Drag Reduction Performance and Mechanism of Hydrophobic Polymers in Fresh Water and Brine
Abstract
1. Introduction
2. Material and Methods
2.1. Raw Materials
2.2. Synthesis of Drag Reducer
2.3. Methods
2.3.1. Characterization
2.3.2. Stability Coefficient
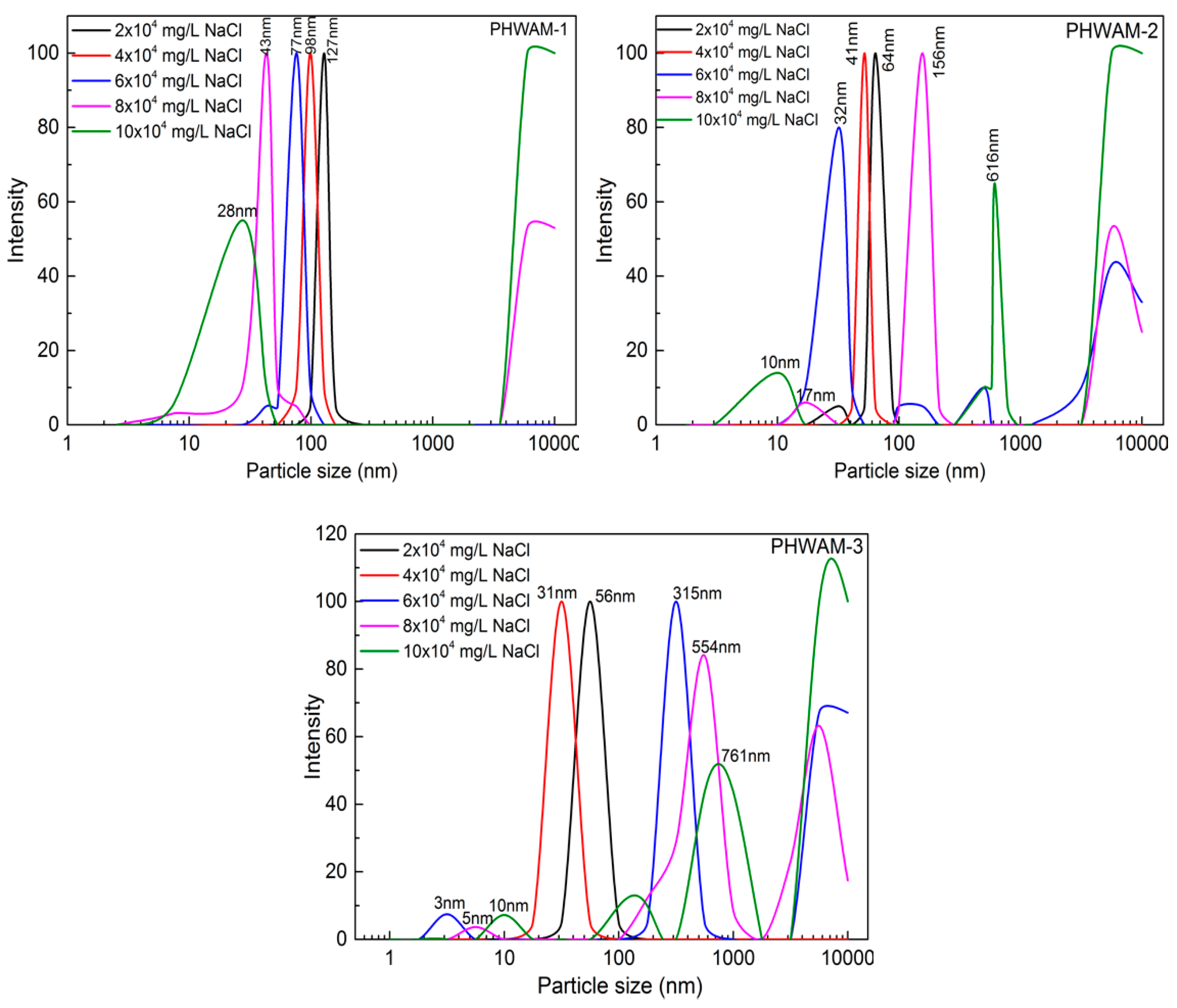
2.3.3. Dynamic Light Scattering Measurements
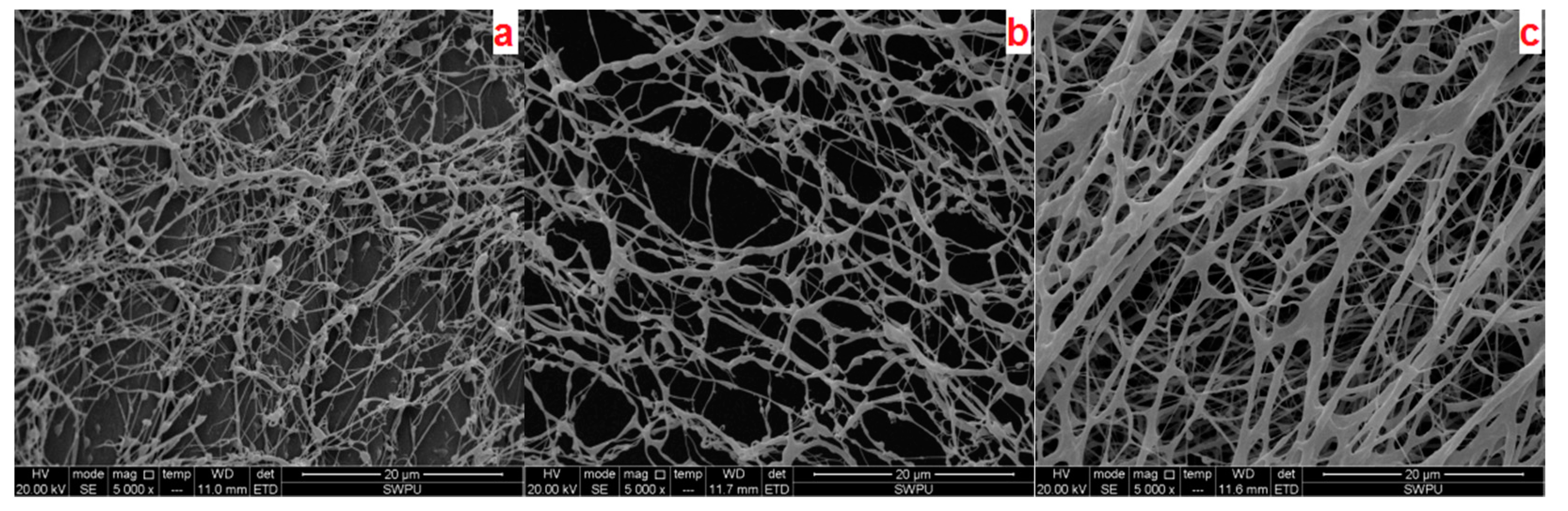
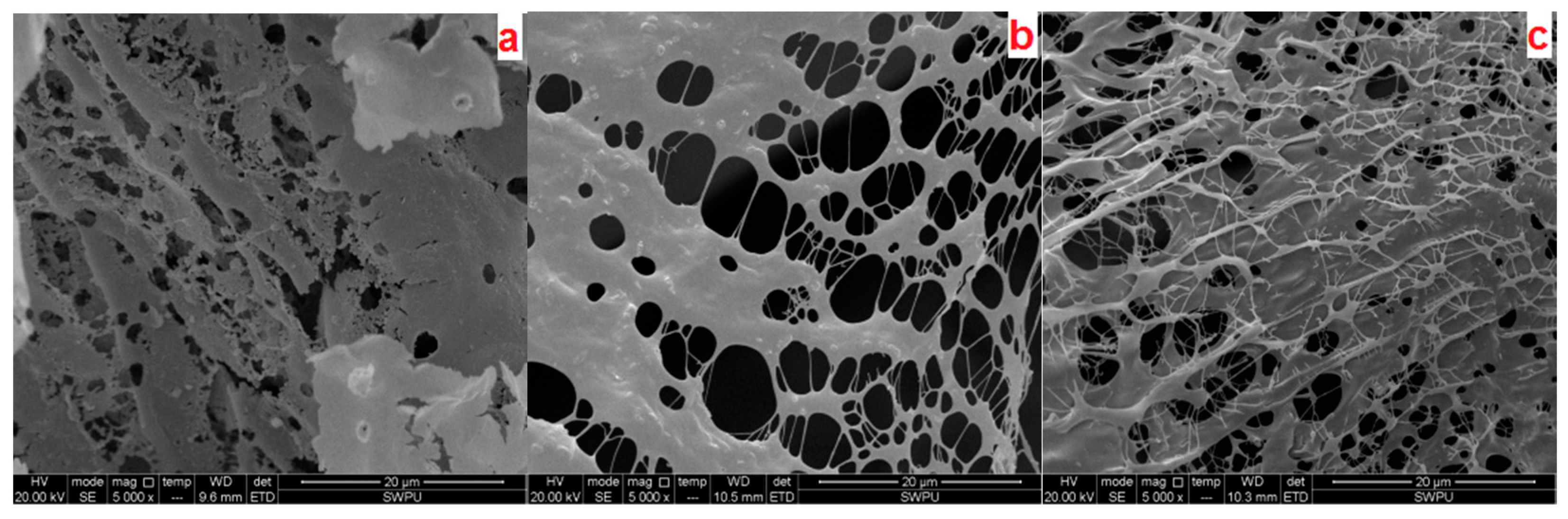
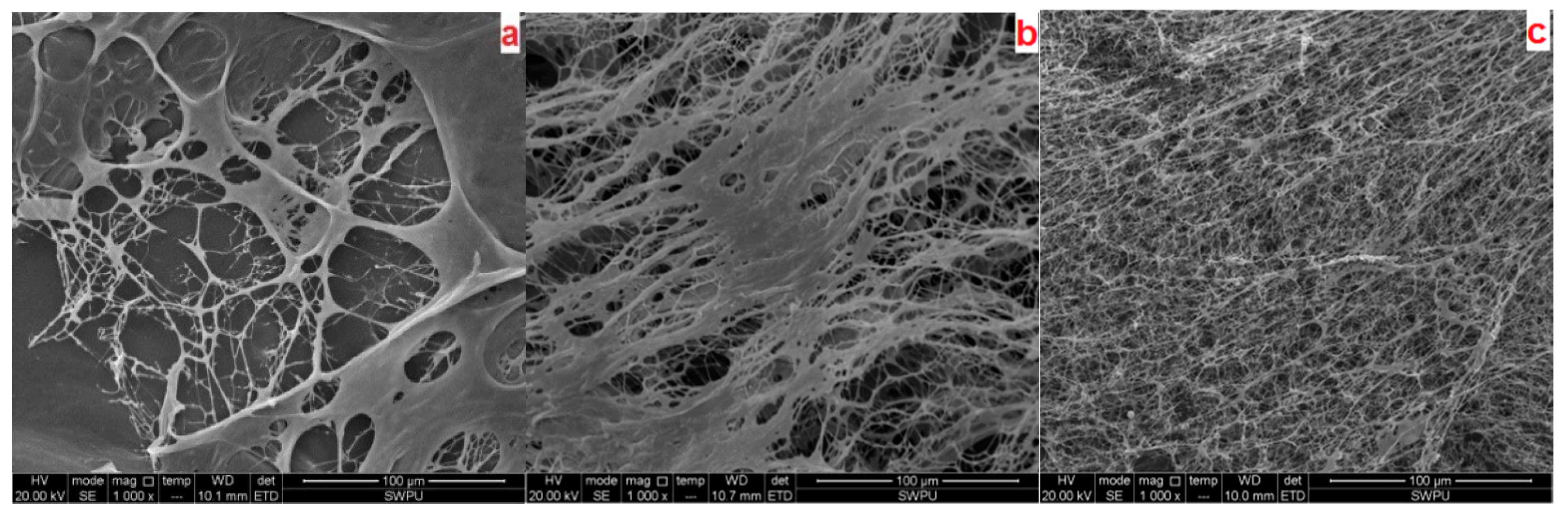
2.3.4. Microstructure Analysis
2.3.5. DR Testing
3. Results and Discussion
3.1. Characterization
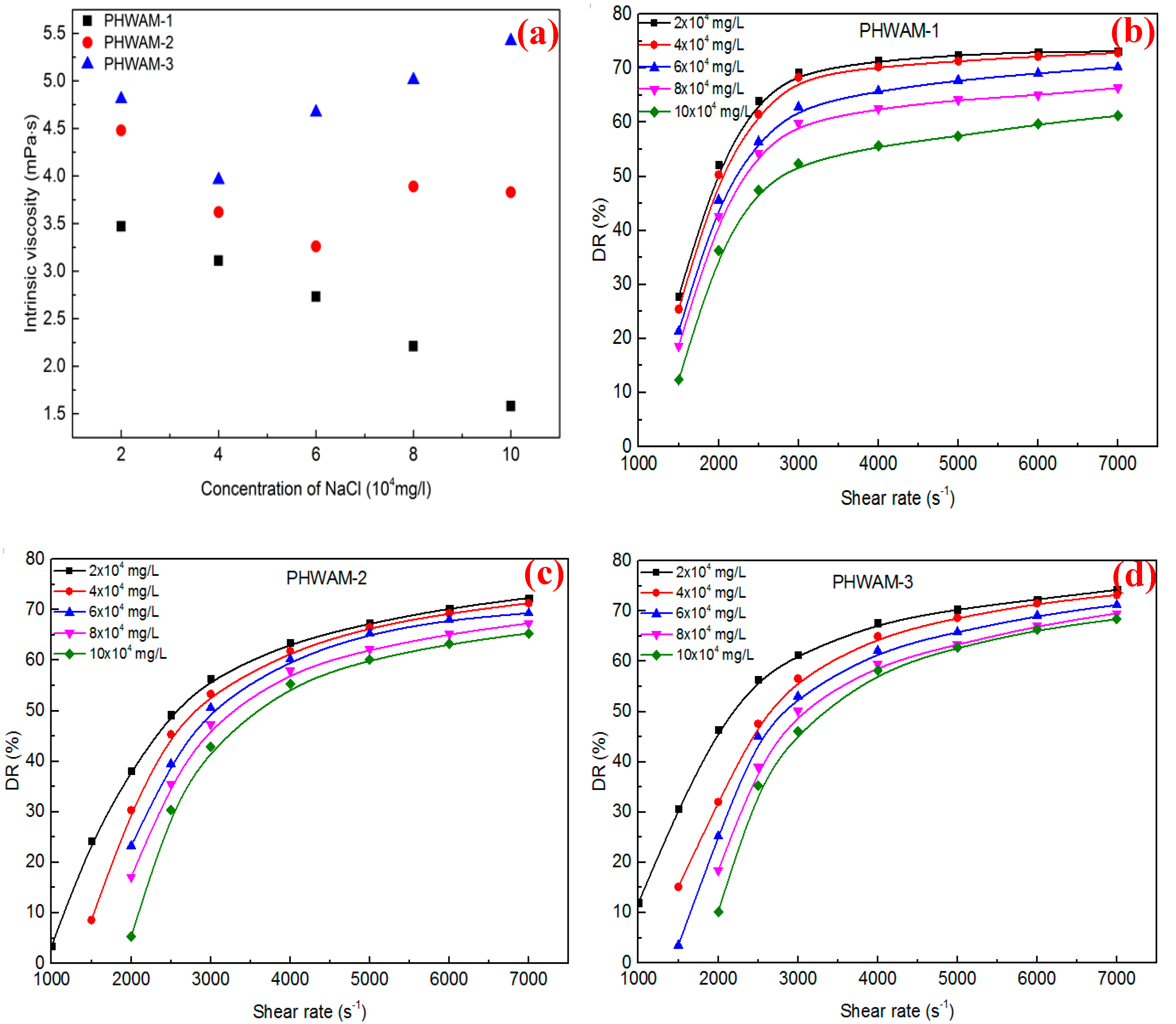
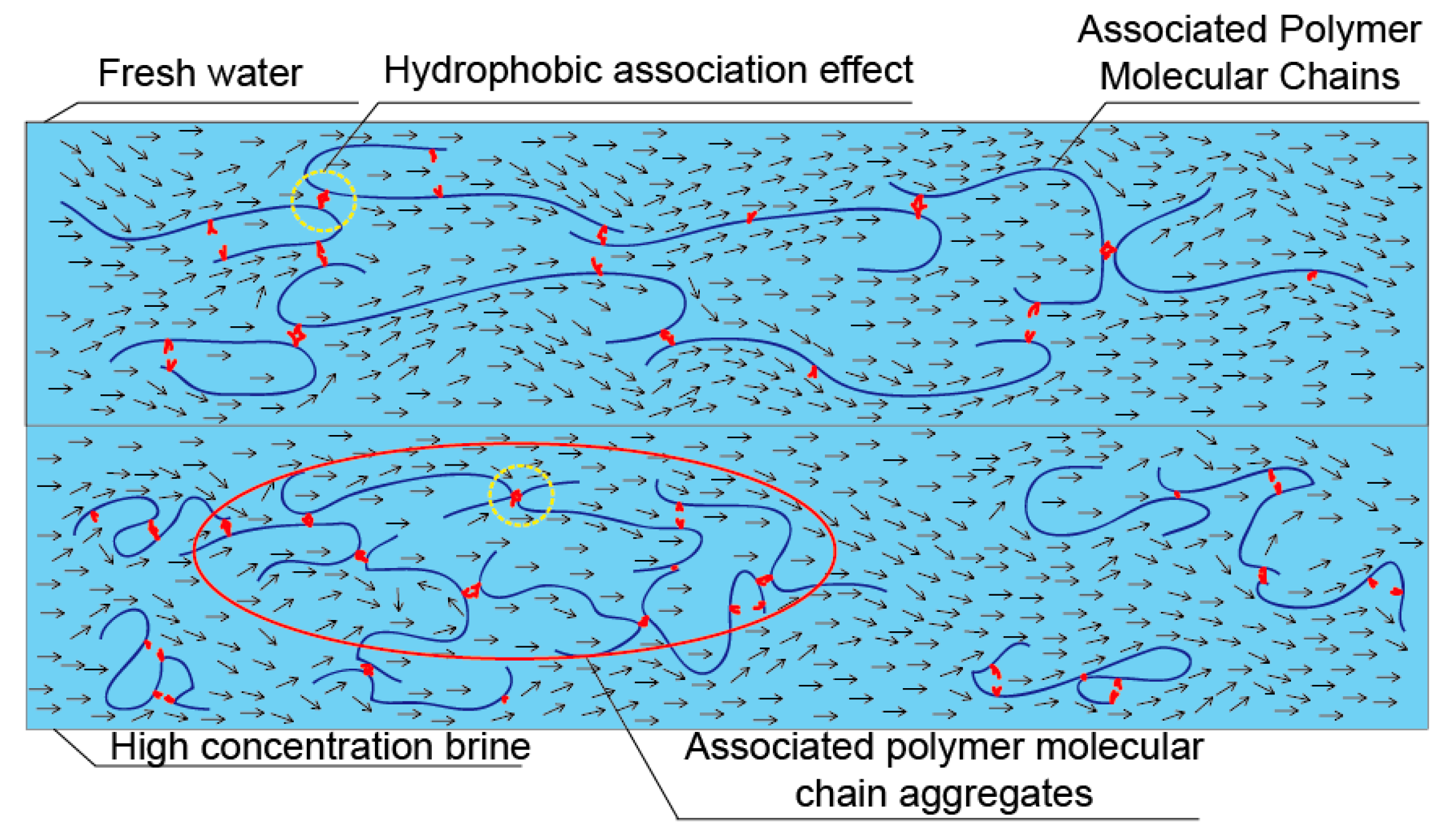
3.2. Critical Association Concentration
3.3. DR in Fresh Water
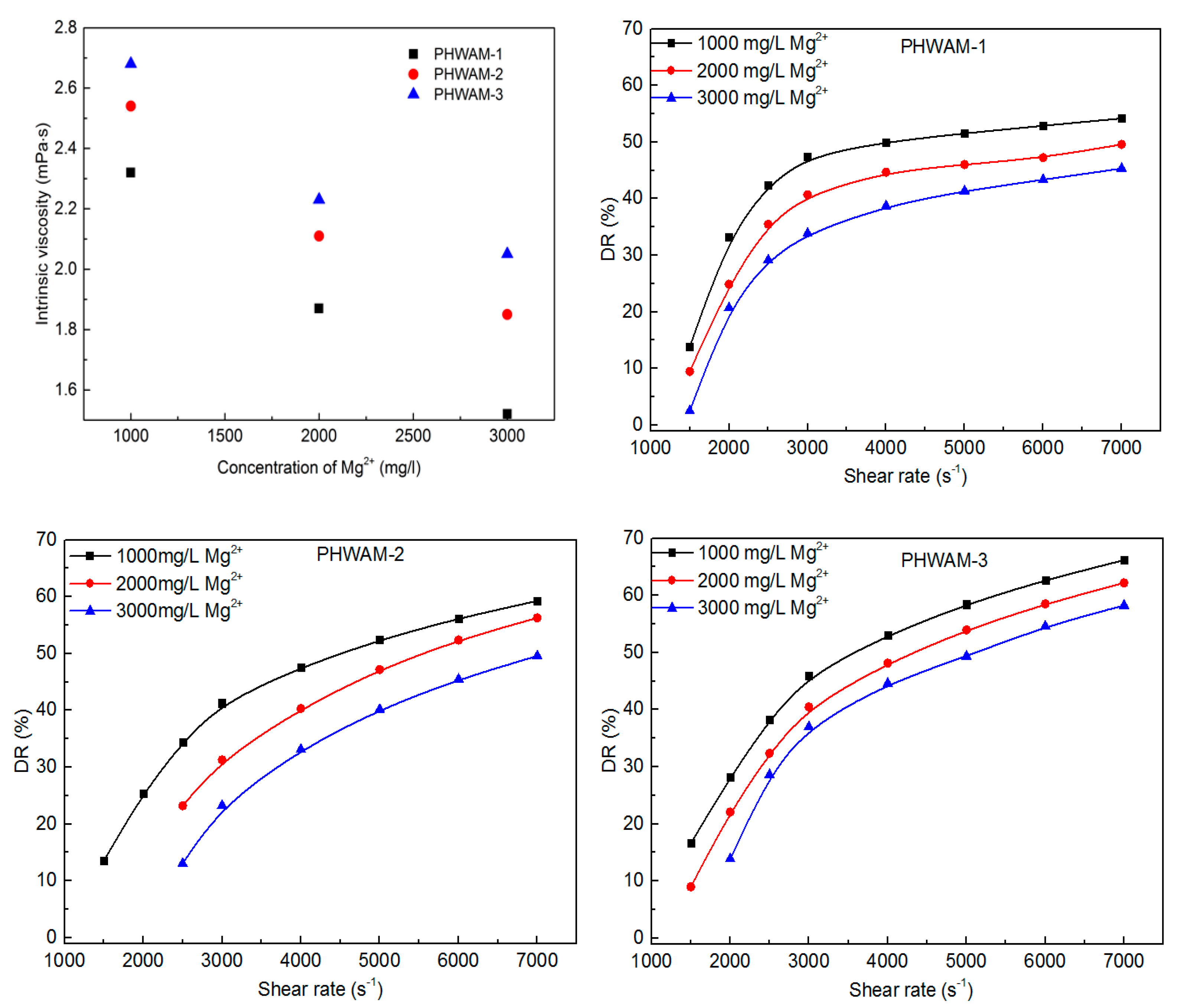
3.4. DR in Brine
3.5. DR of High Mineralization Water Quality
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Toms, B.A. Some Observations on the Flow of Linear Polymer Solutions Through Straight Tubes at Large Reynolds Number; North-Holland Publish Co: American Samoa, AS, USA, 1949; pp. 135–142. [Google Scholar]
- Kim, C.A.; Jo, D.S.; Choi, H.J.; Kim, C.B.; Jhon, M.S. A high-precision rotating disk apparatus for drag reduction characterization. Polym. Test. 2000, 20, 43–48. [Google Scholar] [CrossRef]
- Virk, P.S. Drag reduction fundamentals. AIChE. J. 1975, 21, 625–656. [Google Scholar] [CrossRef]
