Evaluation of Nanocomposite Made of Polylactic Acid and Nanocellulose from Carrot Pomace Modified with Silver Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Silver Nanoparticles Synthesis
2.3. Preparation of Composites
2.4. Characterization of CCNF/AgNPs Colloids
2.4.1. Spectroscopy UV-Vis
2.4.2. Atomic Force Microscope (AFM)
2.5. Composite Characterization
2.5.1. Fourier Transform Infrared Spectroscopy (FTIR)
2.5.2. Scanning Electron Microscopy (SEM)
2.5.3. Differential Scanning Calorimetry (DSC)
2.5.4. Wettability
2.5.5. Gas Transmission Measurements
2.5.6. Mechanical Properties
2.5.7. Antimicrobial Assay
2.5.8. Statistical Analysis
3. Results and Discussion
3.1. Nanocellulose Modified with Silver Nanoparticles
3.2. Characterization of Composites
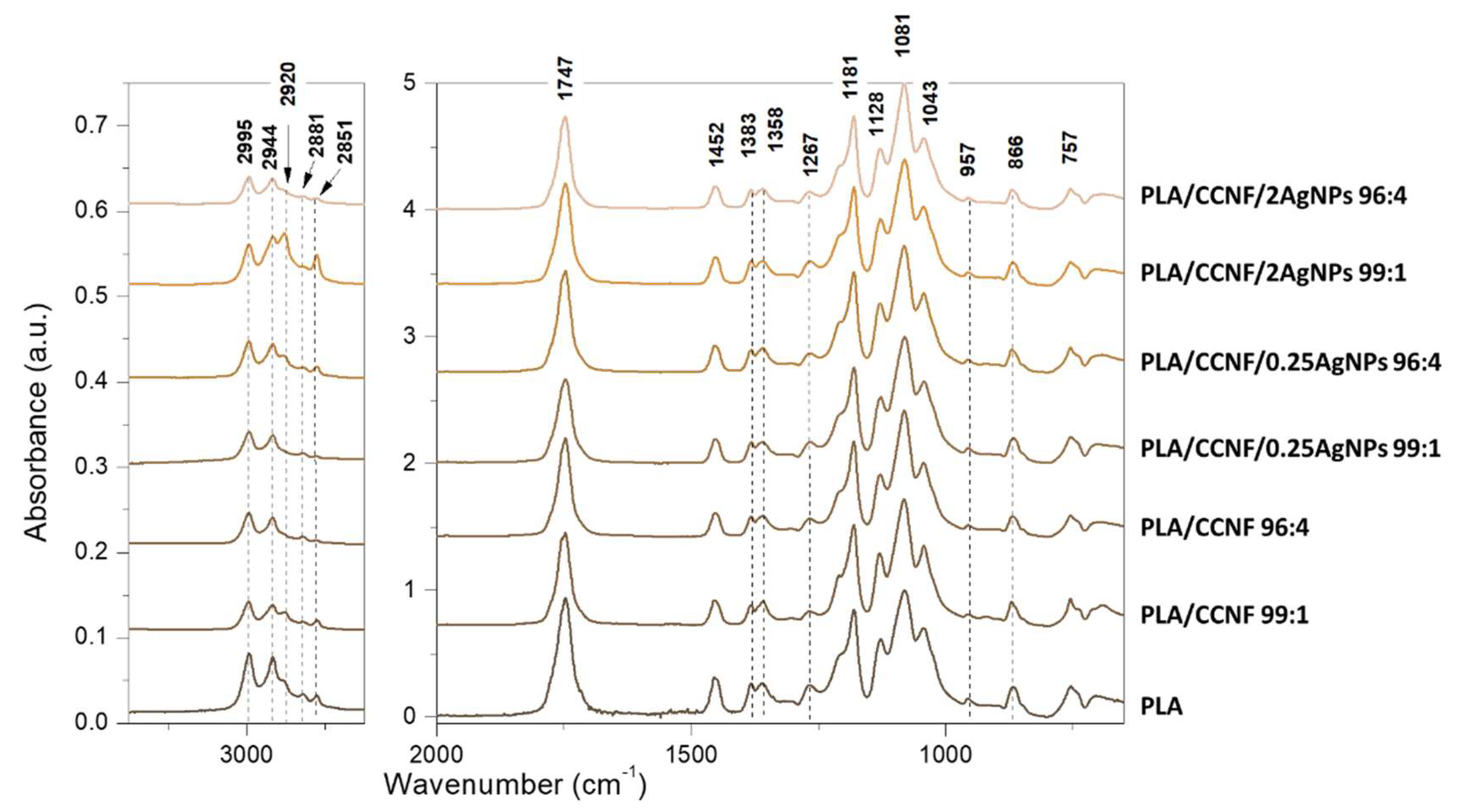
3.2.1. FTIR
3.2.2. Morphology of Obtained Composite
3.2.3. DSC
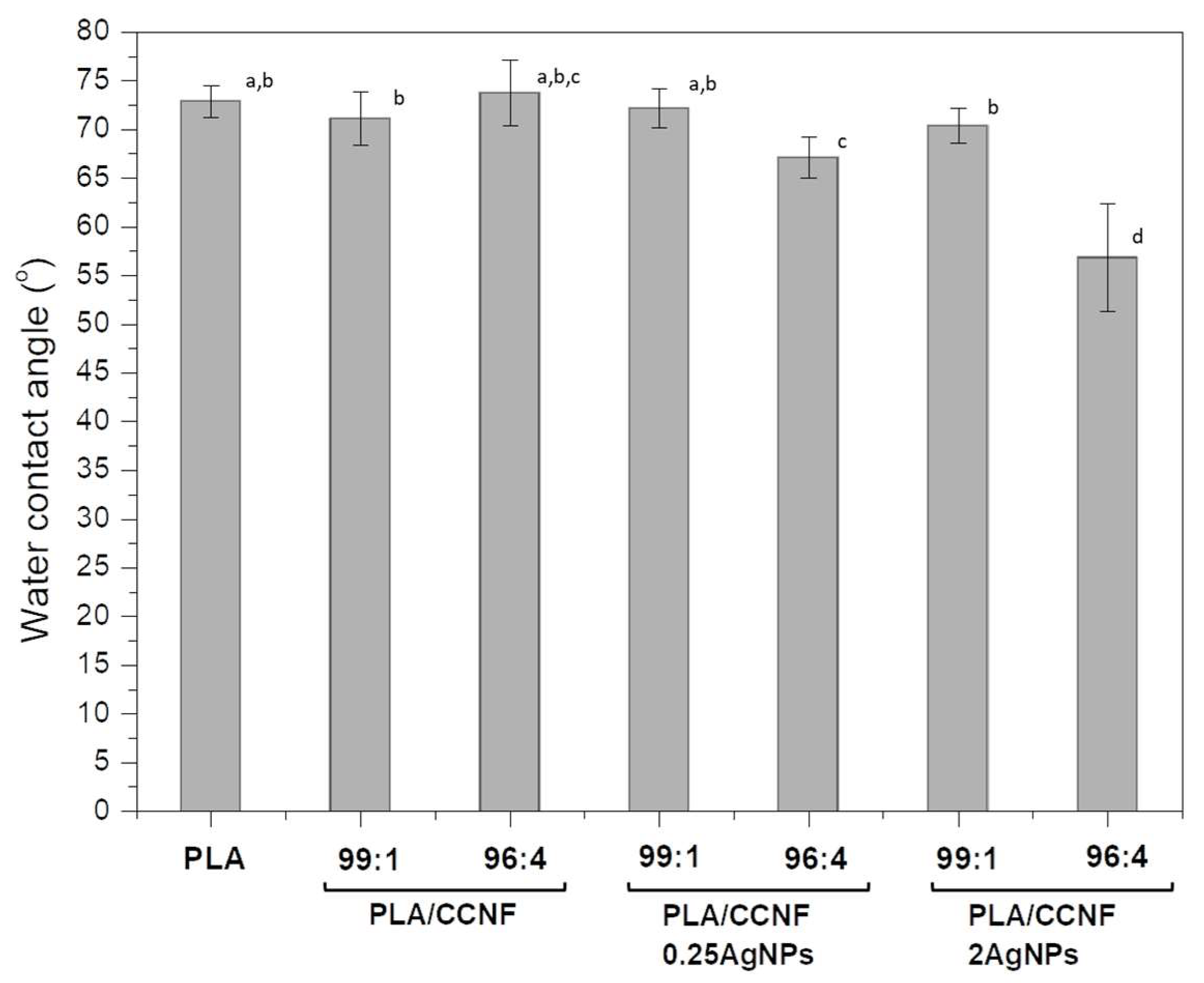
3.2.4. Hydrophobic Properties
3.2.5. Gas Permeability
3.2.6. Antibacterial Properties
3.2.7. Mechanical Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Rhim, J.-W.; Park, H.-M.; Hac, C.-S. Bio-nanocomposites for food packaging applications. Prog. Polym. Sci. 2013, 38, 1629–1652. [Google Scholar] [CrossRef]
- United Nations Environment Programme. Single-Use Plastics: A Roadmap for Sustainability. 2018. Available online: https://wedocs.unep.org/handle/20.500.11822/25496 (accessed on 4 February 2020).
