Thermochromic Fibers via Electrospinning
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Formulation
2.2.1. Liquid Crystal Formulation
2.2.2. Polymer Solution Preparation
2.2.3. Electrospinning Blend Preparation
2.3. Fiber Spinning
2.3.1. Blend Electrospinning
2.3.2. Coaxial Electrospinning
2.4. Characterization
2.4.1. Solution Characterization
2.4.2. Fiber Characterization
3. Results
3.1. Blend Fiber Spinning—Polystyrene (PS)/LC Fibers
3.1.1. Polystyrene/LC Blend Preparation
3.1.2. Effect of LC Concentration
3.1.3. Effect of LC Formulation
3.1.4. Blend Solution Properties
3.2. Coaxial Fiber Spinning—Polyvinylpyrrolidone (PVP)/LC Fibers
3.2.1. PVP Electrospinning
3.2.2. Coaxial Electrospinning
3.3. Thermochromic Fiber Characterization
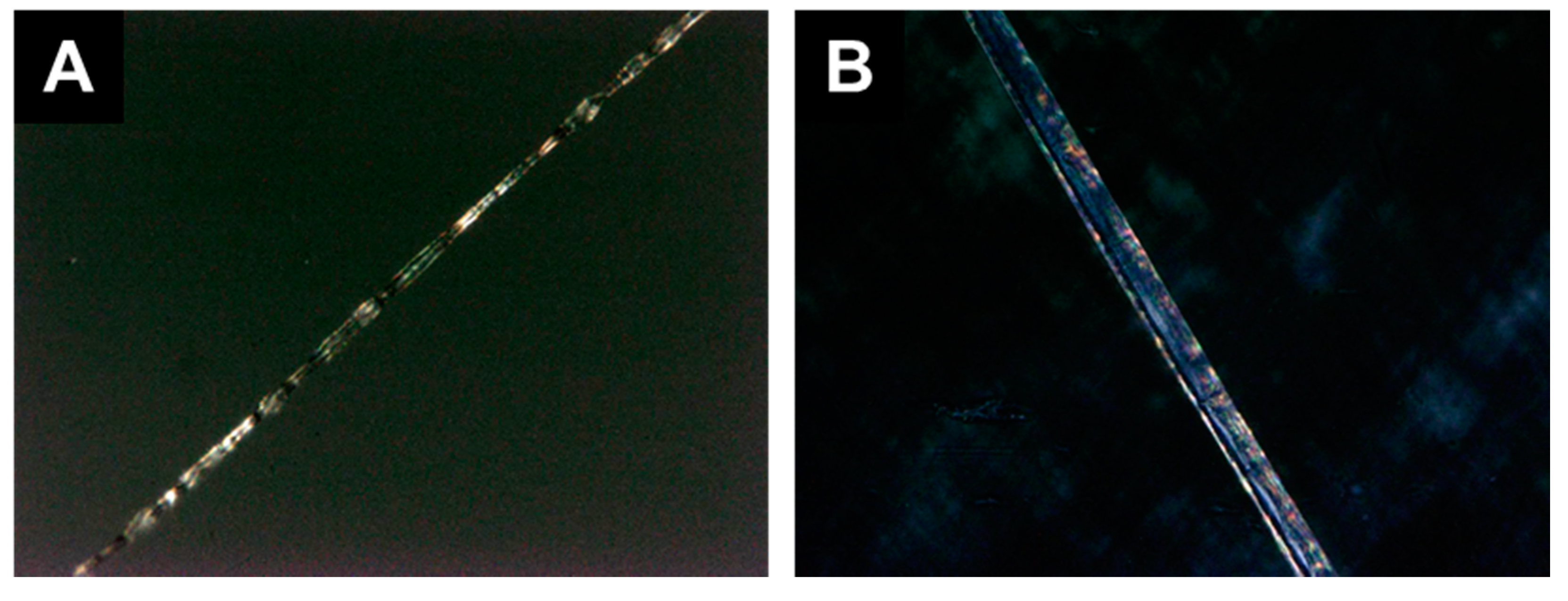
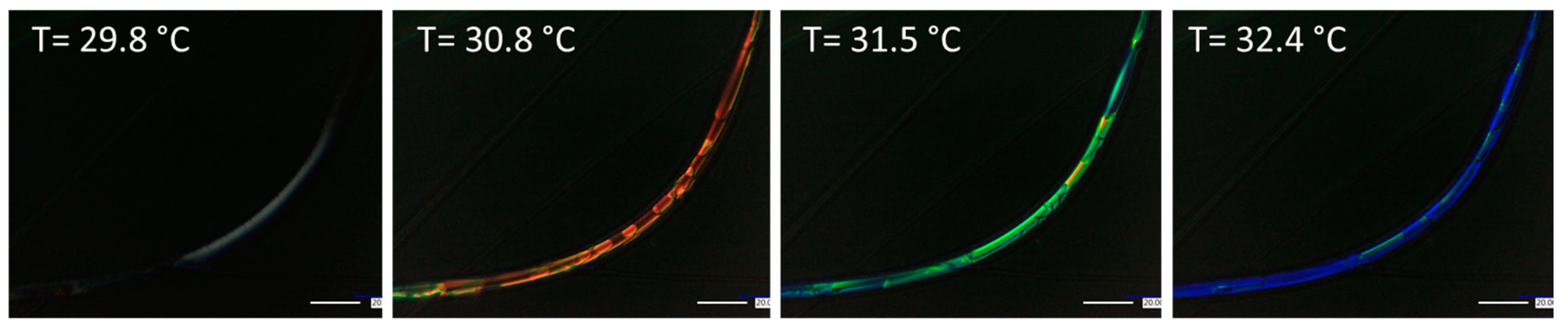
3.3.1. Blend Fibers
3.3.2. Coaxial Fibers
3.4. Fiber Spinning Method Comparison
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kye, Y.; Kim, C.; Lagerwall, J. Multifunctional Responsive Fibers Produced by Dual Liquid Crystal Core Electrospinning. J. Mater. Chem. C 2015, 3, 8979–8985. [Google Scholar] [CrossRef]
- Honaker, L.W.; Vats, S.; Anyfantakis, M.; Lagerwall, J.P.F. Elastic Sheath—Liquid Crystal Core Fibres Achieved by Microfluidic Wet Spinning. J. Mater. Chem. C 2019, 7, 11588–11596. [Google Scholar] [CrossRef]
