Using 3-Isocyanatopropyltrimethoxysilane to Decorate Graphene Oxide with Nano-Titanium Dioxide for Enhancing the Anti-Corrosion Properties of Epoxy Coating
Abstract
1. Introduction
2. Experimental
2.1. Materials
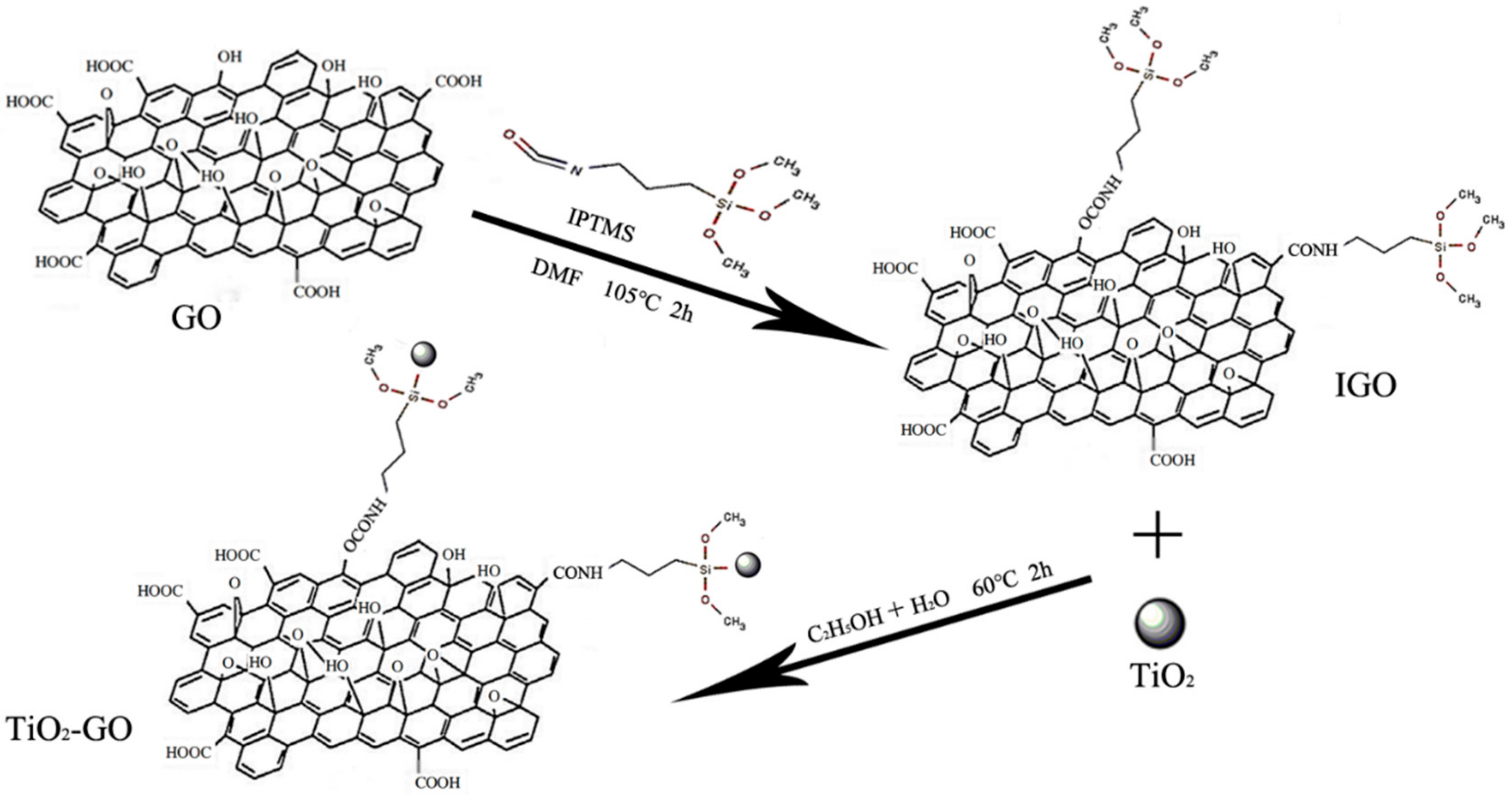
2.2. Preparation of TiO2–GO Nanocomposites
2.3. Preparation of TiO2–GO/Pure Epoxy (EP) Coating
2.4. Characterization
2.4.1. TiO2–GO Nano-Particles Characterizations
2.4.2. Test of TiO2–GO /EP Coatings
3. Results and Discussion
3.1. FT-IR Spectroscopy
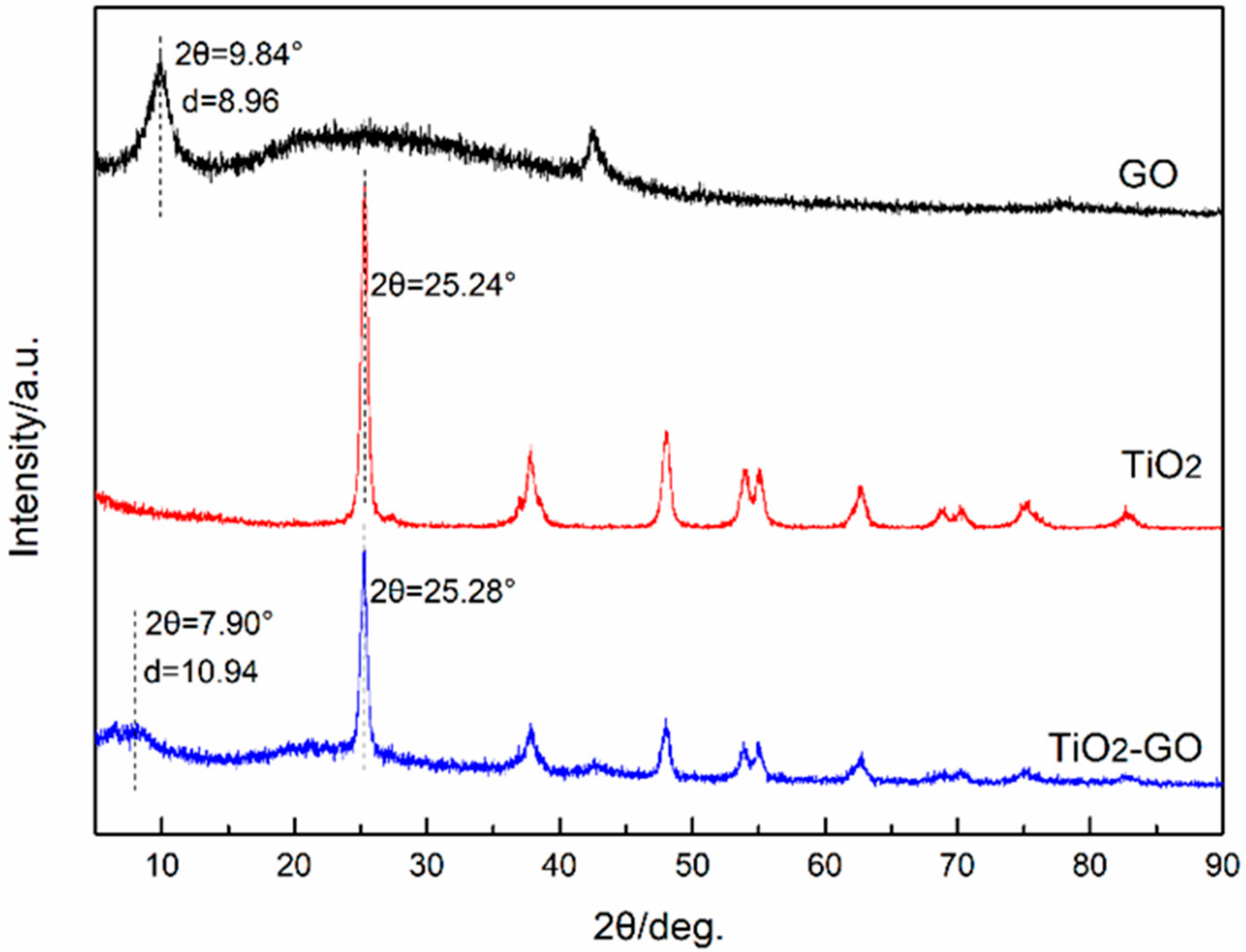
3.2. XRD Analysis
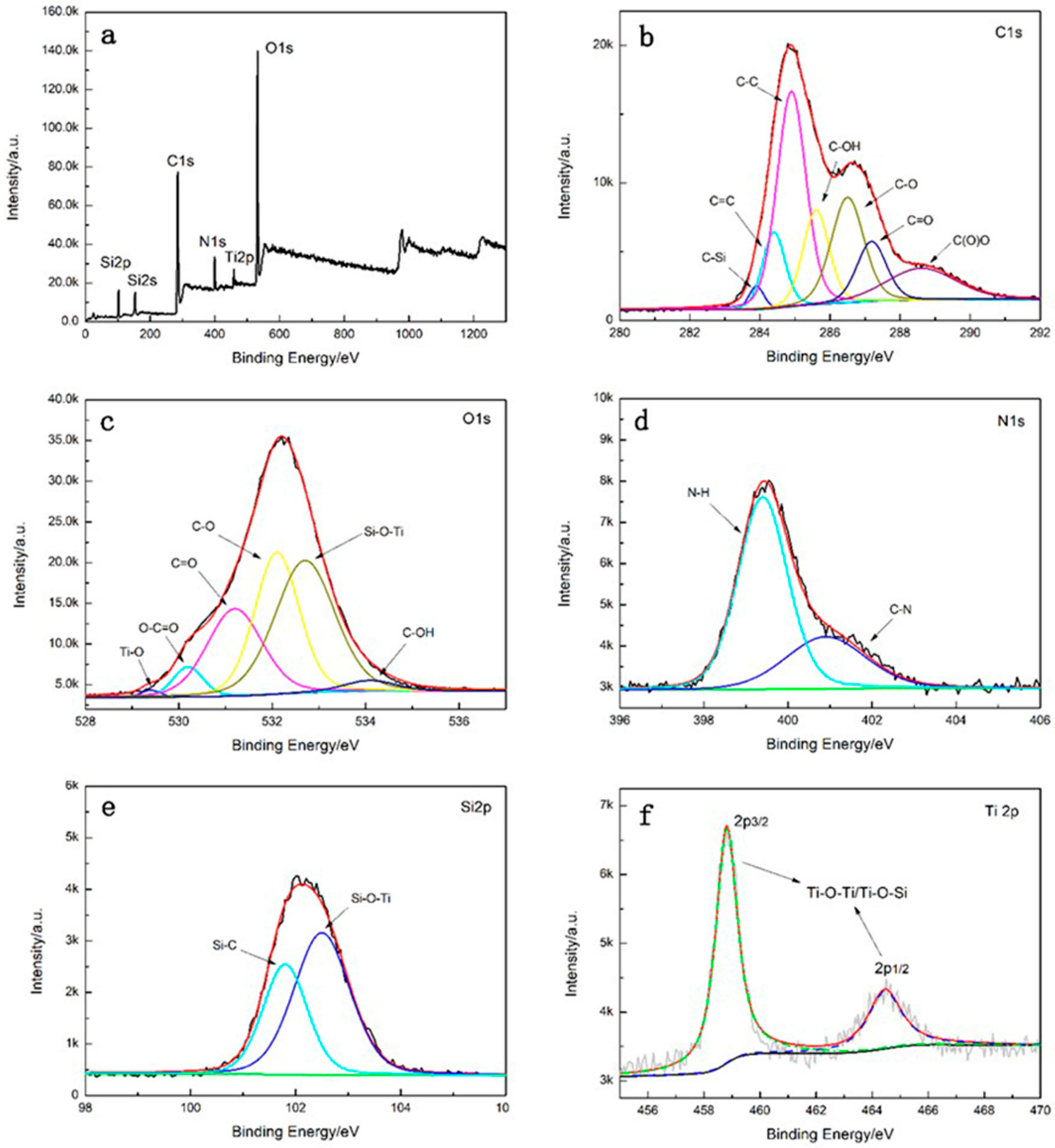
3.3. XPS Analysis
3.4. SEM Morphology
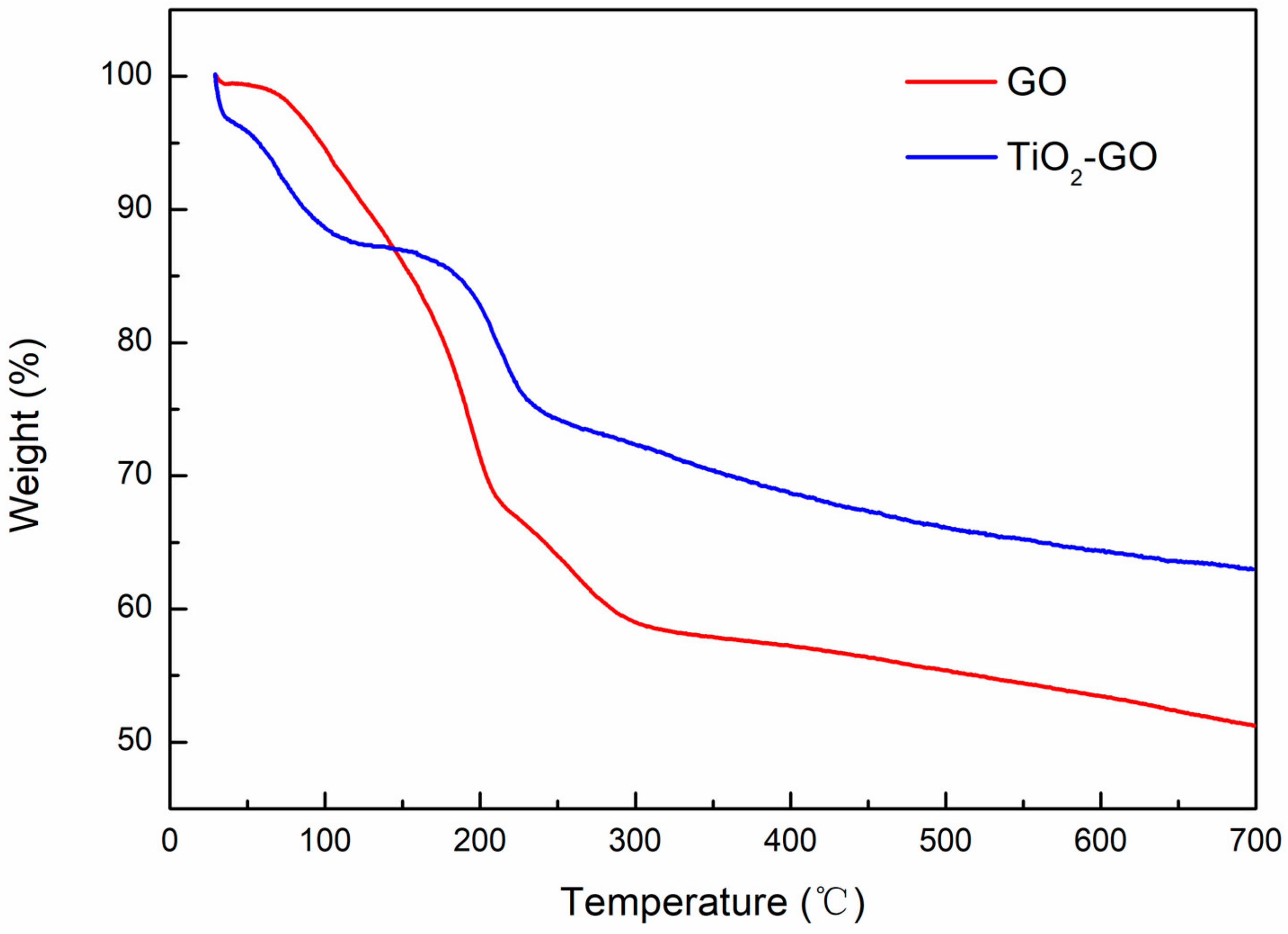
3.5. TG Analysis
3.6. Dispersion Test
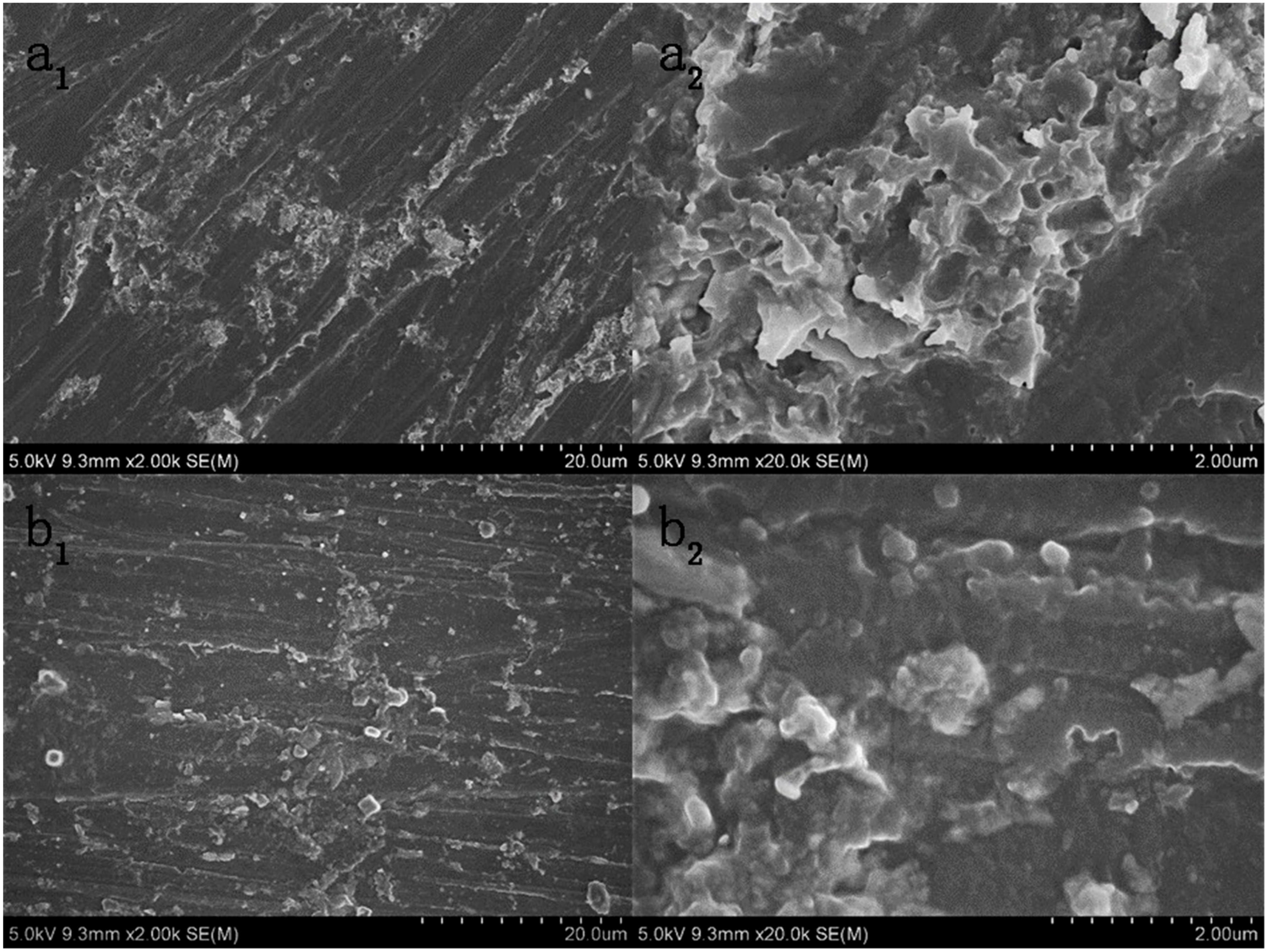
3.7. The Morphology of Epoxy Coatings
3.8. EIS Measurement
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tang, Z.L. Corrosion science general-purpose data model and interface (II): OOD design and corrosion data markup language (CDML). Sci. China Ser. E Technol. Sci. 2008, 51, 1850–1857. [Google Scholar] [CrossRef]
- Gong, L.X.; Zhao, L.; Tang, L.C.; Liu, H.Y.; Mai, Y.W. Balanced electrical, thermal and mechanical properties of epoxy composites filled with chemically reduced graphene oxide and rubber nanoparticles. Compos. Sci. Technol. 2015, 121, 104–114. [Google Scholar] [CrossRef]
- Vaisakh, S.S.; Mahesh, K.V.; Balanand, S.; Metz, R.; Hassanzadeh, M.; Ananthakumar, S. MAX phase ternary carbide derived 2-D ceramic nanostructures [CDCN] as chemically interactive functional fillers for damage tolerant epoxy polymer nanocomposites. RSC Adv. 2015, 5, 16521–16531. [Google Scholar] [CrossRef]