- Siracusa, V.; Rocculi, P.; Romani, S.; Rossa, M.D. Biodegradable polymers for food packaging: A review. Trends Food Sci. Technol. 2008, 19, 634–643. [Google Scholar] [CrossRef]
- Fortunati, E.; Armentano, I.; Zhou, Q.; Puglia, D.; Terenza, A.; Berglund, L.A.; Kenny, J.M. Microstructure and nonisothermal cold crystallization of PLA composites based on silver nanoparticles and nanocrystalline cellulose. Polym. Degrad. Stab. 2012, 97, 2027–2036. [Google Scholar] [CrossRef]
- Chu, Z.; Zhao, T.; Li, L.; Fan, J.; Qin, Y. Characterization of Antimicrobial Poly (Lactic Acid)/Nano-Composite Films with Silver and Zinc Oxide Nanoparticles. Materials 2017, 10, 659. [Google Scholar] [CrossRef]
- De Azeredo, H.M.C. Nanocomposites for food packaging applications. Food Res. Int. 2009, 42, 1240–1253. [Google Scholar] [CrossRef]
- Dufresne, A. Nanocellulose Processing Properties and Potential Applications. Curr. For. Rep. 2019, 5, 76–89. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Fortunati, E.; Dominici, F.; López, J.; Kenny, J.M. Bionanocomposite films based on plasticized PLA–PHB/cellulose nanocrystal blends. Carbohyd. Polym. 2015, 121, 265–275. [Google Scholar] [CrossRef]
- Silvestre, C.; Duraccio, D.; Cimmino, S. Food packaging based on polymer nanomaterials. Prog. Polym. Sci. 2011, 36, 1766–1782. [Google Scholar] [CrossRef]
- Fortunati, E.; Peltzer, M.; Armentano, I.; Jiménez, A.; Kenny, J.M. Combined effects of cellulose nanocrystals and silver nanoparticles on the barrier and migration properties of PLA nano-biocomposites. J. Food Eng. 2013, 118, 117–124. [Google Scholar] [CrossRef]
- Cacciotti, I.; Fortunati, E.; Puglia, D.; Kenny, J.M.; Nanni, F. Effect of silver nanoparticles and cellulose nanocrystals on electrospun poly(lactic) acid mats: Morphology, thermal properties and mechanical behavior. Carbohyd. Polym. 2014, 103, 22–31. [Google Scholar] [CrossRef]
- Fortunati, E.; Rinaldi, S.; Peltzer, M.; Bloise, N.; Visai, L.; Armentano, I.; Jiménez, A.; Latterini, L.; Kenny, J.M. Nano-biocomposite films with modified cellulose nanocrystals and synthesized silver nanoparticles. Carbohyd. Polym. 2014, 101, 1122–1133. [Google Scholar] [CrossRef] [PubMed]
- EFSA. Opinion of the scientific panel on food additives, flavourings. In: Processing aids and Materials in Contact with Food (AFC) on a Request from the Commission Related to a 7th List of Substances for Food Contact Materials. EFSA J. 2005. [Google Scholar] [CrossRef]
- Szymańska-Chargot, M.; Chylińska, M.; Gdula, K.; Kozioł, A.; Zdunek, A. Isolation and Characterization of Cellulose from Different Fruit and Vegetable Pomaces. Polymers 2017, 9, 495. [Google Scholar] [CrossRef] [PubMed]
- Szymańska-Chargot, M.; Chylińska, M.; Pieczywek, P.M.; Zdunek, A. Tailored nanocellulose structure depending on the origin. Example of apple parenchyma and carrot root celluloses. Carbohyd. Polym. 2019, 210, 186–195. [Google Scholar] [CrossRef]