- Bertocchi, M.J.; Ratchford, D.C.; Casalini, R.; Wynne, J.H.; Lundin, G. Electrospun Polymer Fibers Containing a Liquid Crystal Core: Insights into Semi Fl Exible Confinement. J. Phys. Chem. C 2018, 122, 16964–16973. [Google Scholar] [CrossRef]
- Guan, Y.; Zhang, L.; Wang, D.; West, J.L.; Fu, S. Preparation of Thermochromic Liquid Crystal Microcapsules for Intelligent Functional Fi Ber. Mater. Des. 2018, 147, 28–34. [Google Scholar] [CrossRef]
- Wang, J.; Jákli, A.; Guan, Y.; Fu, S.; West, J.; Wang, J.; Jákli, A.; West, J. Developing Liquid-Crystal Functionalized Fabrics for Wearable Sensors Frontline Technology. Inf. Disp. 2017, 33, 16–20. [Google Scholar]
- Enz, E.; La Ferrara, V.; Scalia, G. Confinement-Sensitive Optical Response of Cholesteric Liquid Crystals in Electrospun Fibers. ACS Nano 2013, 7, 6627–6635. [Google Scholar] [CrossRef]
- Lagerwall, J.P.F.; Mccann, J.T.; Formo, E. Coaxial Electrospinning of Microfibres with Liquid Crystal in the Core. Chem. Commun. 2008, 2008, 5420–5422. [Google Scholar] [CrossRef]
- Robinson, A.J.; Kyratzis, I.L. Combinatorial Approach for the Rapid Determination of Thermochromic Behavior of Binary and Ternary Cholesteric Liquid Crystalline Mixtures. ACS Comb. Sci. 2012, 14, 605–612. [Google Scholar] [CrossRef]
- Sage, I. Thermochromic Liquid Crystals Thermochromic Liquid Crystals. Liq. Cryst. 2011, 38, 1551–1561. [Google Scholar] [CrossRef]
