Porous Ultra-Thin Films from Photocleavable Block Copolymers: In-Situ Degradation Kinetics Study of Pore Material
Abstract
1. Introduction
2. Materials and Methods
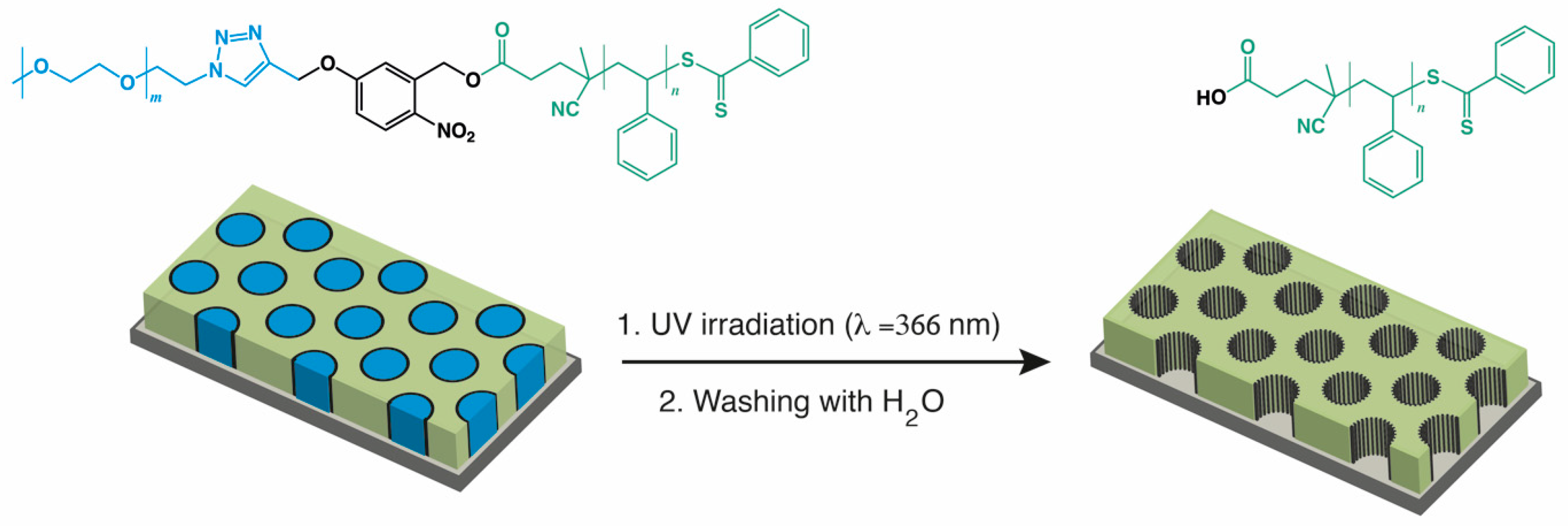
2.1. Thin Film Preparation