- Rinaldi, S.; Fortunati, E.; Taddei, M.; Kenny, J.M.; Armentano, I.; Latterini, L. Integrated PLGA–Agnanocomposite systems to control the degradation rate and antibacterial properties. J. Appl. Polym. Sci. 2013, 130, 1185–1193. [Google Scholar] [CrossRef]
- Fortunati, E.; Armentano, I.; Zhou, Q.; Iannoni, A.; Saino, E.; Visai, L.; Berglund, L.A.; Kenny, J.M. Multifunctional bionanocomposite films of poly(lactic acid), cellulose nanocrystals and silver nanoparticles. Carbohydr. Polym. 2012, 87, 1596–1605. [Google Scholar] [CrossRef]
- Szymańska-Chargot, M.; Chylińska, M.; Pertile, G.; Pieczywek, P.M.; Cieślak, K.J.; Zdunek, A.; Frąc, M. Influence of chitosan addition on the mechanical and antibacterial properties of carrot cellulose nanofibre film. Cellulose 2019, 26, 9613–9629. [Google Scholar] [CrossRef]
- Pinto, R.J.B.; Marques, P.A.A.P.; Neto, C.P.; Trindade, T.; Daina, S.; Sadocco, P. Antibacterial activity of nanocomposites of silver and bacterial or vegetable cellulosic fibers. Acta Biomater. 2009, 5, 2279–2289. [Google Scholar] [CrossRef]
- Walkiewicz, A.; Brzezińska, M. Interactive effects of nitrate and oxygen on methane oxidation in three different soils. Soil Biol. Biochem. 2019, 133, 116–118. [Google Scholar] [CrossRef]
- Yuniarto, K.; Welt, B.A.; Purwanto, A.; Purwadaria, H.K.; Abdellatief, A. Effect of plasticizer on oxygen permeability of cast polylactic acid (PLA) films determined using dynamic accumulation method. J. Appl. Packag. Res. 2014, 6, 51–57. [Google Scholar]
- Mochochoko, T.; Oluwafemi, O.S.; Jumbam, D.N.; Songca, S.P. Green synthesis of silver nanoparticles using cellulose extracted from an aquatic weed; water hyacinth. Carbohyd. Polym. 2013, 98, 290–294. [Google Scholar] [CrossRef] [PubMed]
- Molinaro, S.; Romero, M.C.; Boaro, M.; Sensidoni, A.; Lagazio, C.; Morris, M.; Kerry, J. Effect of nanoclay-type and PLA optical purity on the characteristics of PLA-based nanocomposite films. J. Food Eng. 2013, 117, 113–123. [Google Scholar] [CrossRef]
- Salmieri, S.; Islam, F.; Khan, R.A.; Hossain, F.M.; Ibrahim, H.M.M.; Miao, C.; Hamad, W.Y.; Lacroix, M. Antimicrobial nanocomposite films made of poly(lactic acid)-cellulose nanocrystals (PLA-CNC) in food applications: Part A—Effect of nisin release on the inactivation of Listeria monocytogenes in ham. Cellulose 2014, 21, 1837–1850. [Google Scholar] [CrossRef]
- Pamula, E.; Blazewicz, M.; Paluszkiewicz, C.; Dobrzyński, P. FTIR study of degradation products of aliphatic polyesters-carbon fibres composites. J. Mol. Struct. 2001, 596, 69–75. [Google Scholar] [CrossRef]
