Bio-Based Coatings for Food Metal Packaging Inspired in Biopolyester Plant Cutin
Abstract
1. Introduction
2. Materials and Methods
2.1. Coating Preparation
2.2. Textural Characterization
2.3. Chemical Analysis
2.4. Wettability and Solubility Measurements
2.5. UV-Visible Reflection Spectra
3. Results and Discussion
3.1. Texture and Roughness of Supports and Coatings
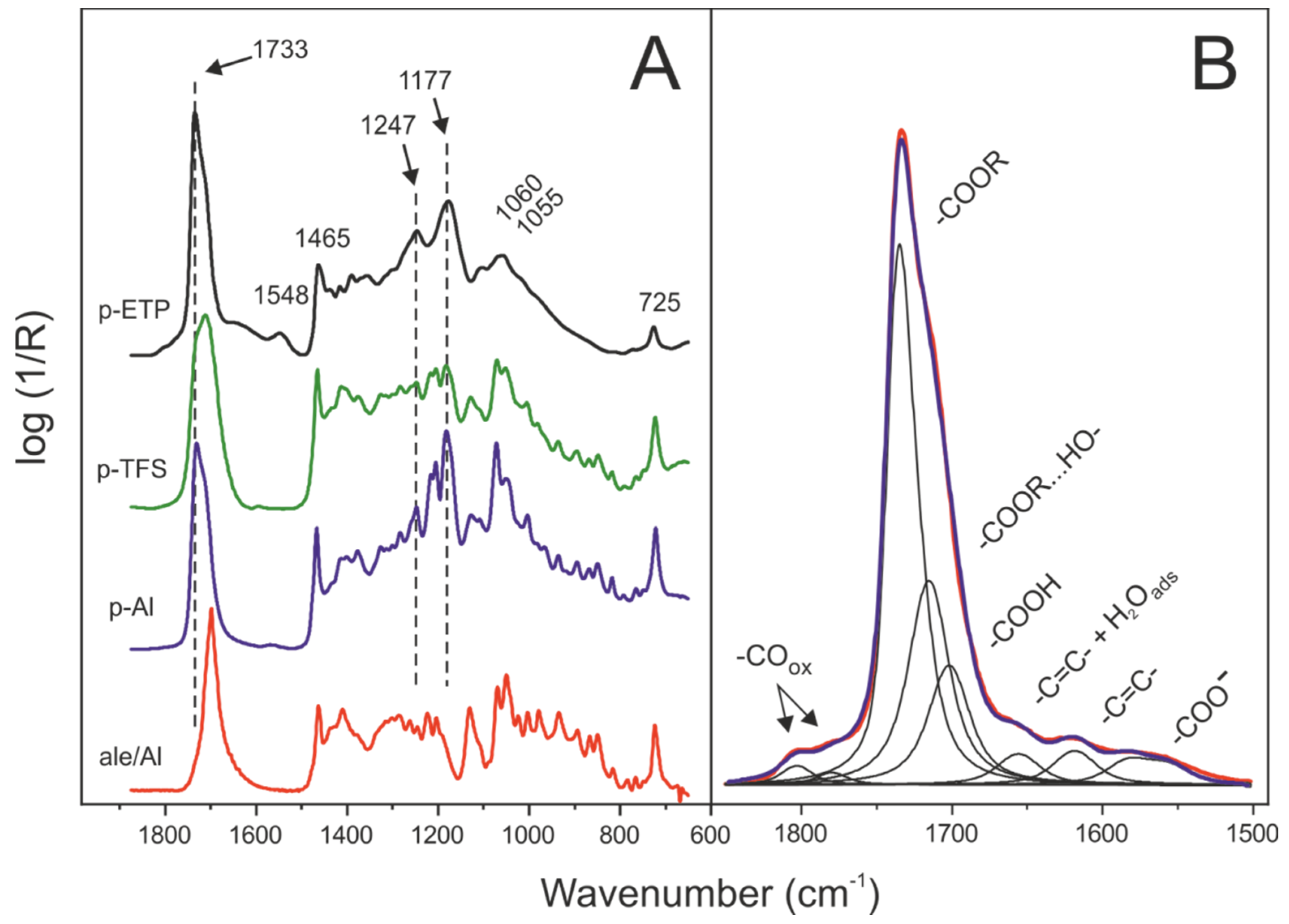
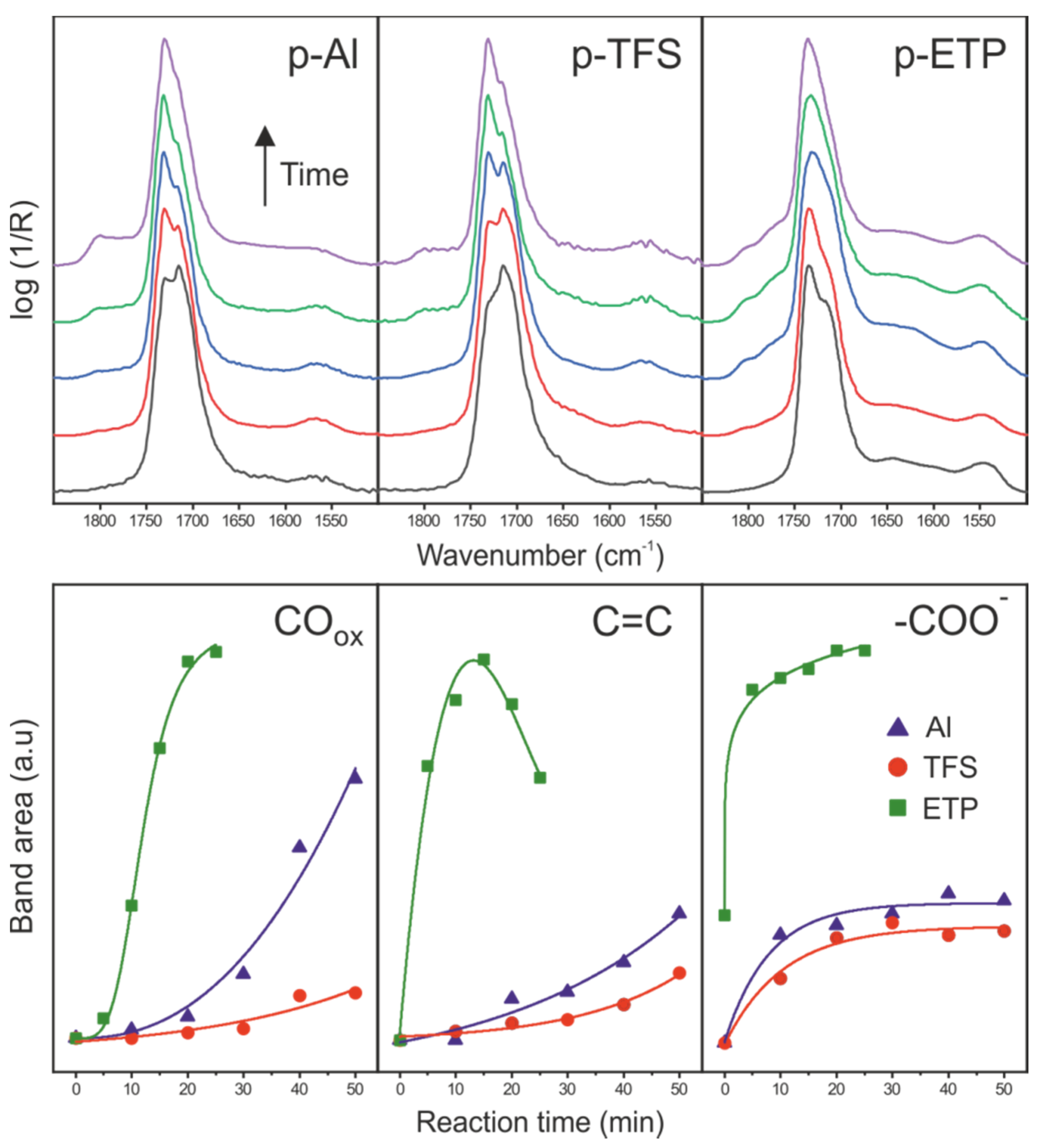
3.2. Comparative Chemical Characterization of Polyaleuritate Coatings