- Holtmyer, M.D.; Chatterji, J. Study of oil soluble polymers as drag reducers. Polym. Eng. Sci. 1980, 20, 473–477. [Google Scholar] [CrossRef]
- Yang, X.; Mao, J.; Zhang, W.; Zhang, H.; Zhang, Y.; Zhang, C.; Ouyang, D.; Chen, Q.; Lin, C.; Zhao, J. Tertiary cross-linked and weighted fracturing fluid enables fracture stimulations in ultra high pressure and temperature reservoir. Fuel 2020, 168. [Google Scholar] [CrossRef]
- Wang, S.; Song, Z.; Cao, T.; Song, X. The methane sorption capacity of Paleozoic shales from the Sichuan Basin, China. Mar. Pet. Geol. 2013, 44, 112–119. [Google Scholar] [CrossRef]
- Kamel, A.; Shah, S.N. Effects of salinity and temperature on drag reduction characteristics of polymers in straight circular pipes. J. Petrol. Sci. Eng. 2009, 67, 23–33. [Google Scholar] [CrossRef]
- Sokhal, K.S.; Gangacharyulu, D.; Bulasara, V.K. Effect of guar gum and salt concentrations on drag reduction and shear degradation properties of turbulent flow of water in a pipe. Carbohydr. Polym. 2018, 181, 1017–1025. [Google Scholar] [CrossRef]
- Aften, C.W. Study of friction reducers for recycled stimulation fluids in environmentally sensitive regions. In Proceedings of the. SPE Eastern Regional Meeting, Morgantown, West Virginia, WV, USA, 13–15 Octomber 2010; pp. 13–15. [Google Scholar]