- Majd, M.T.; Shahrabi, T.; Ramezanzadeh, B. The role of neodymium based thin film on the epoxy/steel interfacial adhesion and corrosion protection promotion. Appl. Surf. Sci. 2019, 464, 516–533. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, L.B.; Zhou, A.G.; Shen, C.J.; Dai, Y.H.; Liu, F.F.; Chen, J.F.; Li, P.; Hu, Q.K. Effects of 2-D transition metal carbide Ti2CTx on properties of epoxy composites. RSC Adv. 2016, 6, 87341–87352. [Google Scholar] [CrossRef]
- Ramezanzadeh, B.; Moghadam, M.H.M.; Shohani, N.; Mandavian, M. Effects of highly crystalline and conductive polyaniline/graphene oxide composites on the corrosion protection performance of a zinc-rich epoxy coating. Chem. Eng. J. 2017, 320, 363–375. [Google Scholar] [CrossRef]
- Ye, Y.W.; Zhang, D.W.; Liu, T.; Liu, Z.Y.; Liu, W.; Pu, J.B.; Chen, H.; Zhao, H.C.; Li, X.G. Improvement of anticorrosion ability of epoxy matrix in simulate marine environment by filled with superhydrophobic POSS-GO nanosheets. J. Hazard. Mater. 2019, 364, 244–255. [Google Scholar] [CrossRef]
- Wang, T.; Ge, H.Y.; Zhang, K.L. A novel core-shell silica@graphene straticulate structured antistatic anticorrosion composite coating. J. Alloy. Compd. 2018, 745, 705–715. [Google Scholar] [CrossRef]
- Zaman, I.; Phan, T.T.; Kuan, H.C.; Meng, Q.S.; La, L.T.B.; Luong, L.; Youssf, O.; Ma, J. Epoxy/graphene platelets nanocomposites with two levels of interface strength. Polymer 2011, 52, 1603–1611. [Google Scholar] [CrossRef]
- Chen, L.; Chai, S.G.; Liu, K.; Ning, N.Y.; Gao, J.; Liu, Q.F.; Chen, F.; Fu, Q. Enhanced epoxy/silica composites mechanical properties by introducing graphene oxide to the interface. ACS Appl. Mater. Interfaces 2012, 4, 4398–4404. [Google Scholar] [CrossRef]