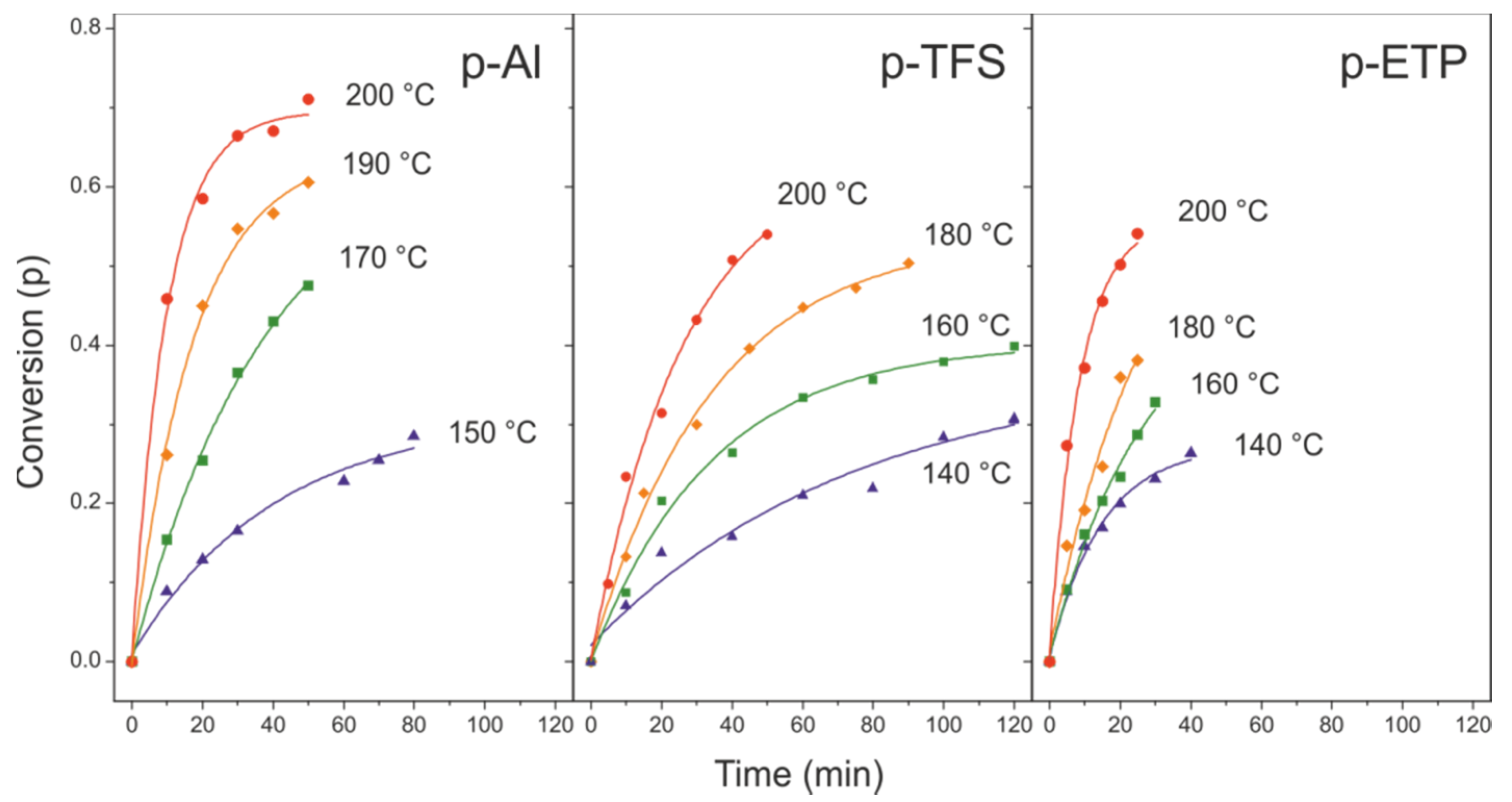
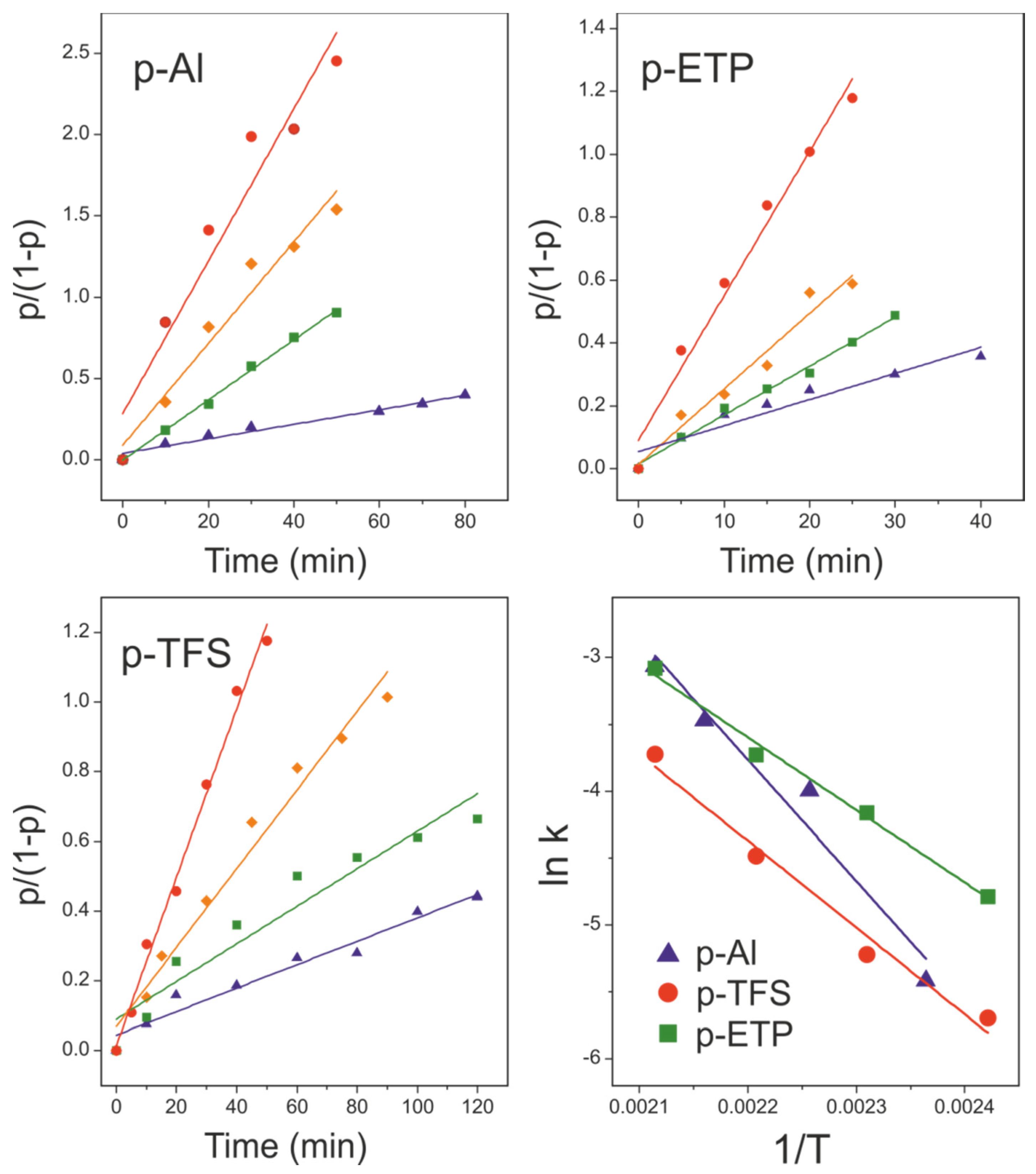
3.3. Kinetic Analysis of the Esterification Reaction in Polyaleuritate Films on Metals
3.3.1. Time-Dependent Method
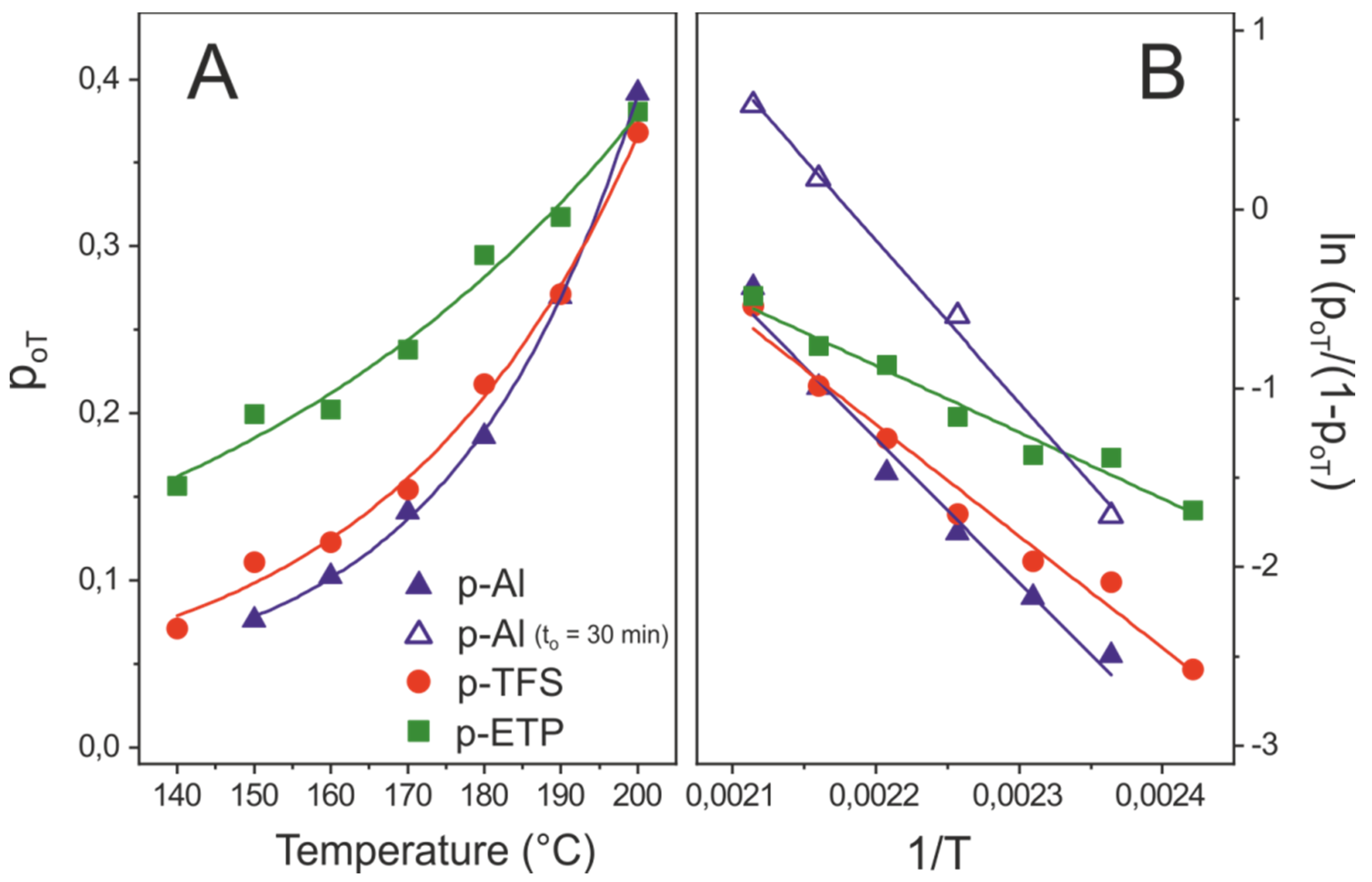
3.3.2. Temperature-Dependent Method
3.4. Side Reactions along the Formation of the Polyaleuritate Film on Metals by Heating in Air
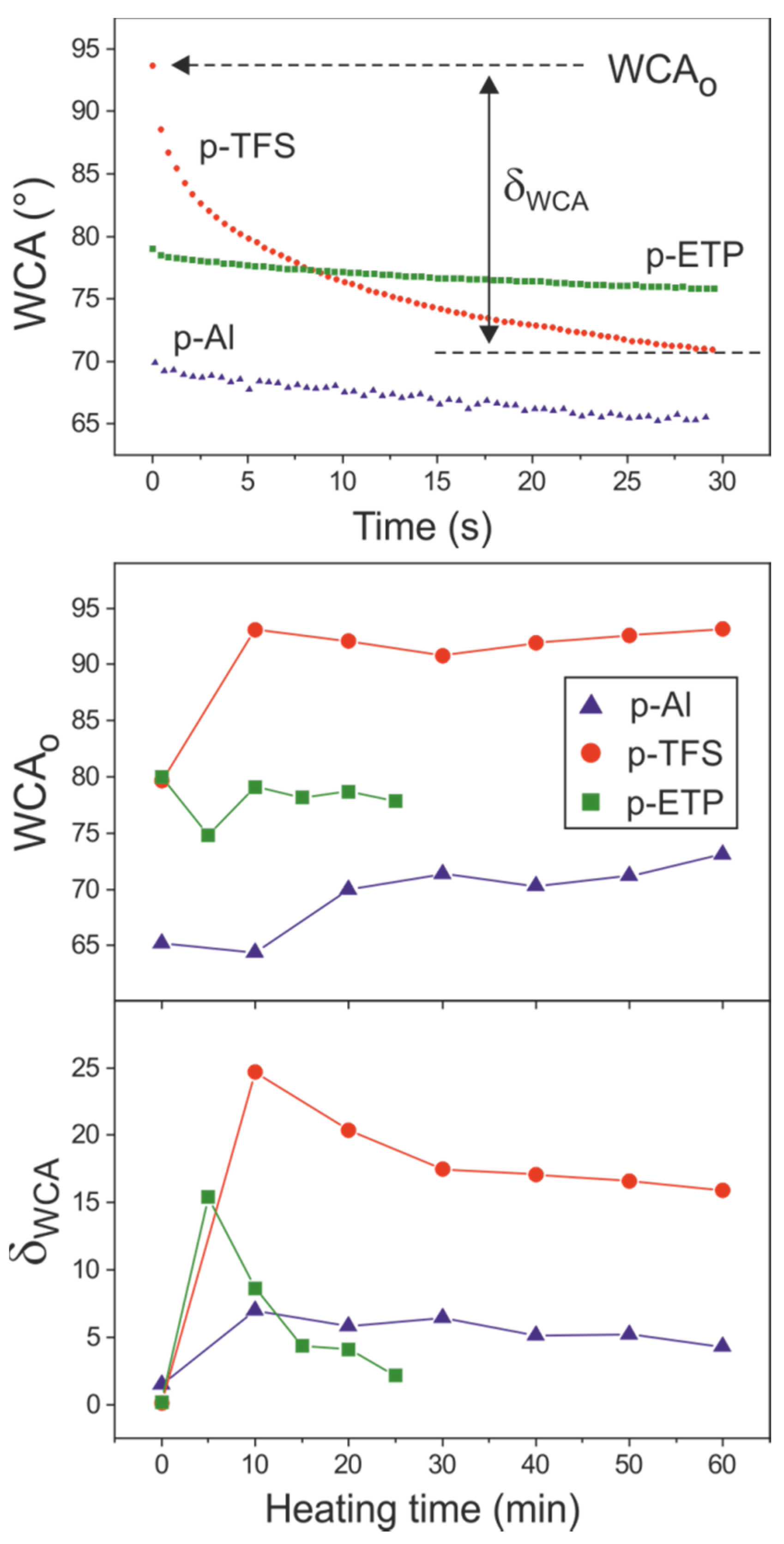
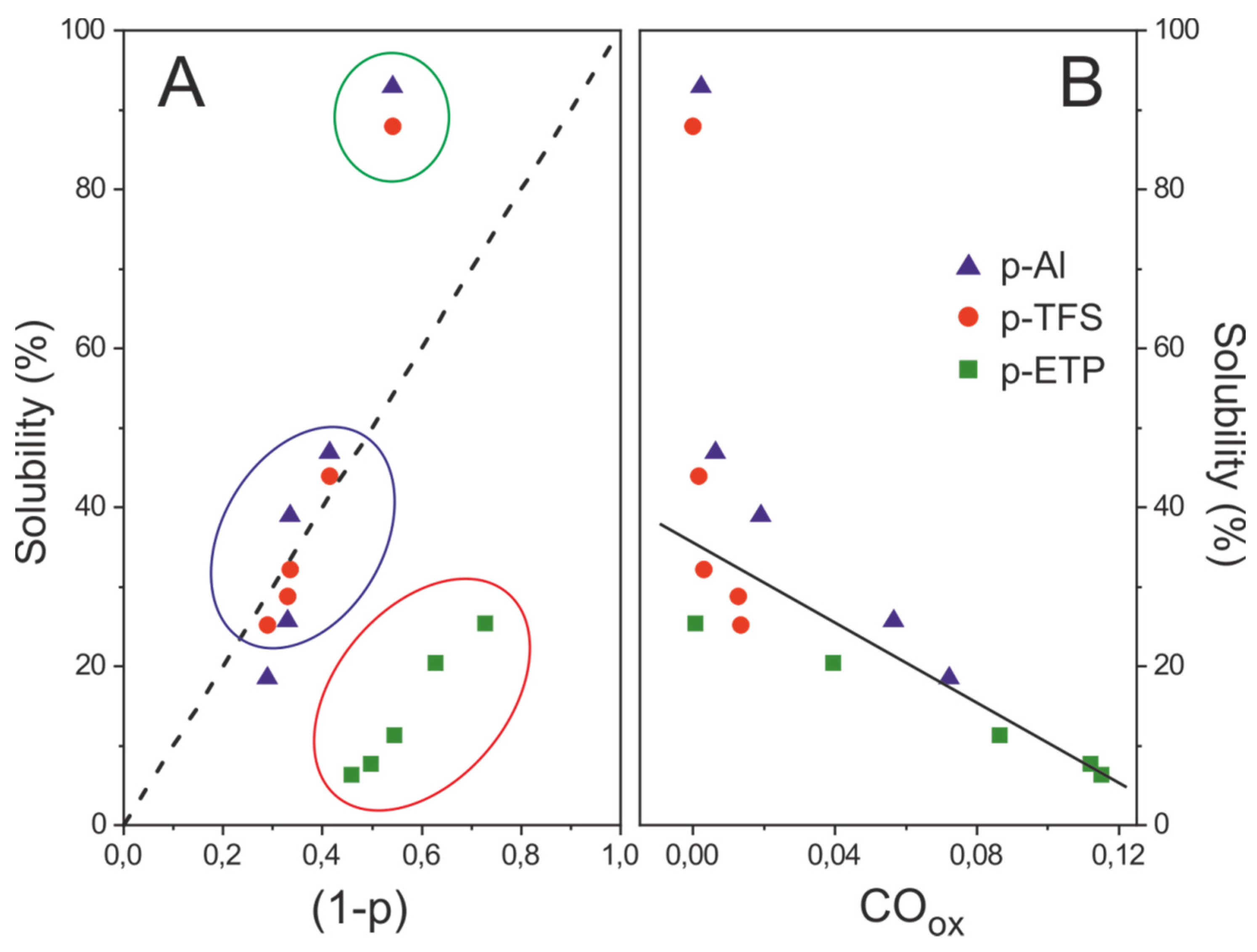