- Kim, D.K.; Hwang, M.; Lagerwall, J.P.F. Liquid Crystal Functionalization of Electrospun Polymer Fibers. J. Polym. Sci. Part B Polym. Phys. 2013, 51, 855–867. [Google Scholar] [CrossRef]
- Pochaczevsky, R.; Wexler, C.E.; Meyers, P.H.; Epstein, J.A.; Marc Joseph, A. Liquid Crystal Thermography of the Spine and Extremities. J. Neurosurg. 1982, 56, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Ochoa, A.D.; Baughn, J.W.; Byerley, A.R. A New Technique for Dynamic Heat Transfer Measurements and Flow Visualization Using Liquid Crystal Thermography. Int. J. Heat Fluid FLow 2005, 26, 264–275. [Google Scholar] [CrossRef]
- Muwanga, R.; Hassan, I. Local Heat Transfer Measurements in Microchannels Using Liquid Crystal Thermography: Methodology Development and Validation. J. Heat Transf. 2006, 128, 617–626. [Google Scholar] [CrossRef]
- Van derWesthuizen, J.E.; Dirker, W.J.; Meyer, J.P. Implementation of Liquid Crystal Thermography to Determine Wall Temperatures and Heat Transfer Coefficients in a Tube-in-Tube Heat Exchanger. Exp. Heat Transf. 2016, 29, 632–656. [Google Scholar] [CrossRef][Green Version]
- Stasiek, J.; Jewartowski, M.; Kowalewski, T.A. The Use of Liquid Crystal Thermography in Selected Technical and Medical Applications—Recent Development. J. Cryst. Process. Technol. 2014, 4, 46–59. [Google Scholar] [CrossRef]