- Liu, Y.W.; Chen, Y. Anticorrosion performance of epoxy-resin coating incorporating talcum powder loaded with sodium tungstate. Int. J. Electrochem. Sci. 2018, 13, 530–541. [Google Scholar] [CrossRef]
- Ahmed, N.M.; El-Gawad, W.M.A.; Souaya, E.R. Study on the corrosion protection performance of new ferrite/kaolin core-shell pigments in epoxy-based paints. Anti-Corros. Methods Mater. 2016, 63, 36–46. [Google Scholar] [CrossRef]
- Wan, Y.J.; Tang, L.C.; Gong, L.X.; Yan, D.; Li, Y.B.; Wu, L.B.; Jiang, J.X.; Lai, G.Q. Grafting of epoxy chains onto graphene oxide for epoxy composites with improved mechanical and thermal properties. Carbon 2014, 69, 467–480. [Google Scholar] [CrossRef]
- Li, G.; Cui, H.Y.; Zhou, J.C.; Hu, W.J. Improvements of nano-TiO2 on the long-term chloride resistance of concrete with polymer coatings. Coatings 2019, 9, 323. [Google Scholar] [CrossRef]
- Ma, Y.; Di, H.H.; Yu, Z.X.; Liang, L.; Lv, L.; Pan, Y.; Zhang, Y.Y.; Yin, D. Fabrication of silica-decorated graphene oxide nanohybrids and the properties of composite epoxy coatings research. Appl. Surf. Sci. 2016, 360, 936–945. [Google Scholar] [CrossRef]
- Palraj, S.; Selvaraj, M.; Maruthan, K.; Rajagopal, G. Corrosion and wear resistance behavior of nano-silica epoxy composite coatings. Prog. Org. Coat. 2015, 81, 132–139. [Google Scholar] [CrossRef]
- Ramezanzadeh, B.; Attar, M.M. Studying the effects of micro and nano sized ZnO particles on the corrosion resistance and deterioration behavior of an epoxy-polyamide coating on hot-dip galvanized steel. Prog. Org. Coat. 2011, 71, 314–328. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef]