- Duncan, T.V. Applications of nanotechnology in food packaging and food safety: Barrier materials, antimicrobials and sensors. J. Colloid Interface Sci. 2011, 363, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Shameli, K.; Ahmad, M.B.; Yunus, W.M.; Ibrahim, N.A.; Rahman, R.A.; Jokar, M.; Darroudi, M. Silver/poly (lactic acid) nanocomposites: Preparation, characterization, and antibacterial activity. Int. J. Nanomed. 2010, 5, 573–579. [Google Scholar] [CrossRef]
- Yang, Z.; Li, X.; Si, J.; Cui, Z.; Peng, K. Morphological, Mechanical and Thermal Properties of Poly(lactic acid) (PLA)/Cellulose Nanofibrils (CNF) Composites Nanofiber for Tissue Engineering. J. Wuhan Univ. Technol.-Mater. Sci. Ed. 2019, 34, 207–215. [Google Scholar] [CrossRef]
- Jonoobi, M.; Harun, J.; Mathew, A.P.; Oksman, K. Mechanical properties of cellulose nanofiber (CNF) reinforced polylactic acid (PLA) prepared by twin screw extrusion. Compos. Sci. Technol. 2010, 70, 1742–1747. [Google Scholar] [CrossRef]
- Dong, J.; Li, M.; Zhou, L.; Lee, S.; Mei, C.; Xu, X.; Wu, Q. The influence of grafted cellulose nanofibers and postextrusion annealing treatment on selected properties of poly(lactic acid) filaments for 3D printing. J. Polym. Sci. Part B Polym. Phys. 2017, 55, 847–855. [Google Scholar] [CrossRef]
- Mokhena, T.; Sefadi, J.; Sadiku, E.; John, M.; Mochane, M.; Mtibe, A. Thermoplastic Processing of PLA/Cellulose Nanomaterials Composites. Polymers 2018, 10, 1363. [Google Scholar] [CrossRef]


| Acronym | PLA (g) | CCNF (g) | AgNO3 (mM) |
|---|---|---|---|
| PLA | 0.500 | - | - |
| PLA/CCNF 99:1 | 0.495 | 0.005 | - |
| PLA/CCNF 96:4 | 0.480 | 0.020 | - |
| PLA/CCNF/0.25AgNPs 99:1 | 0.495 | 0.005 | 0.25 |
| PLA/CCNF/0.25AgNPs 96:4 | 0.480 | 0.020 | 0.25 |
| PLA/CCNF/2AgNPs 99:1 | 0.495 | 0.005 | 2.00 |
| PLA/CCNF/2AgNPs 96:4 | 0.480 | 0.020 | 2.00 |
| Sample | Tg (°C) | Tc (°C) | Ec (J/g) | Tm (°C) | Em (J/g) | Td (°C) | Ed (J/g) |
|---|---|---|---|---|---|---|---|
| PLA | 57.8 | 121.6 | 15.75 | 150.0 | 22.97 | 369.2 | 1123.90 |
| PLA/CCNF 99:1 | 57.9 | 123.7 | 7.72 | 149.8 | 12.27 | 367.9 | 975.73 |
| PLA/CCNF 96:4 | 58.6 | 122.3 | 7.49 | 149.7 | 9.98 | 362.1 | 1023.60 |
| PLA/CCNF/0.25AgNPs 99:1 | 58.4 | 123.6 | 11.47 | 149.8 | 9.72 | 366.4 | 1002.90 |
| PLA/CCNF/0.25AgNPs 96:4 | 58.8 | 123.5 | 8.48 | 149.7 | 14.44 | 354.9 | 823.03 |
| PLA/CCNF/2AgNPs 99:1 | 57.8 | 124.9 | 7.2 | 149.2 | 9.78 | 363.4 | 865.58 |
| PLA/CCNF/2AgNPs 96:4 | 58.0 | 124.4 | 9.99 | 148.6 | 11.34 | 348.4 | 668.55 |
| Sample | Oxygen (mL·mm·min−1·m−2) | Nitrogen (mL·mm·min−1·m−2) | Carbon Dioxide (mL·mm·min−1·m−2) |
|---|---|---|---|
| PLA | 39.24 ± 5.47 a | 155.91 ± 5.59 a,c | 0.30 ± 0.01 a |
| PLA/CCNF 99:1 | 85.48 ± 2.93 a | 320.46 ± 13.44 a,b | 0.76 ± 0.39 a |
| PLA/CCNF 96:4 | 63.00 ± 2.11 a,b | 235.65 ± 7.50 a,b,c | 0.55 ± 0.20 b |
| PLA/CCNF/0.25AgNPs 99:1 | 50.58 ± 4.09 a | 189.55 ± 11.11 b | 0.64 ± 0.13 c |
| PLA/CCNF/0.25AgNPs 96:4 | 66.58 ± 3.44 a,c | 246.81 ± 9.47 a,b,c | 0.80 ± 0.15 a |