- Popov, V.M.; Klimenko, A.S.; Pokanevich, A.P.; Gavrilyuk, I.I.; Moshel, N.V. Liquid-Crystal Thermography of Hot Spots on Electronic Components. Russ. Microelectron. 2007, 36, 392–401. [Google Scholar] [CrossRef]
- Ekkad, S.V.; Han, J. A Transient Liquid Crystal Thermography Technique for Gas Turbine Heat Transfer Measurements. Meas. Sci. Technol. 2000, 11, 957–968. [Google Scholar] [CrossRef]
- Reneker, D.H.; Chun, I. Nanometre Diameter Fibres of Polymer, Produced by Electrospinning. Nanotechnology 1996, 7, 216–223. [Google Scholar] [CrossRef]
- Helgeson, M.E.; Grammatikos, K.N.; Deitzel, J.M.; Wagner, N.J. Theory and Kinematic Measurements of the Mechanics of Stable Electrospun Polymer Jets. Polymer 2008, 49, 2924–2936. [Google Scholar] [CrossRef]
- Rutledge, G.C.; Fridrikh, S.V. Formation of Fibers by Electrospinning. Adv. Drug Deliv. Rev. 2007, 59, 1384–1391. [Google Scholar] [CrossRef]
- Thompson, C.J.; Chase, G.G.; Yarin, A.L.; Reneker, D.H. Effects of Parameters on Nanofiber Diameter Determined from Electrospinning Model. Polymer 2007, 48, 6913–6922. [Google Scholar] [CrossRef]
- Teo, W.E.; Ramakrishna, S. A Review on Electrospinning Design and Nanofibre Assemblies. Nanotechnology 2006, 17, R89–R106. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Xia, Y. Electrospinning of Nanofibers: Reinventing the Wheel? Adv. Mater. 2004, 16, 1151–1170. [Google Scholar] [CrossRef]
