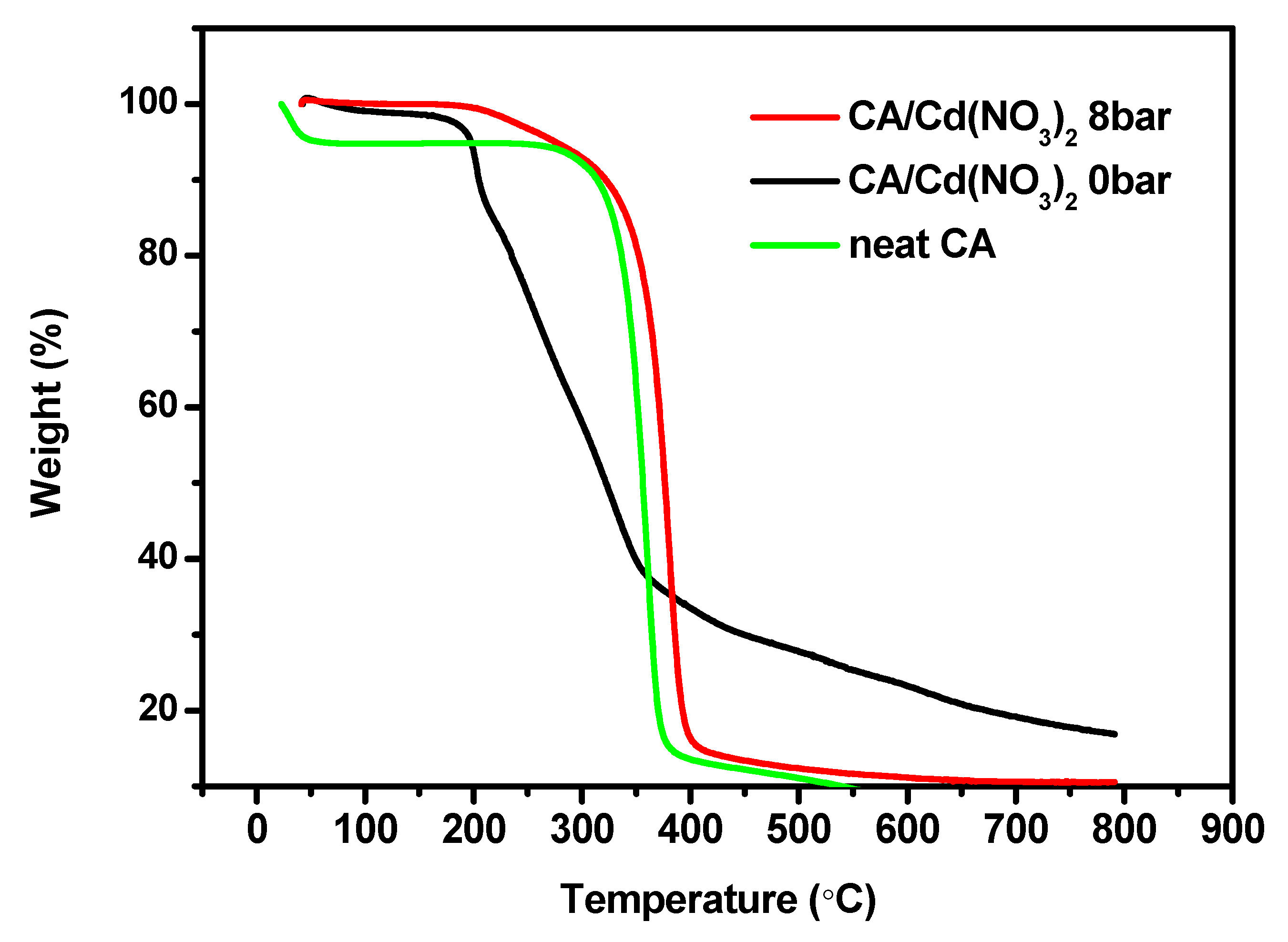
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fehrmann, R.; Riisager, A.; Haumann, M. Supported Ionic Liquids: Fundamentals and Applications; Willey-VCH: Weinheim, Germany, 2014. [Google Scholar]
- Prajapati, A.K.; Mondal, M.K. Hazardous As(III) removal using nanoporous activated carbon of waste garlic stem as adsorbent: Kinetic and mass transfer mechanisms. Korean J. Chem. Eng. 2019, 36, 1900–1914. [Google Scholar] [CrossRef]
- Qian, L.; Ahmed, A.; Zhang, H. Formation of organic nanoparticles by solvent evaporation within porous polymeric materials. Chem. Commun. 2011, 47, 10001–10003. [Google Scholar] [CrossRef]
- Dubinsky, S.; Petukhova, A.; Gourevich, I.; Kumacheva, E. Hybrid porous material produced by polymerization-induced phase separion. Chem. Commun. 2010, 46, 2578–2580. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Vejerano, E.; Khan, A.; Lisak, G.; Ahn, J.H.; Kim, K.-H. Metal organic frameworks (MOFs): Current trends and challenges in control and management of air quality. Korean J. Chem. Eng. 2019, 36, 1839–1853. [Google Scholar] [CrossRef]
- Li, W.; Zhang, G.; Zhang, C.; Meng, Q.; Fan, Z.; Gao, C. Synthesis of trinity metal-organic framework membranes for CO2 capture. Chem. Commun. 2014, 50, 3214–3216. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.-H.; Chen, Q.; Sui, Z.-Y.; Pan, L.; Yu, J.; Han, B.-H. Preparation and adsorption performance of crosslinked porous polycarbazoles. Chem. Commun. 2014, 2, 16181–16189. [Google Scholar]