3.5. Surface Wettability and Solubility of Polyaleuritate Coatings
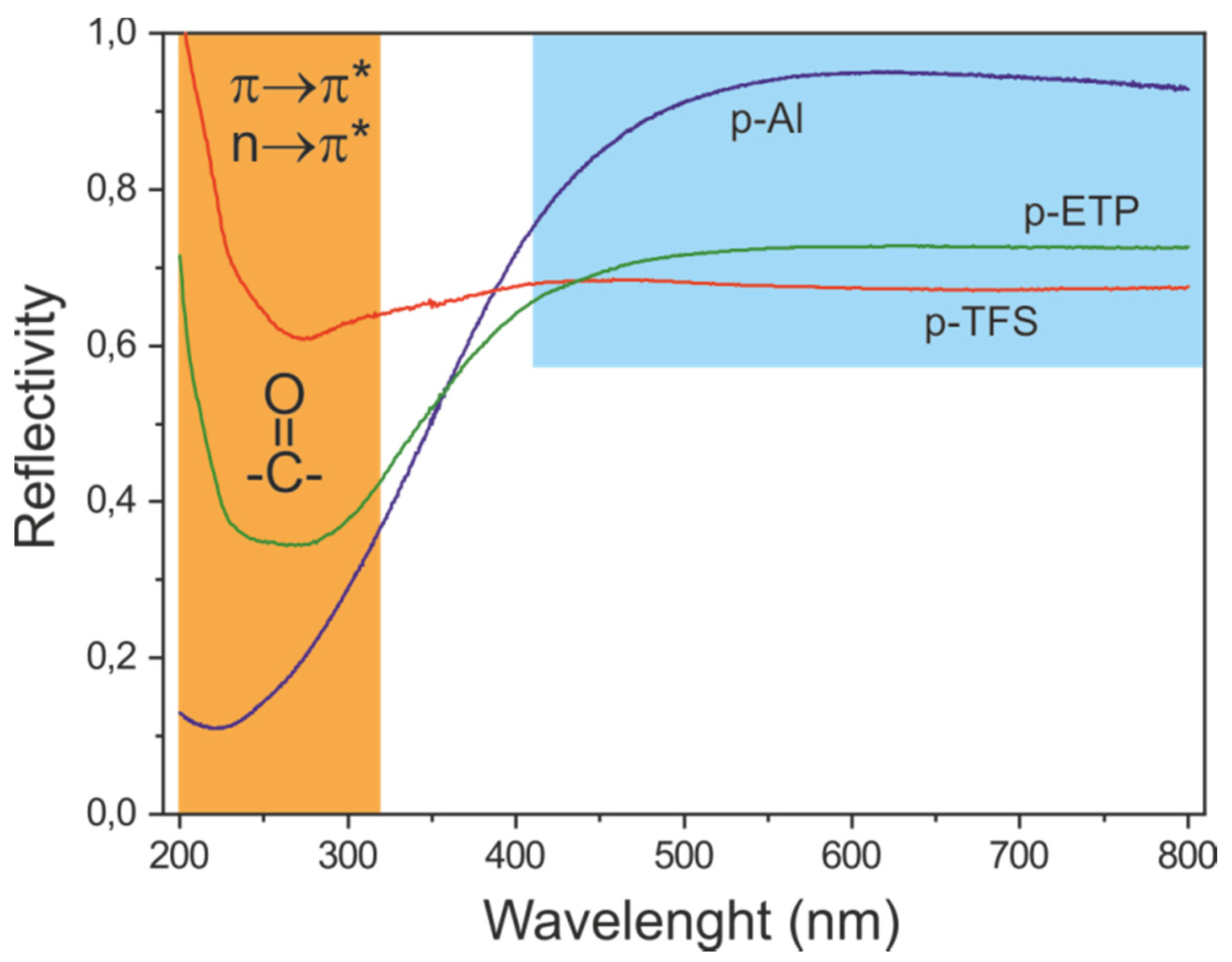
3.6. Light Absorption and Reflection of Polyaleuritate-Coated Metals
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Available online: https://www.researchandmarkets.com/reports/4544662/global-canned-food-market-growth-trends-and (accessed on 2 March 2020).
- Center for the Evaluation of Risks to Human Reproduction, National Toxicology Program, US Department of Health and Human Services. NTP-CERHR Monograph on the potential human reproductive and development effects of bisphenol A. Ntp Cerhr Mon 2008, 22, v–vii. [Google Scholar]
- Joint FAO/WHO Expert Meeting to Review Toxicological and Health Aspects of Bisphenol A; Food and Agriculture Organization of the United Nations and World Health Organization: Ottawa, ON, Canada, 2010.
- Opinion of the French Food Safety Agency on the Critical Analysis of the Results of a Developmental Neurotoxicity Study of Bisphenol A Together with Other Recently-Published Data on Its Toxic Effects; AFSSA-Request no. 2009-SA-0270; Agence Française de Sécurité Sanitaire des Aliments: Maisons-Alfort, France, 2010.
- Opinion of the French Agency for Food, Environmental and Occupational Health & Safety on the Assessment of the Risks Associated with Bisphenol A for Human Health, and on Toxicological Data and Data on the Use of Bisphenols S, F, M, AP, AF and BADGE; ANSES Opinions request no. 2009-SA-0331 and no. 2010-SA-0197; ANSES: Maisons-Alfort, France, 2013.