- Zhou, J.; Baltazar, M.; Sun, H. Water-Based Environmentally Preferred Friction Reducer in Ultrahigh-TDS Produced Water for Slick-Water Fracturing in Shale Reservoirs; SPE 167775; Society of Petroleum Engineers: Vienna, Austria, 2014. [Google Scholar]
- Paul, W.; Russell, T. Process for Achieving Improved Friction Reduction in Hydraulic Fracturing and Cooled Tubing Applications in High Salinity Conditions. U.S. Patent 2012/0214714 A1, 23 August 2012. [Google Scholar]
- Stevens, M.J.; Kremer, K. The nature of flexible linear polyelectrolytes in salt free solution: A molecular dynamics study. J. Chem. Phys. 1995, 103, 1669. [Google Scholar] [CrossRef]
- Sato, Y.; Hashidzume, A.; Morishima, Y. Self-Association in Water of Copolymers of Acrylic Acid and N-Dodecylmeth acrylamide As Studied by Fluorescence, Dynamic Light Scattering, and Rheological Techniques. Macromolecules 2001, 34, 6121–6130. [Google Scholar] [CrossRef]
- Hill, A.; Candau, F.; Selb, J. Properties of hydrophobically associating polyacrylamides: Influence of the method of synthesis. Macromolecules 1993, 26, 4521–4532. [Google Scholar] [CrossRef]
- Kriwet, B.; Walter, E.; Kissel, T. Synthesis of bio-adhesive poly (acrylic acid) nano-and micro particles using an inverse emulsion polymerization method for the entrapment of hydrophilic drug candidates. J. Controll. Release 1998, 56, 149–158. [Google Scholar] [CrossRef]