- Guan, Y.; Zhang, L.; Li, M.; West, J.L.; Fu, S. Preparation of Temperature-Response Fi Bers with Cholesteric Liquid Crystal Dispersion. Colloids Surfaces A 2018, 546, 212–220. [Google Scholar] [CrossRef]
- Yu, H.J.; Fridrikh, S.V.; Rutledge, G.C. Production of Submicrometer Diameter Fibers by Two-Fluid Electrospinning. Adv. Mater. 2004, 16, 1562–1566. [Google Scholar] [CrossRef]
- Moghe, A.K.; Gupta, B.S. Co-Axial Electrospinning for Nanofiber Structures: Preparation and Applications. Polym. Rev. 2008, 48, 353–377. [Google Scholar] [CrossRef]
- Enz, E.; Lagerwall, J. Electrospun Microfibres with Temperature Sensitive Iridescence from Encapsulated Cholesteric Liquid Crystal Electrospun Microfibres with Temperature Sensitive Iridescence from Encapsulated Cholesteric Liquid Crystal. J. Mater. Chem. 2010, 20, 6866–6872. [Google Scholar] [CrossRef]
- Tang, C.; Saquing, C.D.; Morton, S.W.; Glatz, B.N.; Kelly, R.M.; Khan, S.A. Cross-Linked Polymer Nanofibers for Hyperthermophilic Enzyme Immobilization: Approaches to Improve Enzyme Performance. ACS Appl. Mater. Interfaces 2014, 6, 11899–11906. [Google Scholar] [CrossRef]
- Forward, K.M.; Flores, A.; Rutledge, G.C. Production of Core/Shell Fibers by Electrospinning from a Free Surface. Chem. Eng. Sci. 2013, 104, 250–259. [Google Scholar] [CrossRef]
- Buyuktanir, E.A.; Frey, M.W.; West, J.L. Self-Assembled, Optically Responsive Nematic Liquid Crystal/Polymer Core-Shell Fi Bers: Formation and Characterization. Polymer 2010, 51, 4823–4830. [Google Scholar] [CrossRef]