- Rochester, J.R. Bisphenol A and human health: A review of the literature. Reprod. Toxicol. 2013, 42, 132–135. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; Kondo, F.; Katayama, Y. Human exposure to bisphenol A. Toxicology 2016, 226, 79–89. [Google Scholar] [CrossRef]
- Lakind, J.S.; Naiman, D.Q. Daily intake of bisphenol A and potential sources of exposure: 2005–2006 national health and nutrition examination survey. J. Expo. Sci. Environ. Epid. 2011, 21, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Carwile, J.L.; Ye, X.; Zhou, X.; Calafat, A.M.; Michels, K.B. Canned soup consumption and urinary bisphenol A: A randomized crossover trial. JAMA-J. Am. Med. Assoc. 2011, 306, 2218–2219. [Google Scholar] [CrossRef]
- Rudel, R.A.; Gray, J.M.; Engel, C.L.; Rawsthorne, T.W.; Dodson, R.E.; Ackerman, J.M.; Rizzo, J.; Nudelman, J.L.; Brody, J.G. Food packing and bisphenol A and bis(2-ethyhexyl) phthalate exposure: Findings from a dietary intervention. Environ. Health Perspect. 2011, 119, 914–920. [Google Scholar] [CrossRef]
- Cao, X.-L.; Corriveau, J.; Popovic, S. Bisphenol A in canned food products from Canadian markets. J. Food Protect. 2010, 73, 1085–1089. [Google Scholar] [CrossRef]
- Noonan, G.O.; Ackerman, L.K.; Begley, T.H. Concentration of bisphenol A in highly consumed canned foods on the U.S. market. J. Agric. Food Chem. 2011, 59, 7178–7185. [Google Scholar] [CrossRef]
- Lakind, J.S. Can coatings for foods and beverages: Issues and options. Int. J. Technol. Policy Manag. 2013, 13, 80–95. [Google Scholar] [CrossRef]
- Robertson, G.L. Food Packaging: Principles and Practice; Taylor & Francis: Boca Raton, FL, USA, 2006. [Google Scholar]
- Oldring, P.T.K.; Nehring, U. Packaging Materials 7, Metal Packaging for Foodstuff, Packaging Materials; ILSI Europe Report Series 2007; ILSI: Washington, DC, USA, 2007. [Google Scholar]
- Crimmins, M.; Malotky, D.L.; Romick, J.D. Polyolefin Dispersion Compositions for Making High Vapor Transport Hydrophobic Coatings. US Patent US9701824B2, 11 July 2017. [Google Scholar]
- Domínguez, E.; Heredia-Guerrero, J.A.; Heredia, A. The biophysical design of plant cuticles: An overview. New Phytol. 2011, 189, 938–949. [Google Scholar] [CrossRef]
- Benítez, J.J.; Heredia-Guerrero, J.A.; Guzmán-Puyol, S.; Domínguez, E.; Heredia, A. Polyester films obtained by noncatalyzed melt-condensation polymerization of aleuritic (9,10,16-trihydroxyhexadecanoic) acid in air. J. Appl. Polym. Sci. 2015, 132, 41328. [Google Scholar] [CrossRef]
- Benítez, J.J.; Guzmán-Puyol, S.; Cruz-Carrillo, M.A.; Ceseracciu, L.; González Moreno, A.; Heredia, A.; Heredia-Guerrero, J.A. Insoluble and thermostable polyhydroxyesters from a renewable natural occurring polyhydroxylated fatty acid. Front. Chem. 2019, 7, 643. [Google Scholar] [CrossRef] [PubMed]
- Pinkasova, P.; Chen, H.; Verhoeven, M.W.G.M.; Sukhishvili, S.; Du, H. Thermally annealed Ag nanoparticles on anodized aluminium oxide for SERS sensing. RSC Adv. 2013, 3, 17954–17961. [Google Scholar] [CrossRef]