- Çalhan, A.; Deniz, S.; Romero, J.; Hasanoğlu, A. Development of metal organic framework filled PDMS/PI composite membranes for biobutanol recovery. Korean J. Chem. Eng. 2019, 36, 1489–1498. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, B.; Williams, K.; Gao, W.-Y.; Ma, S. A new microporous carbon material synthesized via thermolysis of a porous aromatic framework embedded with an extra carbon source for low-pressure CO2 uptake. Chem. Commun. 2013, 49, 10269–10271. [Google Scholar] [CrossRef]
- Takase, A.; Kanoh, H.; Ohba, T. Wide carbon nanopores as efficient sites for the separation of SF6 from N2. Sci. Rep. 2015, 5, 11994–11998. [Google Scholar] [CrossRef]
- Xu, Q.; Kong, Q.; Liu, Z.; Zhang, J.; Wang, X.; Yue, R.L.L.; Cui, G. Polydopamine-coated cellulose microfibrillated membrane as high performance lithium-ion battery separator. RSC Adv. 2014, 4, 7845–7850. [Google Scholar] [CrossRef]
- Lee, M.J.; Kim, J.H.; Lim, H.-S.; Lee, S.Y.; Yu, H.K.; Kim, J.H.; Lee, J.S.; Sun, Y.-K.; Guiver, M.D.; Suh, K.D.; et al. Highly lithium-ion conductive battery separators from thermally rearranged polybenzoxazole. Chem. Commun. 2015, 51, 2068–2071. [Google Scholar] [CrossRef]
- Peng, K.; Wang, B.; Li, Y.; Ji, C. Magnetron sputtering deposition of TiO2 particles on polypropylene separators for lithium-ion batteries. RSC Adv. 2015, 5, 81468–81473. [Google Scholar] [CrossRef]