- Vanderhoff, J.W. Inverse Emulsion Polymerization; American Chemical Society: Washington, DC, USA, 1962. [Google Scholar]
- Lee, D.H.; Akhavan, R. Skin-Friction Drag Reduction in Turbulent Channel Flow by a Scale-Dependent Molecular Viscosity, Meeting of the Aps Division of Fluid Dynamics. American Physical Society. Int. J. Heat Fluid Flow 2011, 63, 28–43. [Google Scholar]
- Graham, M.D. Drag reduction and the dynamics of turbulence in simple and complex fluid. Phys. Fluids 2014, 26, S3–S8. [Google Scholar] [CrossRef]
- Ricco, P.; Hahn, S. Turbulent drag reduction through rotating discs. J. Fluid. Mech. 2013, 722, 267–290. [Google Scholar] [CrossRef]
- Diamond, P.; Harvey, J.; Katz, J.; Nelson, D.; Steinhardt, P. Drag Reduction by Polymer Additives. J. Org. Chem. 1992, 103, 105–121. [Google Scholar]
- L’vov, V.S.; Pomyalov, A.; Procaccia, I.; Tiberkevich, V. Drag Reduction by Polymers in Wall Bounded Turbulence. Phys. Rev. Lett. 2004, 92, 244503. [Google Scholar] [CrossRef]
- Benzi, R. A short review on drag reduction by polymers in wall bounded turbulence. Phys. D Nonlinear Phenom. 2010, 239, 1338–1345. [Google Scholar] [CrossRef]