- Wang, J.; West, J.L. Morphology Tuning of Electrospun Liquid Crystal/Polymer Fibers. ChemPhysChem 2016, 17, 3080–3085. [Google Scholar] [CrossRef] [PubMed]
- Dotivala, A.C.; Puthuveetil, K.P.; Tang, C. Shear Force Fiber Spinning: Process Parameter and Polymer Solution Property Considerations. Polymers 2019, 11, 294. [Google Scholar] [CrossRef] [PubMed]
- Andreas, J.M.; Hauser, E.A.; Tucker, W.B. Boundary tension by pendant drops. J. Phys. Chem. 1937, 42, 1001–1019. [Google Scholar] [CrossRef]
- Lin, K.; Chua, K.N.; Christopherson, G.T.; Lim, S.; Mao, H.Q. Reducing Electrospun Nanofiber Diameter and Variability Using Cationic Amphiphiles. Polymer 2007, 48, 6384–6394. [Google Scholar] [CrossRef]
- Singer, J.C.; Giesa, R.; Schmidt, H.W. Shaping Self-Assembling Small Molecules into Fibres by Melt Electrospinning. Soft Matter 2012, 8, 9972–9976. [Google Scholar] [CrossRef]
- Singer, J.C.; Ringk, A.; Giesa, R.; Schmidt, H.W. Melt Electrospinning of Small Molecules. Macromol. Mater. Eng. 2015, 300, 259–276. [Google Scholar] [CrossRef]
- Manasco, J.L.; Saquing, C.D.; Tang, C.; Khan, S.A. Cyclodextrin Fibers via Polymer-Free Electrospinning. RSC Adv. 2012, 2. [Google Scholar] [CrossRef]