- Zhao, M.; Wang, J.; Chong, C.; Yu, X.; Wang, L.; Shi, Z. An electrospun lignin/polyacrylonitrile nonwoven composite separator with high porosity and thermal stability for lithium-ion batteries. RSC Adv. 2015, 5, 101115–101120. [Google Scholar] [CrossRef]
- Sun, L.; Tang, S. Synthesis of bi-functionalized ionic liquid mesoporous alumina composite material and its CO2 capture capacity. Korean J. Chem. Eng. 2019, 36, 1708–1715. [Google Scholar] [CrossRef]
- Scrosati, B.; Hassoun, J.; Sun, Y.-K. Lithium-ion batteries. A look into the future. Energy Environ. Sci. 2011, 4, 3287–3295. [Google Scholar] [CrossRef]
- Chung, S.-H.; Manthiram, A. High-performance Li-S batteries with an ultra-lightweight MWCNT-coated separator. J. Phys. Chem. Lett. 2014, 5, 1978–1983. [Google Scholar] [CrossRef]
- He, M.; Zhang, X.; Jiang, K.; Wang, J.; Wang, Y. Pure inorganic separator for lithium ion batteries. ACS Appl. Mater. Interfaces 2015, 7, 738–742. [Google Scholar] [CrossRef]
- Lee, J.; Lee, C.L.; Park, K.; Kim, I.D. Synthesis of an Al2O3-coated polyimide nanofiber mat and its electrochemical charateristics as a separator for lithium ion batteries. Power Source 2014, 248, 1211–1217. [Google Scholar] [CrossRef]
- Lloyd, D.R.; Kim, S.S.; Kinzer, K.E. Microporous membrane formation via thermally-induced phase separation. II. Liquid-Liquid phase separation. J. Membr. Sci. 1990, 52, 239–250. [Google Scholar] [CrossRef]
- Mo, D.; Liu, J.D.; Duan, J.L.; Yao, H.J.; Latif, H.; Cao, D.L.; Chen, Y.H.; Zhang, S.X.; Zhai, P.F.; Liu, J. Fabrication of different pore shapes by multi-step etching technique in ion-irradiated PET membranes. J. Membr. Sci. 2014, 333, 58–63. [Google Scholar] [CrossRef]