- Kim, K.; Li, C.F.; Sureshkumar, R.; Balachandar, S.; Adrian, R.J. Effects of polymer stresses on eddy structures in drag-reduced turbulent channel flow. J. Fluid Mech. 2007, 584, 281. [Google Scholar] [CrossRef]
- Hong, C.H.; Choi, H.J.; Zhang, K.; Renou, F.; Grisel, M. Effect of salt on turbulent drag reduction of xanthan gum. Carbohydr. Polym. 2015, 121, 342–347. [Google Scholar] [CrossRef]
- Guo, L.; Tam, K.C.; Jenkins, R.D. Effects of salt on the intrinsic viscosity of model alkali-soluble associative polymers. Macromol. Chem. Phys. 1998, 199, 1175–1184. [Google Scholar] [CrossRef]
- Ruckenstein, E.; Park, J.S. Hydrophilic-hydrophobic polymer composites. J. Polym. Sci. Pol. Lett. 1988, 26, 529–536. [Google Scholar] [CrossRef]
- Lee, D.H. Skin-Friction Drag Reduction by Dilute Polymer Solutions in Turbulent Channel Flow. Diss. Theses Grad. 2010, 486, 213–238. [Google Scholar]
- Jovanovic, J.; Frohnapfel, B.; Pashtrapanska, M.; Durst, F. The effect of polymers on the dynamics of turbulence in a drag reduced flow. Therm. Sci. 2004, 9, 13–41. [Google Scholar] [CrossRef]
- Virk, P.S. Drag reduction in rough pipes. J. Fluid Mech. 1971, 45, 22. [Google Scholar] [CrossRef]
- Dragan, S.; Ghimici, L. Viscometric behaviour of some hydrophobically modified cationic polyelectrolytes. Polymer 2001, 42, 2887–2891. [Google Scholar] [CrossRef]