| PLA/CCNF/2AgNPs 99:1 | 47.95 ± 1.36 a,d | 182.07 ± 4.49 c | 0.31 ± 0.01 a,b,c,d |
| PLA/CCNF/2AgNPs 96:4 | 49.00 ± 3.25 a,e | 183.16 ± 12.98 a,b,c | 0.34 ± 0.03 d |
| PLA:CCNF | Bacillus cerreus | Staphylococcus aureus | Escherichia coli |
|---|---|---|---|
| PLA | - | - | - |
| PLA/CCNF 99:1 | -/+ | -/+ | -/+ |
| PLA/CCNF 96:4 | -/+ | -/+ | -/+ |
| PLA/CCNF/0.25AgNPs 99:1 | + | + | + |
| PLA/CCNF/0.25AgNPs 96:4 | + | -/+ | + |
| PLA/CCNF/2AgNPs 99:1 | + | - | + |
| PLA/CCNF/2AgNPs 96:4 | + | - | + |
| Sample | Young’s Modulus | Yield Strength | Yield Strain | Tensile Stress at Break | Strain at Break |
|---|---|---|---|---|---|
| (MPa) | (MPa) | (%) | (MPa) | (%) | |
| PLA | 1940.9 ± 271.3 a | 21.6 ± 4.7 a,b,c,d | 1.92 ± 0.48 b,c | 26.39 ± 5.30 a,b | 2.76 ± 0.88 a |
| PLA/CCNF 99:1 | 1923.7 ± 238.1 a | 19.9 ± 3.9 a,b,c | 1.31 ± 0.21 a | 28.66 ± 4.63 a,b,c | 2.19 ± 0.32 a |
| PLA/CCNF 96:4 | 1769.4 ± 170.3 a,b | 16.9 ± 2.9 a | 1.20 ± 0.08 a | 23.45 ± 3.88 a | 2.06 ± 0.40 a |
| PLA/CCNF/0.25AgNPs 99:1 | 2295.4 ± 203.1 c | 25.4 ± 6.3 d | 1.46 ± 0.40 a | 34.30 ± 7.16 c | 2.16 ± 0.48 a |
| PLA/CCNF/0.25AgNPs 96:4 | 2199.4 ± 107.6 c | 23.8 ± 3.5 c,d | 1.37 ± 0.27 a | 31.36 ± 4.44 b,c | 2.06 ± 0.39 a |
| PLA/CCNF/2AgNPs 99:1 | 1789.4 ± 253.5 a,b | 18.7 ± 1.9 a,b | 1.61 ± 0.36 a,b | 25.30 ± 2.81 a | 5.16 ± 2.33 b |
| PLA/CCNF/2AgNPs 96:4 | 1659.8 ± 166.1 b | 21.7 ± 2.0 b,c,d | 2.04 ± 0.42 c | 26.57 ± 1.82 a,b | 3.13 ± 0.91 a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szymańska-Chargot, M.; Chylińska, M.; Pieczywek, P.M.; Walkiewicz, A.; Pertile, G.; Frąc, M.; Cieślak, K.J.; Zdunek, A. Evaluation of Nanocomposite Made of Polylactic Acid and Nanocellulose from Carrot Pomace Modified with Silver Nanoparticles. Polymers 2020, 12, 812. https://doi.org/10.3390/polym12040812
Szymańska-Chargot M, Chylińska M, Pieczywek PM, Walkiewicz A, Pertile G, Frąc M, Cieślak KJ, Zdunek A. Evaluation of Nanocomposite Made of Polylactic Acid and Nanocellulose from Carrot Pomace Modified with Silver Nanoparticles. Polymers. 2020; 12(4):812. https://doi.org/10.3390/polym12040812
Chicago/Turabian StyleSzymańska-Chargot, Monika, Monika Chylińska, Piotr M. Pieczywek, Anna Walkiewicz, Giorgia Pertile, Magdalena Frąc, Krystian J. Cieślak, and Artur Zdunek. 2020. "Evaluation of Nanocomposite Made of Polylactic Acid and Nanocellulose from Carrot Pomace Modified with Silver Nanoparticles" Polymers 12, no. 4: 812. https://doi.org/10.3390/polym12040812
APA StyleSzymańska-Chargot, M., Chylińska, M., Pieczywek, P. M., Walkiewicz, A., Pertile, G., Frąc, M., Cieślak, K. J., & Zdunek, A. (2020). Evaluation of Nanocomposite Made of Polylactic Acid and Nanocellulose from Carrot Pomace Modified with Silver Nanoparticles. Polymers, 12(4), 812. https://doi.org/10.3390/polym12040812