- Apeal, P.Y.; Ramirez, P.; Blonskaya, I.V.; Orelovitch, O.L.; Sartowska, B.A. Accurate characterization of single track-etched, conical nanopores. Chem. Commun. 2014, 16, 15214–15223. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Luo, G.; Wu, J.; Xia, H. Preparation of microporous silicone rubber membrane with tunable pore size via solvent evaporation-induced phase separation. ACS Appl. Mater. Interfaces 2013, 5, 2040–2046. [Google Scholar] [CrossRef] [PubMed]
- Hermsdorf, N.; Sahre, K.; Volodin, P.; Stamm, M.; Eichhorn, K.J.; Cunis, S.; Gehrke, R.; Panagiotou, P.; Titz, T.; Muller-Buschbaum, P. Supported particle track eyched polyimide membranes: A grazing incidence small-angle X-ray scattering study. Langmuir 2004, 20, 10303–10310. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.G.; Hwang, J.; Kang, S.W. Control of nanoporous polymer matrix by an ionic liquid and water pressure for applications to water-treatment and separator. J. Chem. Eng. 2016, 284, 37–40. [Google Scholar] [CrossRef]
- Lee, W.G.; Kim, D.H.; Jeon, W.C.; Kwak, S.K.; Kang, S.J.; Kang, S.W. Facile control of nanoporosity in Cellulose Acetate using Nickel(II) nitrate additive and water pressure treatment for highly efficient battery gel separators. Sci. Rep. 2017, 7, 1287–1295. [Google Scholar] [CrossRef]
- Klemm, D.; Heublein, B.; Fink, H.P.; Bohn, A. Cellulose: Fascinating biopolymer and sustainable raw material. Angew. Chem. Int. Ed. Engl. 2005, 44, 3358–3393. [Google Scholar] [CrossRef]