- Stefanov, P.; Stoychev, D.; Stoycheva, M.; Marinova, T. XPS and SEM studies of chromium oxide films chemically formed on stainless steel 316L. Mater. Chem. Phys. 2000, 65, 212–215. [Google Scholar] [CrossRef]
- Che, Y.; Han, Z.; Luo, B.; Xia, D.; Shi, J.; Gao, Z.; Wang, J. Corrosion mechanism differences of tinplate in aerated and deaerated citric acid solution. Int. J. Electrochem. Sci. 2012, 7, 9997–10007. [Google Scholar]
- Bellamy, L.J. The Infrared Spectra of Complex Molecules, 3rd ed.; Chapman and Hall: London, UK, 1975. [Google Scholar]
- Benítez, J.J.; Heredia-Guerrero, J.A.; de Vargas-Parody, M.I.; Cruz-Carrillo, M.A.; Morales-Flórez, V.; de la Rosa-Fox, N.; Heredia, A. Biodegradable polyester films from renewable aleuritic acid: Surface modifications induced by melt-polycondensation in air. J. Phys. D Appl. Phys. 2016, 49, 175601. [Google Scholar] [CrossRef]
- Benítez, J.J.; Heredia-Guerrero, J.A.; Guzmán-Puyol, S.; Barthel, J.M.; Domínguez, E.; Heredia, A. Polyhydroxyester films obtained by non-catalyzed melt-polycondensation of natural occurring fatty polyhydroxyacids. Front. Mater. 2015, 2, 59. [Google Scholar] [CrossRef]
- Haque, M.Z.; Faruq, M.O. Chemical investigation on thermally polymerized aleuritic acid. J. Bangladesh Acad. Sci. 2000, 24, 171–177. [Google Scholar]
- Tiwari, N.J.; Sawant, S.B. Behenic acid esters: Kinetics and properties. Eur. J. Lipid Sci. Technol. 2005, 107, 30–35. [Google Scholar] [CrossRef]
- Chang, W.L.; Karalis, T. Polyesterification reactions of adipic acid-based polyesters. J. Polym. Sci. A Polym. Chem. 1993, 31, 493–504. [Google Scholar] [CrossRef]
- Maliger, R.; Halley, P.J.; Cooper-White, J.J. Poly(glycerol-sebacate) bioelastomers-kinetics of step-growth reactions using Fourier Transform (FT)-Raman spectroscopy. J. Appl. Polym. Sci. 2013, 127, 3980–3986. [Google Scholar] [CrossRef]
- Bikiaris, D.N.; Achilias, D.S. Synthesis of poly(alkylene succinate) biodegradable polyesters, part II: Mathematical modelling of the polycondensation reaction. Polymer 2008, 49, 3677–3685. [Google Scholar] [CrossRef]
- Bawn, C.E.H.; Huglin, M.B. The kinetics of the polycondensation of 12-hydroxystearic acid. Polymer 1962, 3, 257–262. [Google Scholar] [CrossRef]
- Masutani, K.; Kimura, Y. PLA synthesis. From the monomer to the polymer. In Poly(lactic acid) Science and Technology: Processing, Properties, Additives and Applications; Jiménez, A., Peltzer, M., Ruseckaite, R., Eds.; RSC Polymer Chemistry Series; Royal Society of Chemistry: London, UK, 2015; Volume 12, pp. 3–36. [Google Scholar]
- Mello, V.M.; Pousa, G.P.A.G.; Pereira, M.S.C.; Dias, I.M.; Suarez, P.A.Z. Metal oxides as heterogeneous catalysts for esterification of fatty acids obtained from soybean oil. Fuel Process. Technol. 2011, 92, 53–57. [Google Scholar] [CrossRef]
- Wan, Z.; Lim, J.K.; Hameed, B.H. Chromium-tungsten heterogeneous catalysts for the esterification of palm fatty acid distillate to fatty acid methyl ester. J. Taiwan Inst. Chem. E. 2015, 54, 64–70. [Google Scholar] [CrossRef]
- Martinez-Urrutia, A.; Fernandez de Arroiabe, P.; Ramirez, M.; Martinez-Aguirre, M.; Mounir Bou-Ali, M. Contact angle measurement for LiBr aqueous solutions on different surface materials used in absorption systems. Int. J. Refrig. 2018, 95, 182–188. [Google Scholar] [CrossRef]