- Park, K.S.; Yi, K.S. Bipolar supercurrent, differential conductance and critical current in a nano transistor of a graphene-based junction. J. Korean Phys. Soc. 2007, 50, 1873–1877. [Google Scholar] [CrossRef]
- Balandin, A.A.; Ghosh, S.; Bao, W.Z.; Calizo, I.; Teweldebrhan, D.; Miao, F.; Lau, C.N. Superior thermal conductivity of single-layer graphene. Nano Lett. 2008, 8, 902–907. [Google Scholar] [CrossRef]
- Lee, C.; Wei, X.D.; Kysar, J.W.; Hone, J. Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science 2008, 321, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Nair, R.R.; Blake, P.; Grigorenko, A.N.; Novoselov, K.S.; Booth, T.J.; Stauber, T.; Peres, N.M.R.; Geim, A.K. Fine structure constant defines visual transparency of graphene. Science 2008, 320, 1308. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.H.; Ocola, L.E.; Chen, J.H. Reduced graphene oxide for room-temperature gas sensors. Nanotechnology 2009, 20, 445502. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Cai, W.M.; Long, M.C.; Zhou, B.X.; Wu, Y.H.; Wu, D.Y.; Feng, Y.J. Synthesis of Visible-Light Responsive Graphene Oxide/TiO2 Composites with p/n Heterojunction. ACS Nano 2010, 4, 6425–6432. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.S.; Zhou, G.M.; Yin, L.C.; Ren, W.; Li, F.; Cheng, H.M. Graphene/metal oxide composite electrode materials for energy storage. Nano Energy 2012, 1, 107–131. [Google Scholar] [CrossRef]
- Lin, Y.T.; Don, T.M.; Wong, C.J.; Meng, F.C.; Lin, Y.J.; Lee, S.Y.; Lee, C.F.; Chiu, W.Y. Improvement of mechanical properties and anticorrosion performance of epoxy coatings by the introduction of polyaniline/graphene composite. Surf. Coat. Technol. 2019, 374, 1128–1138. [Google Scholar] [CrossRef]