- Saito, S. Salt effect on polymer solutions. J. Polym. Sci. Pol. Chem. 1969, 7, 1789–1802. [Google Scholar] [CrossRef]
- Nakano, Y.; Seida, Y. Effect of Water Structure on Adsorption of Thermos Sensitive Polymer Hydrogel in Salt Solutions. J. Chem. Eng. Jpn. 1996, 29, 5. [Google Scholar]
- Ma, J.; Cui, P.; Zhao, L.; Huang, R. Synthesis and solution behavior of hydrophobic association water-soluble polymers containing arylalkyl group. Eur. Polym. J. 2002, 38, 1627–1633. [Google Scholar] [CrossRef]
- Podhajecka, K.; Prochazka, K.; Hourdet, D. Synthesis and viscoelastic behavior of water-soluble polymers modified with strong hydrophobic side chains. Polymer 2007, 48, 1586–1595. [Google Scholar] [CrossRef]
- Fu, Z.; Otsuki, T.; Motozawa, M.; Kurosawa, T.; Yu, B.; Kawaguchi, Y. Experimental investigation of polymer diffusion in the drag-reduced turbulent channel flow of inhomogeneous solution. Int. J. Heat Mass Transf. 2014, 77, 860–873. [Google Scholar] [CrossRef]
- Gupta, V.K.; Sureshkumar, R.; Khomami, B. Passive scalar transport in polymer drag-reduced turbulent channel flow. AIChE J. 2005, 51, 1938–1950. [Google Scholar] [CrossRef]
- Guan, X.L.; Yao, S.Y.; Jiang, N. A study on coherent structures and drag-reduction in the wall turbulence with polymer additives by TRPIV. Acta Mech. Sin. 2013, 29, 485–493. [Google Scholar] [CrossRef]