- Villa, f.; Marengo, M.; De Coninck, J. A new model to predict the influence of surface temperature on contact angle. Sci. Rep. 2018, 8, 6549. [Google Scholar] [CrossRef]
- Kunst, S.R.; Ludwig, G.A.; Piaggio Cardoso, H.R.; Andrade Santana, J.; Vitorino Sarmento, V.H.; de Fraga Malfatti, C. Hybrid films with (trimethoxysilylpropyl) methacrylate (TMSM), poly (methyl methacrylate) PMMA and tetraethoxysilane (TEOS) applied on tinplate. Mater. Res. 2014, 17, 75–81. [Google Scholar] [CrossRef]
- Bao, Z.; Wang, C.; Zhang, Y.; Zhao, Q.-Z. Modification of wettability of stainless steel by picosecond laser surface microstructuring. Photon. Res. 2015, 3, 180–183. [Google Scholar] [CrossRef]
- Prajitno, D.H. Effect of surface roughness on contact angle measurement of nanofluid on surface stainless steel 304 by sessile drop method. J. Phys. Conf. Ser. 2016, 739, 012029. [Google Scholar] [CrossRef]
- Pavia, D.L.; Lampman, G.M.; Kriz, G.S.; Vyvyan, J.A. Introduction to Spectroscopy; Cengage Learning: Belmont, MA, USA, 2009; Chapter 7; Brooks/Cole. [Google Scholar]
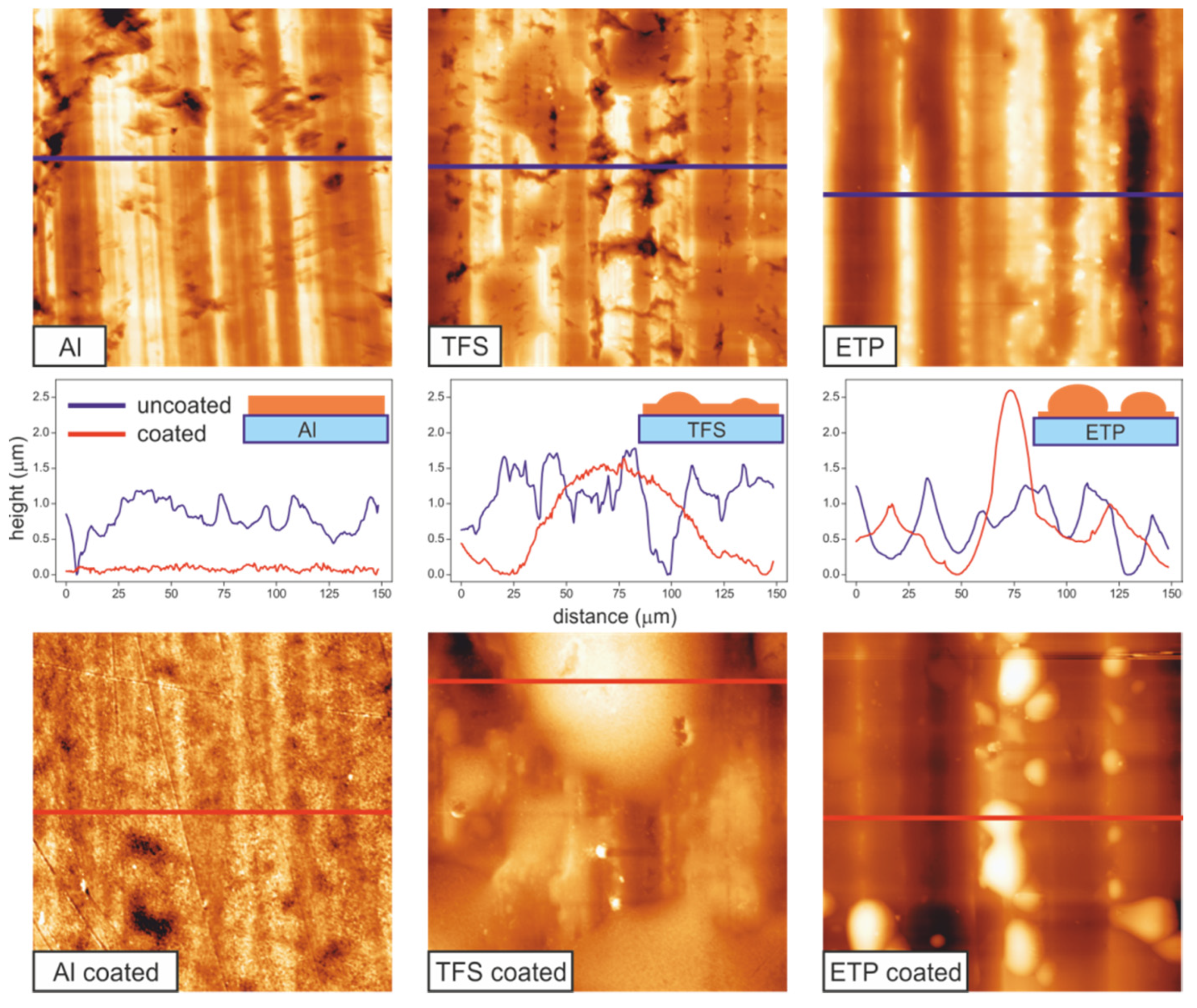
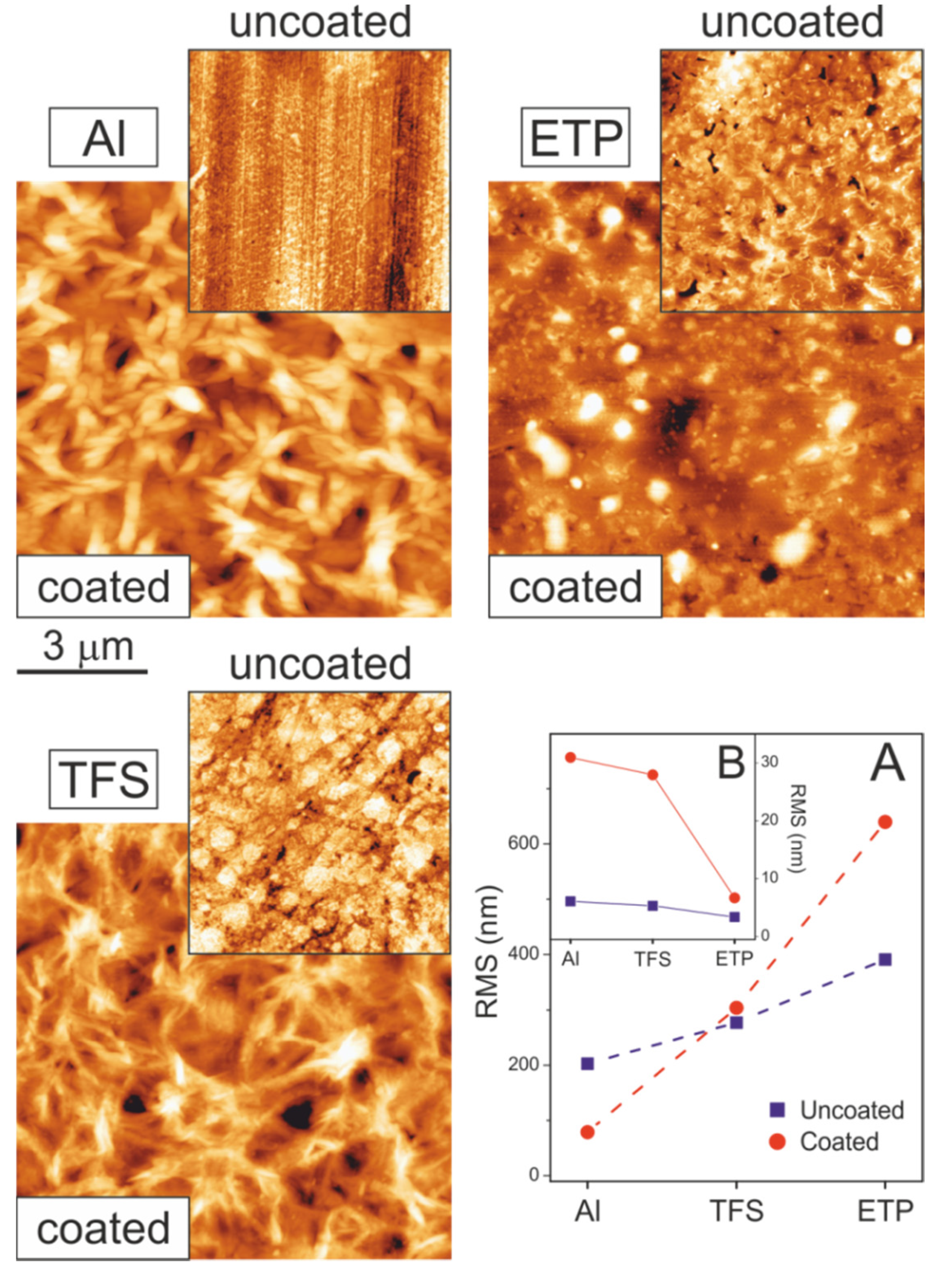
| Large | Small | ||||
|---|---|---|---|---|---|
| RMS (nm) | havg. (nm) | hmax. (nm) | RMS (nm) | SF | |
| Uncoated | |||||
| Al | 203 ± 13 | 1083 ± 86 | 1676 ± 90 | 6.1 ± 0.2 | 1.0782 |
| TFS | 277 ± 63 | 1145 ± 141 | 2175 ± 128 | 5.3 ± 0.8 | 1.0453 |
| ETP | 391 ± 116 | 934 ± 288 | 1901 ± 387 | 3.4 ± 0.5 | 1.0094 |
| Coated | |||||
| p-Al | 79 ± 80 | 494 ± 278 | 1054 ± 319 | 31 ± 2 | 1.0368 |
| p-TFS | 304 ± 65 | 1179 ± 335 | 1936 ± 249 | 28 ± 2 | 1.0483 |
| p-ETP | 640 ± 102 | 1415 ± 329 | 3249 ± 322 | 6.7 ± 0.5 | 1.0117 |
| Support | Time-dependent method | Temperature-dependent method | ||
|---|---|---|---|---|
| lnA | Eact (kJ/mol) | lnA | Eact (kJ/mol) | |
| Al | 15.5 | 75 ± 11 | 13.4 (15.7) * | 67 ± 5 (76 ± 3) * |
| TFS | 9.2 | 54 ± 5 | 9.5 | 52 ± 4 |
| ETP | 7.7 | 45 ± 3 | 4.3 | 31 ± 2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benítez, J.J.; Osbild, S.; Guzman-Puyol, S.; Heredia, A.; Heredia-Guerrero, J.A. Bio-Based Coatings for Food Metal Packaging Inspired in Biopolyester Plant Cutin. Polymers 2020, 12, 942. https://doi.org/10.3390/polym12040942
Benítez JJ, Osbild S, Guzman-Puyol S, Heredia A, Heredia-Guerrero JA. Bio-Based Coatings for Food Metal Packaging Inspired in Biopolyester Plant Cutin. Polymers. 2020; 12(4):942. https://doi.org/10.3390/polym12040942
Chicago/Turabian StyleBenítez, José J., Sonja Osbild, Susana Guzman-Puyol, Antonio Heredia, and José A. Heredia-Guerrero. 2020. "Bio-Based Coatings for Food Metal Packaging Inspired in Biopolyester Plant Cutin" Polymers 12, no. 4: 942. https://doi.org/10.3390/polym12040942
APA StyleBenítez, J. J., Osbild, S., Guzman-Puyol, S., Heredia, A., & Heredia-Guerrero, J. A. (2020). Bio-Based Coatings for Food Metal Packaging Inspired in Biopolyester Plant Cutin. Polymers, 12(4), 942. https://doi.org/10.3390/polym12040942