- Pourhashem, S.; Vaezi, M.R.; Rashidi, A.; Bagherzadeh, M.R. Distinctive roles of silane coupling agents on the corrosion inhibition performance of graphene oxide in epoxy coatings. Prog. Org. Coat. 2017, 111, 47–56. [Google Scholar] [CrossRef]
- Wan, Y.J.; Gong, L.X.; Tang, L.C.; Wu, L.B.; Jiang, J.X. Mechanical properties of epoxy composites filled with silane-functionalized graphene oxide. Compos. Part A Appl. Sci. Manuf. 2014, 64, 79–89. [Google Scholar] [CrossRef]
- Yu, Z.X.; Di, H.H.; Ma, Y.; He, Y.; Liang, L.; Lv, L.; Ran, X.; Pan, Y.; Luo, Z. Preparation of graphene oxide modified by titanium dioxide to enhance the anti-corrosion performance of epoxy coatings. Surf. Coat. Technol. 2015, 276, 471–478. [Google Scholar] [CrossRef]
- Liu, J.H.; Yu, Q.; Yu, M.; Li, S.M.; Zhao, K.; Xue, B.; Zu, H. Silane modification of titanium dioxide-decorated graphene oxide nanocomposite for enhancing anticorrosion performance of epoxy coatings on AA-2024. J. Alloy. Compd. 2018, 744, 728–739. [Google Scholar] [CrossRef]
- Shi, H.W.; Liu, F.C.; Yang, L.H.; Han, E.H. Characterization of protective performance of epoxy reinforced with nanometer-sized TiO2 and SiO2. Prog. Org. Coat. 2008, 62, 359–368. [Google Scholar]
- Duong, H.P.; Hung, C.H.; Dao, H.C.; Le, M.D.; Chen, C.Y. Modification of TiO2 nanotubes with 3-aminopropyl triethoxysilane and its performances in nanocomposite coatings. New J. Chem. 2018, 42, 8745–8751. [Google Scholar]
- Botas, C.; Alvarez, P.; Blanco, P.; Granda, M.; Blanco, C.; Santamaria, R.; Romasanta, L.J.; Verdejo, R.; Lopez-Manchado, M.A.; Menendez, R. Graphene materials with different structures prepared from the same graphite by the Hummers and Brodie methods. Carbon 2013, 65, 156–164. [Google Scholar]