- Andrady, A.L. Science and Technology of Polymer Nanofibers; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Li, Z.; Wang, C. Effects of Working Parameters on Electrospinning. In One-Dimensional Nanostructures: Electrospinning Technique and Unique Nanofibers; Li, Z., Wang, C., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 15–28. [Google Scholar] [CrossRef]
- Ramakrishna, S. Electrospinning Process. In An Introduction to Electrospinning and Nanofibers; World Scientific: Singapore, 2005. [Google Scholar]
- Levit, S.L.; Nguyen, J.; Hattrup, N.P.; Rabatin, B.E.; Stwodah, R.; Vasey, C.L.; Zeevi, M.P.; Gillard, M.; Angelo, P.A.D.; Swana, K.W.; et al. Color Space Transformation-Based Algorithm for Evaluation of Thermochromic Behavior of Cholesteric Liquid Crystals Using Polarized Light Microscopy. ACS Omega 2020. [Google Scholar] [CrossRef]
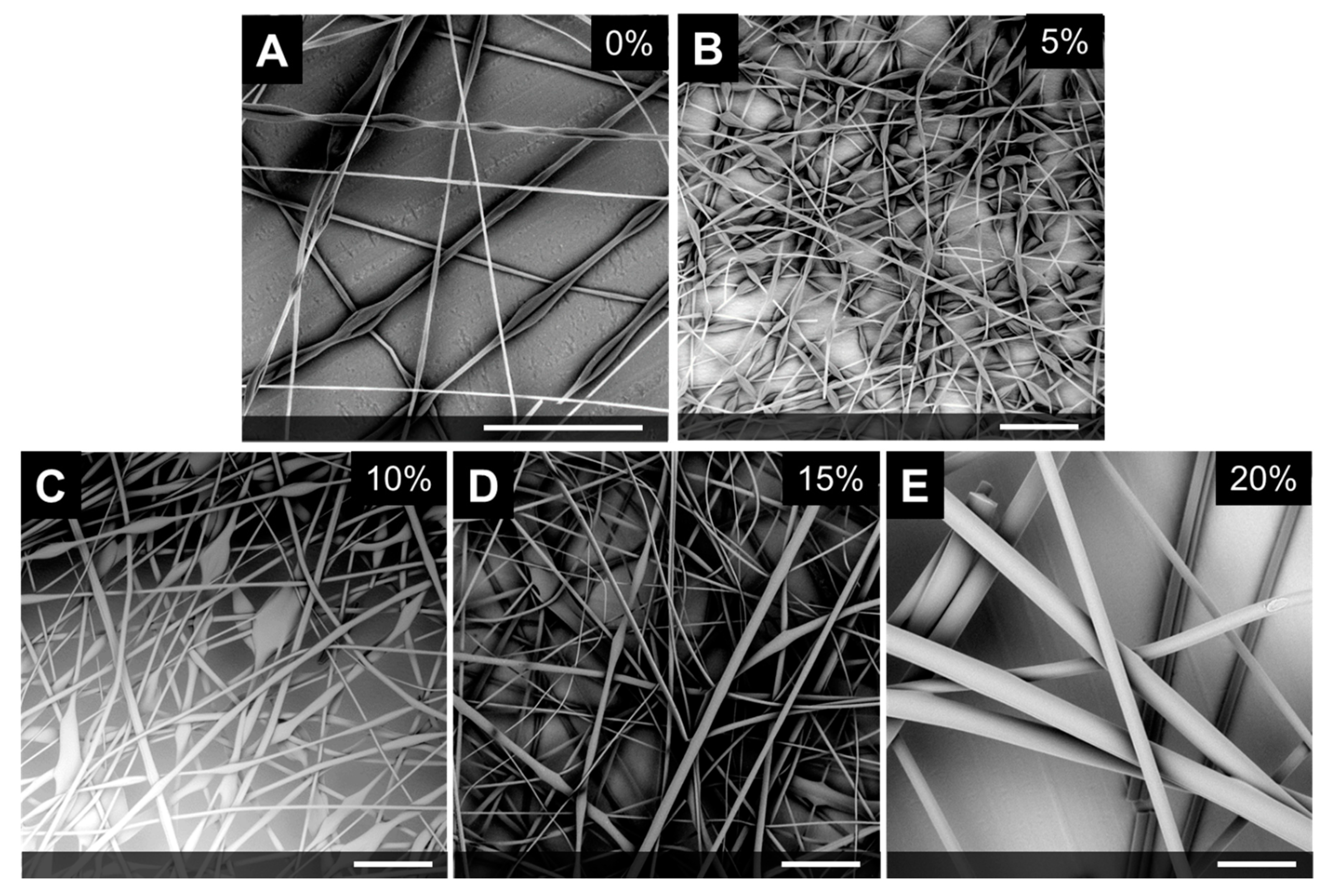
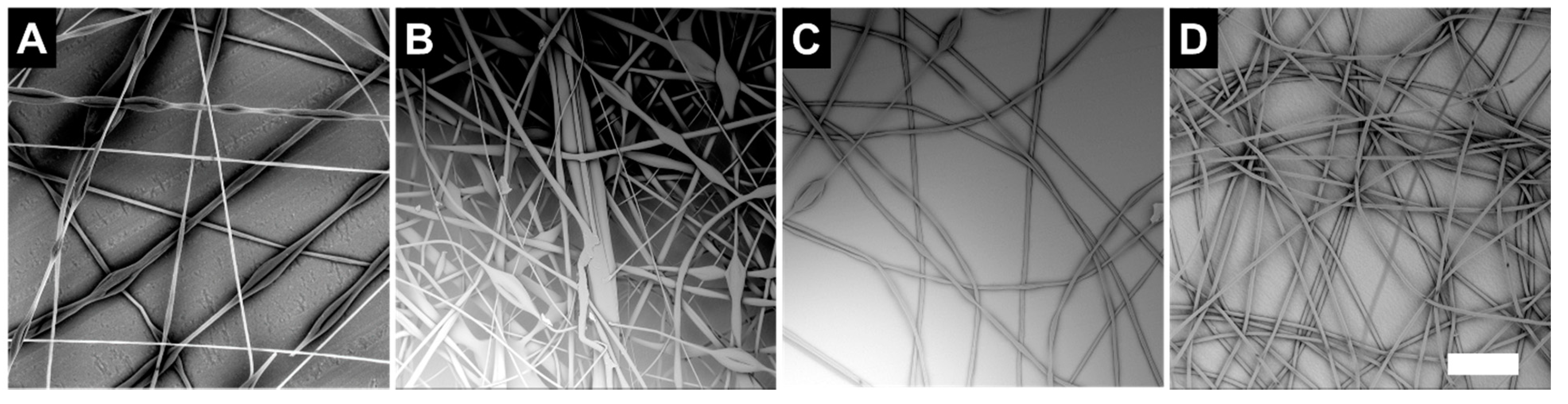
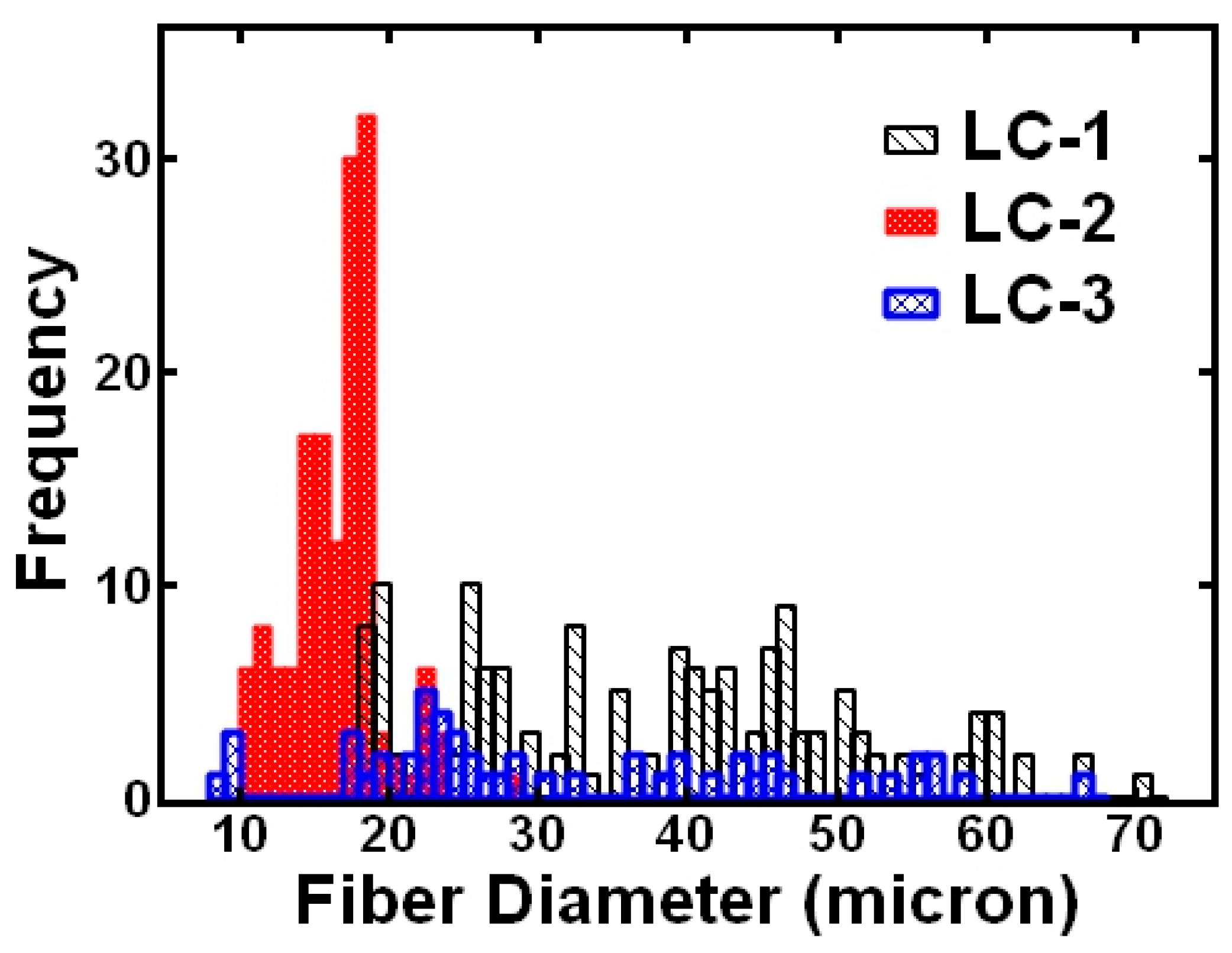

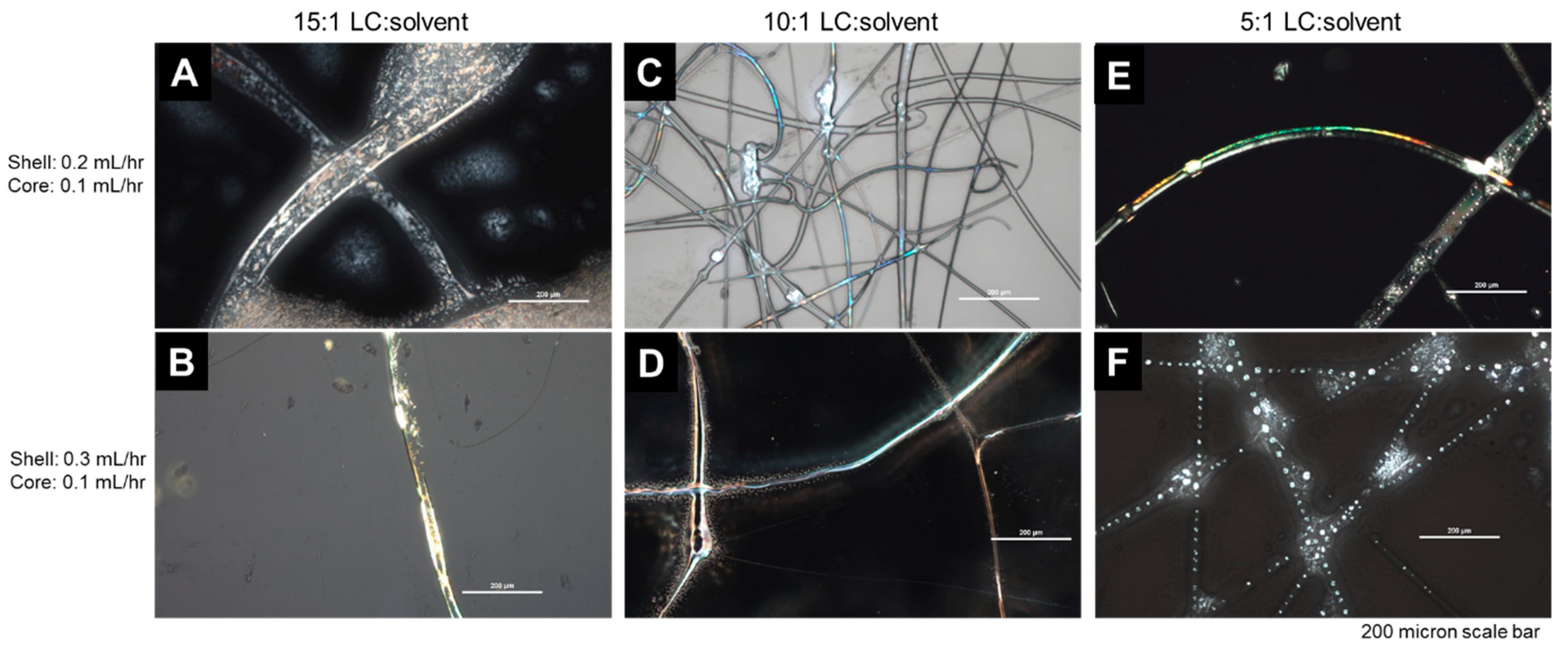

| Sample | COC (wt.%) | CP (wt.%) | CB (wt.%) |
|---|---|---|---|
| LC-1 | 60 | 30 | 10 |
| LC-2 | 45 | 45 | 10 |
| LC-3 | 30 | 60 | 10 |
| Sample | Avg. Fiber Diameter (μm) | Std. Dev. (μm) | Coefficient of Variation (%) |
|---|---|---|---|
| 20 wt.% PS | 4.8 | 2.6 | 53 |
| 20 wt.% PS, 20 wt.% LC-1 | 38 | 13 | 34 |
| 20 wt.% PS, 20 wt.% LC-2 | 17 | 3 | 18 |
| 20 wt.% PS, 20 wt.% LC-3 | 32 | 15 | 47 |
| Sample | Viscosity (Pa*s) | Surface Tension (mN/m) | Conductivity (μS/cm) |
|---|---|---|---|