- Katz, E.R.; Rubinstein, C.L.; Hubert, N.C.; Blew, A. Effect of Salt Concentration on the Drag Reduction Efficiency of Polyethylene Oxide Polymers. Nature 1973, 242, 79–80. [Google Scholar]
- Shah, D.; Ecanow, B. Effect of temperature and sodium chloride on drag reduction by sodium carboxymethyl cellulose and guar gum. J. Pharm. Sci. USA 2010, 63, 1164–1165. [Google Scholar] [CrossRef] [PubMed]











| Samples | Water Phase (110 g, pH = 7) | Oil Phase (110 g, HLB = 6) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| AM (mol) | AA (mol) | AMPS (mol) | C12AM (mol) | DiC12AM (mol) | SDS (mol) | White Oil (g) | Tween-60 (g) | Span-80 (g) | |
| PHWAM-1 | 0.4 | 0.14 | 0.03 | \ | \ | 0.01 | 100 | 1.6 | 8.4 |
| PHWAM-2 | 0.4 | 0.14 | 0.03 | 0.001 | \ | 0.01 | 100 | 1.6 | 8.4 |
| PHWAM-3 | 0.4 | 0.14 | 0.03 | \ | 0.001 | 0.01 | 100 | 1.6 | 8.4 |
| Samples | Intrinsic Viscosity (mPa·s) | Solid Content (%) | Stability Coefficient |
|---|---|---|---|
| PHWAM-1 | 16.6 | 20.0 | 0.91 |
| PHWAM-2 | 15.6 | 19.1 | 0.87 |
| PHWAM-3 | 15.4 | 18.9 | 0.84 |
| Sample of Reducers | DR of Reducers | ||
|---|---|---|---|
| 0 mg/L Ca2+ | 5000 mg/L Ca2+ | 15,000 mg/L Ca2+ | |
| PHWAM-1 | 61.24% | 55.03% | 41.12% |
| PHWAM-2 | 64.38% | 63.33% | 53.41% |
| PHWAM-3 | 68.44% | 66.73% | 57.35% |