- Huang, N.M.; Lim, H.N.; Chia, C.H.; Yarmo, M.A.; Muhamad, M.R. Simple room-temperature preparation of high-yield large-area graphene oxide. Int. J. Nanomed. 2011, 6, 3443–3448. [Google Scholar]
- Remyamol, T.; John, H.; Gopinath, P. Synthesis and nonlinear optical properties of reduced graphene oxide covalently functionalized with polyaniline. Carbon 2013, 59, 308–314. [Google Scholar]
- Li, A.P.; Kan, C.Y.; Du, Y.; Liu, D.S. Study on the evolvement of structure in synthesis of urea-formaldehyde resins by FTIR. Acta Phys. Chim. Sin. 2006, 22, 873–877. [Google Scholar]
- Parhizkar, N.; Ramezanzadeh, B.; Shahrabi, T. Corrosion protection and adhesion properties of the epoxy coating applied on the steel substrate pre-treated by a sol-gel based silane coating filled with amino and isocyanate silane functionalized graphene oxide nanosheets. Appl. Surf. Sci. 2018, 439, 45–59. [Google Scholar]
- Parhizkar, N.; Shahrabi, T.; Ramezanzadeh, B. Synthesis and characterization of a unique isocyanate silane reduced graphene oxide nanosheets; screening the role of multifunctional nanosheets on the adhesion and corrosion protection performance of an amido-amine cured epoxy composite. J. Taiwan Inst. Chem. Eng. 2018, 82, 281–299. [Google Scholar]
- Pourhashem, S.; Vaezi, M.R.; Rashidi, A. Investigating the effect of SiO2-graphene oxide hybrid as inorganic nanofiller on corrosion protection properties of epoxy coatings. Surf. Coat. Technol. 2017, 311, 282–294. [Google Scholar]
- Ramezanzadeh, B.; Ahmadi, A.; Mandavian, M. Enhancement of the corrosion protection performance and cathodic delamination resistance of epoxy coating through treatment of steel substrate by a novel nanometric sol-gel based silane composite film filled with functionalized graphene oxide nanosheets. Corros. Sci. 2016, 109, 182–205. [Google Scholar]