| 20 wt.% PS | 1.1 ± 0.3 | 23 ± 1 | N.D. |
| 20 wt.% PS, 20 wt.% LC-1 | 1.3 ± 0.1 | 31 ± 2 | N.D. |
| 20 wt.% PS, 20 wt.% LC-2 | 1.7 ± 0.1 | 33 ± 2 | N.D. |
| 20 wt.% PS, 20 wt.% LC-3 | 1.0 ± 0.2 | 22 ± 2 | N.D. |
| Blend | Coaxial | |
|---|---|---|
| Fiber Diameter | 17 ± 3 μm | 23 ± 20 μm |
| Nominal Fiber Throughput (g/hr) | 0.2 | 0.5 |
| Nominal LC loading (wt. LC/wt. polymer) | 50% | 60% |
| Thermochromic behavior | No | Yes |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, J.; Stwodah, R.M.; Vasey, C.L.; Rabatin, B.E.; Atherton, B.; D’Angelo, P.A.; Swana, K.W.; Tang, C. Thermochromic Fibers via Electrospinning. Polymers 2020, 12, 842. https://doi.org/10.3390/polym12040842
Nguyen J, Stwodah RM, Vasey CL, Rabatin BE, Atherton B, D’Angelo PA, Swana KW, Tang C. Thermochromic Fibers via Electrospinning. Polymers. 2020; 12(4):842. https://doi.org/10.3390/polym12040842
Chicago/Turabian StyleNguyen, Jimmy, Ratib M. Stwodah, Christopher L. Vasey, Briget E. Rabatin, Benjamin Atherton, Paola A. D’Angelo, Kathleen W. Swana, and Christina Tang. 2020. "Thermochromic Fibers via Electrospinning" Polymers 12, no. 4: 842. https://doi.org/10.3390/polym12040842
APA StyleNguyen, J., Stwodah, R. M., Vasey, C. L., Rabatin, B. E., Atherton, B., D’Angelo, P. A., Swana, K. W., & Tang, C. (2020). Thermochromic Fibers via Electrospinning. Polymers, 12(4), 842. https://doi.org/10.3390/polym12040842