| Sample of Reducers | DR of Reducers | ||
|---|---|---|---|
| 1000 mg/L Ca2+ | 2000 mg/L Ca2+ | 3000 mg/L Ca2+ | |
| PHWAM-1 | 54.18% | 48.77% | 45.32% |
| PHWAM-2 | 59.27% | 57.12% | 49.58% |
| PHWAM-3 | 66.18% | 62.51% | 58.24% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, H.; Mao, J.; Zhang, W.; Yang, B.; Yang, X.; Zhang, Y.; Lin, C.; Feng, J.; Zhang, H. Drag Reduction Performance and Mechanism of Hydrophobic Polymers in Fresh Water and Brine. Polymers 2020, 12, 955. https://doi.org/10.3390/polym12040955
Tan H, Mao J, Zhang W, Yang B, Yang X, Zhang Y, Lin C, Feng J, Zhang H. Drag Reduction Performance and Mechanism of Hydrophobic Polymers in Fresh Water and Brine. Polymers. 2020; 12(4):955. https://doi.org/10.3390/polym12040955
Chicago/Turabian StyleTan, Hongzhong, Jincheng Mao, Wenlong Zhang, Bo Yang, Xiaojiang Yang, Yang Zhang, Chong Lin, Jianfa Feng, and Hao Zhang. 2020. "Drag Reduction Performance and Mechanism of Hydrophobic Polymers in Fresh Water and Brine" Polymers 12, no. 4: 955. https://doi.org/10.3390/polym12040955
APA StyleTan, H., Mao, J., Zhang, W., Yang, B., Yang, X., Zhang, Y., Lin, C., Feng, J., & Zhang, H. (2020). Drag Reduction Performance and Mechanism of Hydrophobic Polymers in Fresh Water and Brine. Polymers, 12(4), 955. https://doi.org/10.3390/polym12040955