- Lee, W.G.; Kang, S.W. Control of Pore in Cellulose Acetate containing Mg salt by Water Pressure Treatment for Applications to Separators. J. Ind. Eng. Chem. 2019, 70, 103–106. [Google Scholar] [CrossRef]

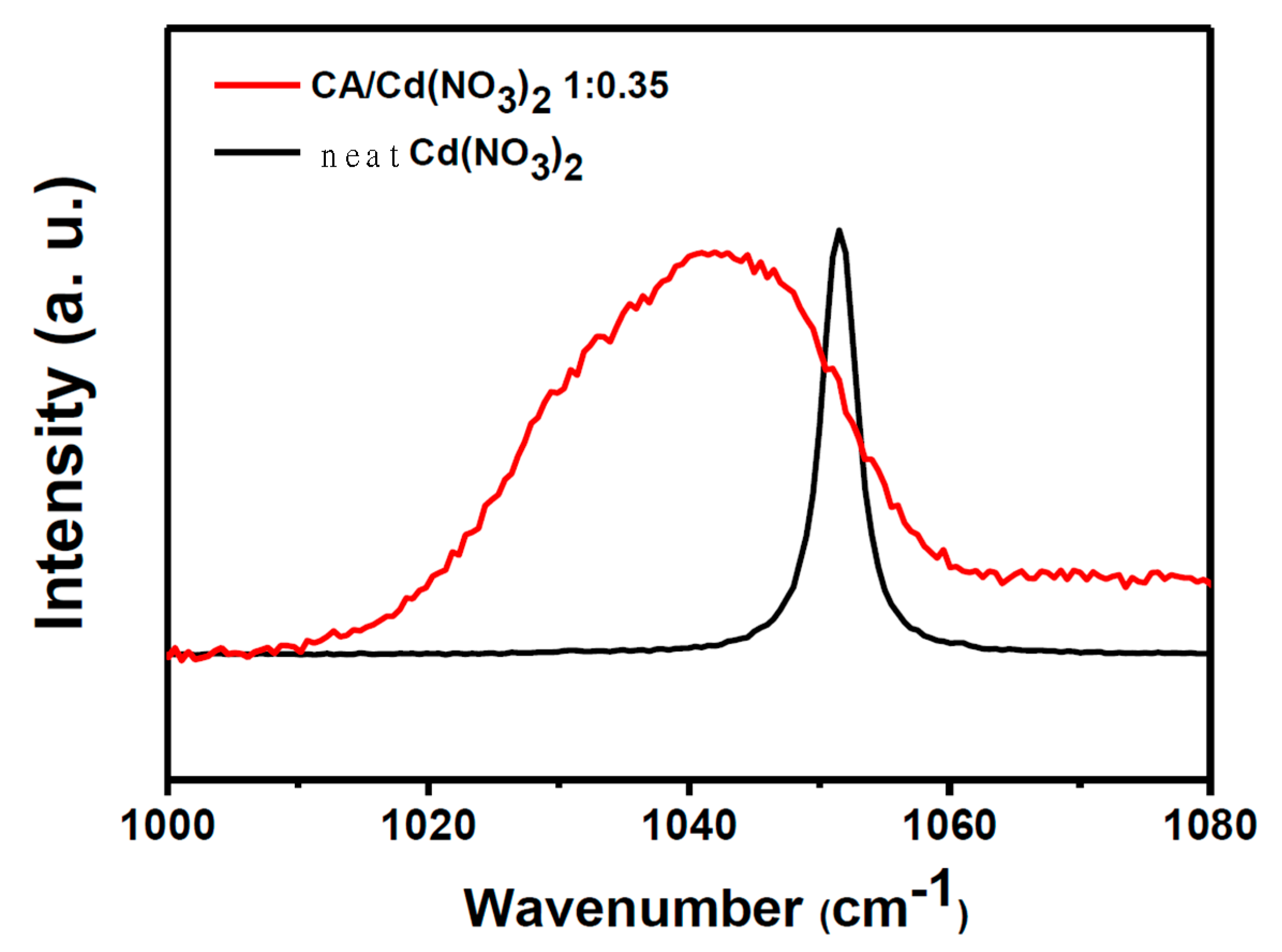
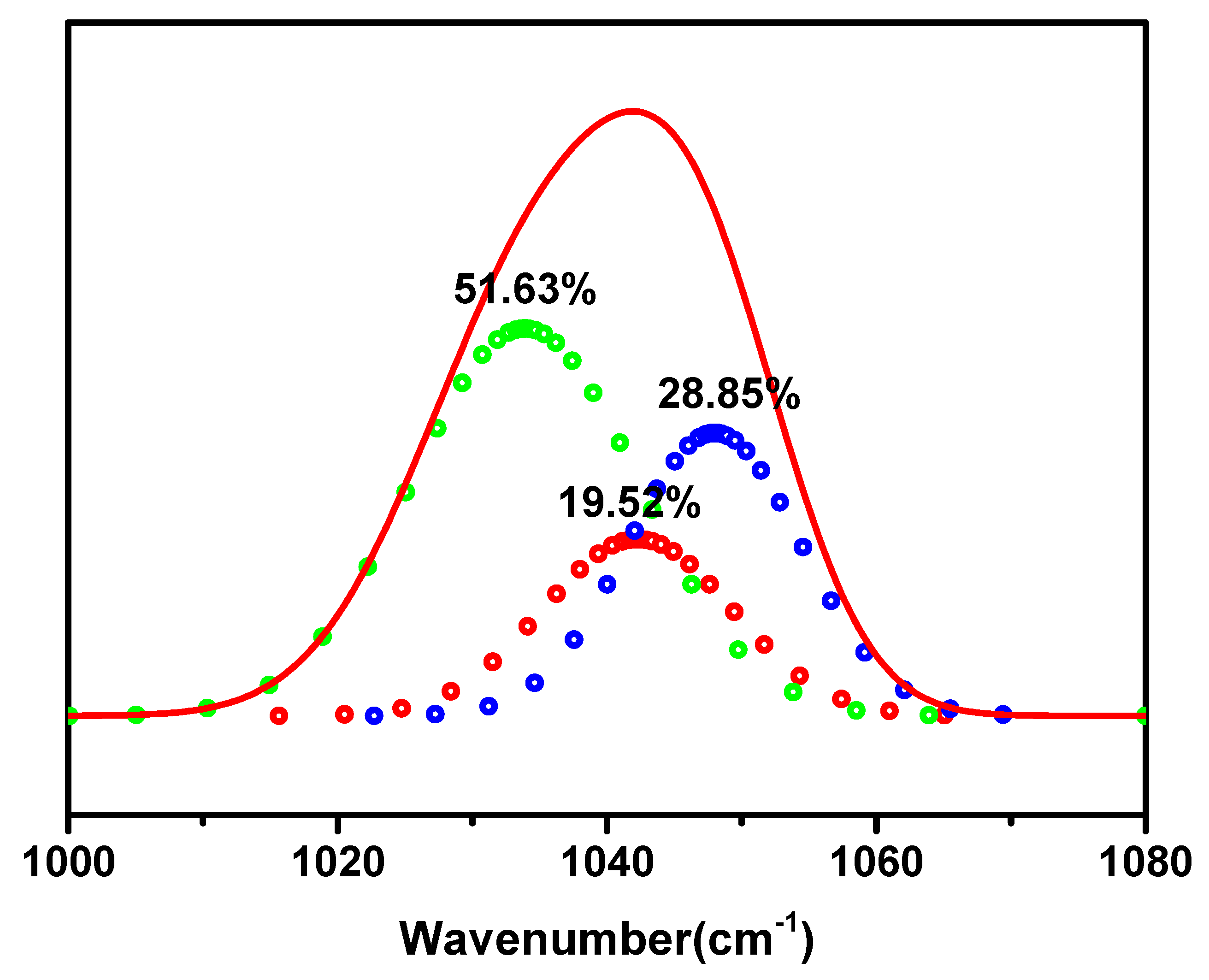

| Elements | Atomic Radius (pm) | Ionic Radius (pm) |
|---|---|---|
| Cd | 155 | 109 |
| Ni | 135 | 83 |
| Mg | 150 | 86 |
| Zn | 135 | 88 |
| Free ions % | Ion Pair % | Ionic Aggregates % | |
|---|---|---|---|
| Cd(NO3)2 in CA | 51.63% | 19.52% | 28.85% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, W.G.; Cho, Y.; Kang, S.W. Effect of Ionic Radius in Metal Nitrate on Pore Generation of Cellulose Acetate in Polymer Nanocomposite. Polymers 2020, 12, 981. https://doi.org/10.3390/polym12040981
Lee WG, Cho Y, Kang SW. Effect of Ionic Radius in Metal Nitrate on Pore Generation of Cellulose Acetate in Polymer Nanocomposite. Polymers. 2020; 12(4):981. https://doi.org/10.3390/polym12040981
Chicago/Turabian StyleLee, Woong Gi, Younghyun Cho, and Sang Wook Kang. 2020. "Effect of Ionic Radius in Metal Nitrate on Pore Generation of Cellulose Acetate in Polymer Nanocomposite" Polymers 12, no. 4: 981. https://doi.org/10.3390/polym12040981
APA StyleLee, W. G., Cho, Y., & Kang, S. W. (2020). Effect of Ionic Radius in Metal Nitrate on Pore Generation of Cellulose Acetate in Polymer Nanocomposite. Polymers, 12(4), 981. https://doi.org/10.3390/polym12040981