- Park, S.; An, J.; Piner, R.D.; Jung, I.; Yang, D.; Velamakanni, A.; Nguyen, S.T.; Ruoff, R.S. Aqueous suspension and characterization of chemically modified graphene sheets. Chem. Mater. 2008, 20, 6592–6594. [Google Scholar] [CrossRef]
- Yu, Y.-H.; Lin, Y.-Y.; Lin, C.-H.; Chan, C.-C.; Huang, Y.-C. High-performance polystyrene/graphene-based nanocomposites with excellent anti-corrosion properties. Polym. Chem. 2014, 5, 535–550. [Google Scholar]
- Chang, K.C.; Ji, W.F.; Lai, M.C.; Hsiao, Y.R.; Hsu, C.H.; Chuang, T.L.; Wei, Y.; Yeh, J.M.; Liu, W.R. Correction: Synergistic effects of hydrophobicity and gas barrier properties on the anticorrosion property of PMMA nanocomposite coatings embedded with graphene nanosheets. Polym. Chem. 2014, 5, 1049–1056. [Google Scholar]
- Sun, W.; Wang, L.; Wu, T.; Pan, Y.; Liu, G. Synthesis of low-electrical-conductivity graphene/pernigraniline composites and their application in corrosion protection. Carbon 2014, 79, 605–614. [Google Scholar]
- Brusciotti, F.; Snihirova, D.V.; Xue, H.; Montemor, M.F.; Lamaka, S.V.; Ferreira, M.G.S. Hybrid epoxy–silane coatings for improved corrosion protection of Mg alloy. Corros. Sci. 2013, 67, 82–90. [Google Scholar]



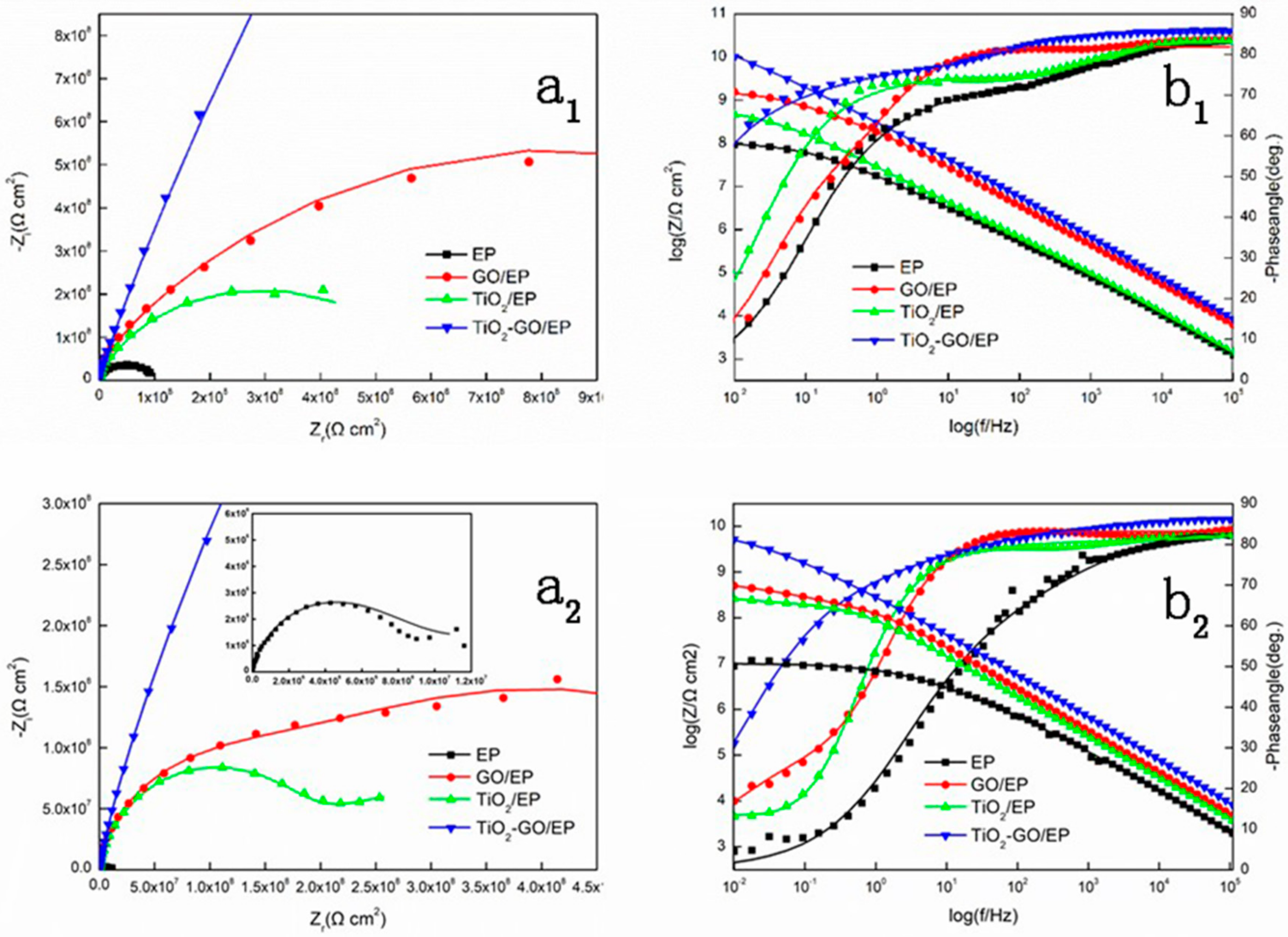

| Sample | Time/h | Rs(Ω cm2) | Qc | Rcr(Ω cm2) | Qdl | Rct(Ω cm2) | ||
|---|---|---|---|---|---|---|---|---|
| Y0(Ω−1 cm−2 sn) | n | Y0(Ω−1 cm−2 sn) | n | |||||
| EP | 1 h | 0.01 | 6.766 × 10−8 | 0.8895 | 5.64 × 107 | |||
| GO/EP | 1 h | 0.01 | 9.365 ×10−9 | 0.894 | 1.173 × 109 | |||
| TiO2/EP | 1 h | 0.01 | 5.854 × 10−9 | 0.8639 | 4.939 × 109 | |||
| TiO2−GO/EP | 1 h | 0.01 | 5.869 × 10−10 | 0.9014 | 1.501 × 1010 | |||
| EP | 120 h | 0.01 | 2.702 × 10−9 | 0.9102 | 9.947 × 105 | 2.572 × 10−11 | 0.4752 | 1.098 × 107 |
| GO/EP | 120 h | 0.01 | 3.119 × 10−9 | 0.9243 | 7.939 × 108 | 4.92 × 10−9 | 0.7169 | 5.988 × 108 |
| TiO2/EP | 120 h | 0.01 | 9.607 × 10−9 | 0.9218 | 1.625 × 108 | 6.08 × 10−9 | 0.5517 | 5.44 × 108 |
| TiO2−GO/EP | 120 h | 0.01094 | 6.24 × 10−10 | 0.8986 | 5.149 × 109 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Song, B.; Zhang, S.; Zhang, F.; Liu, C.; Zhang, N.; Yao, H.; Shi, Y. Using 3-Isocyanatopropyltrimethoxysilane to Decorate Graphene Oxide with Nano-Titanium Dioxide for Enhancing the Anti-Corrosion Properties of Epoxy Coating. Polymers 2020, 12, 837. https://doi.org/10.3390/polym12040837
Li W, Song B, Zhang S, Zhang F, Liu C, Zhang N, Yao H, Shi Y. Using 3-Isocyanatopropyltrimethoxysilane to Decorate Graphene Oxide with Nano-Titanium Dioxide for Enhancing the Anti-Corrosion Properties of Epoxy Coating. Polymers. 2020; 12(4):837. https://doi.org/10.3390/polym12040837
Chicago/Turabian StyleLi, Weihang, Bojun Song, Shirui Zhang, Fan Zhang, Chang Liu, Nan Zhang, Huiling Yao, and Yuanchang Shi. 2020. "Using 3-Isocyanatopropyltrimethoxysilane to Decorate Graphene Oxide with Nano-Titanium Dioxide for Enhancing the Anti-Corrosion Properties of Epoxy Coating" Polymers 12, no. 4: 837. https://doi.org/10.3390/polym12040837
APA StyleLi, W., Song, B., Zhang, S., Zhang, F., Liu, C., Zhang, N., Yao, H., & Shi, Y. (2020). Using 3-Isocyanatopropyltrimethoxysilane to Decorate Graphene Oxide with Nano-Titanium Dioxide for Enhancing the Anti-Corrosion Properties of Epoxy Coating. Polymers, 12(4), 837. https://doi.org/10.3390/polym12040837