2.2. In-Situ X-ray Reflectivity (XRR) Experiments
2.3. In-Situ Scanning Force Microscopy Measurements
2.4. Grazing Incidence Small-Angle X-ray Scattering Measurements
3. Results and Discussion
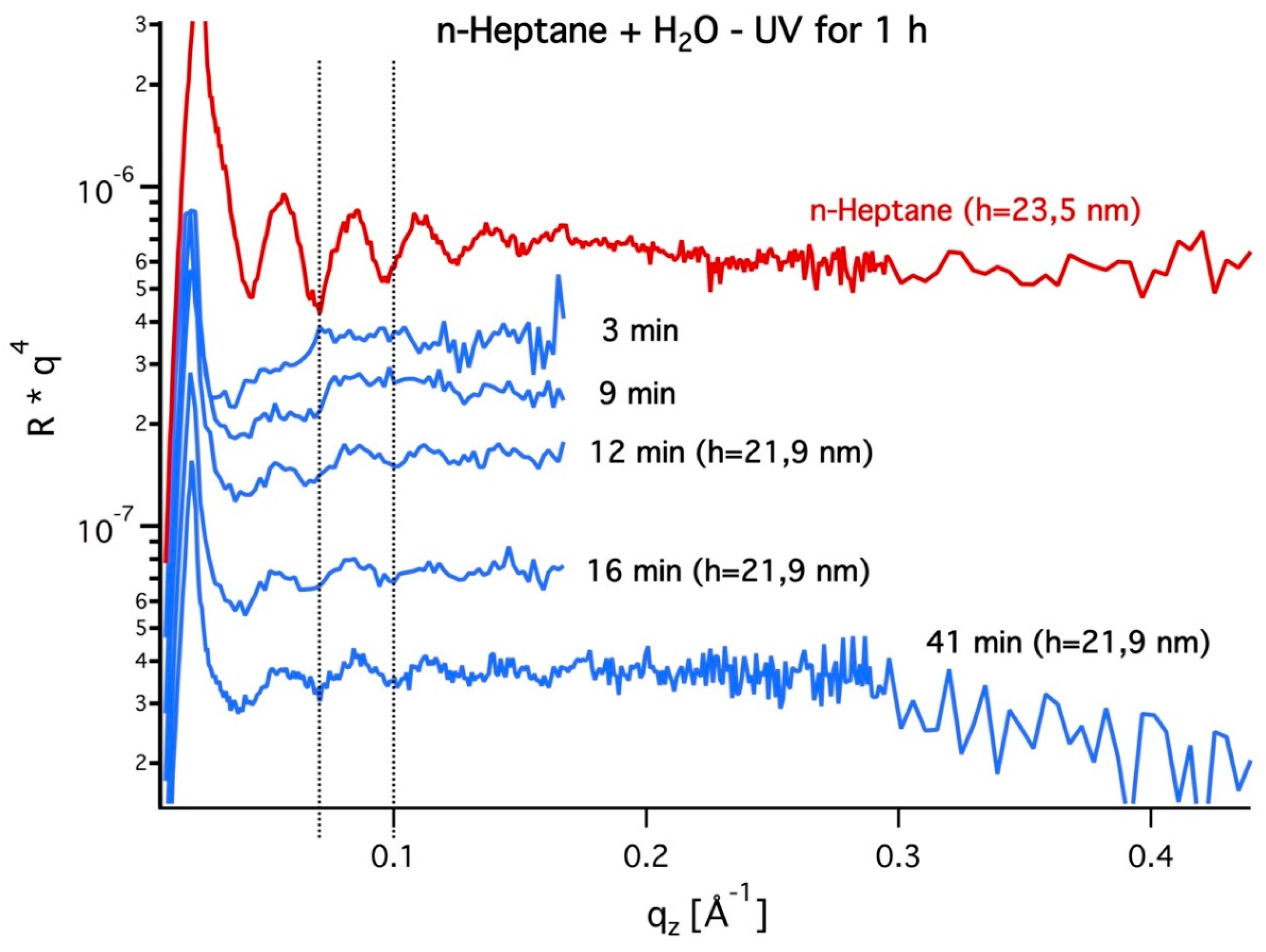
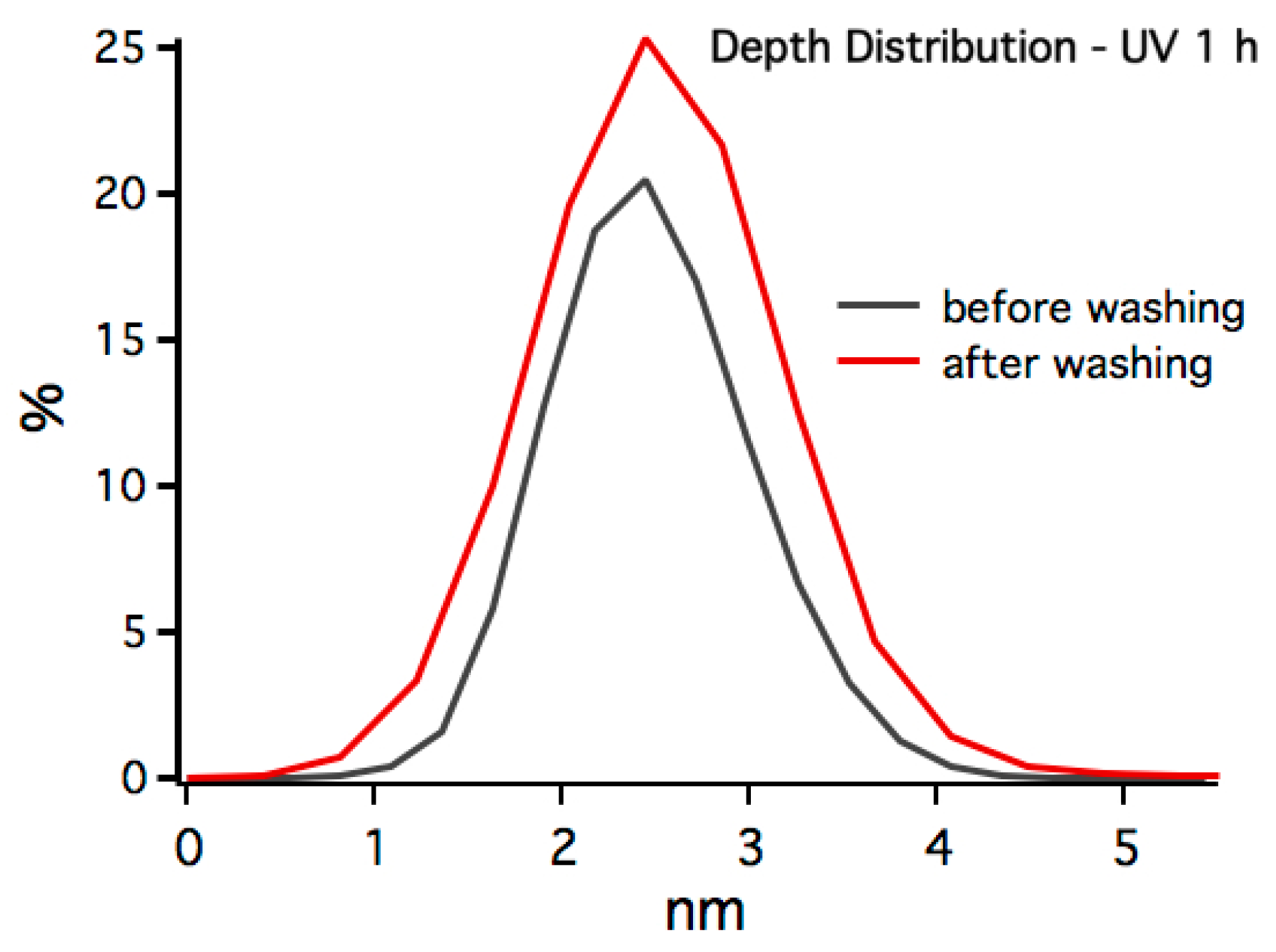
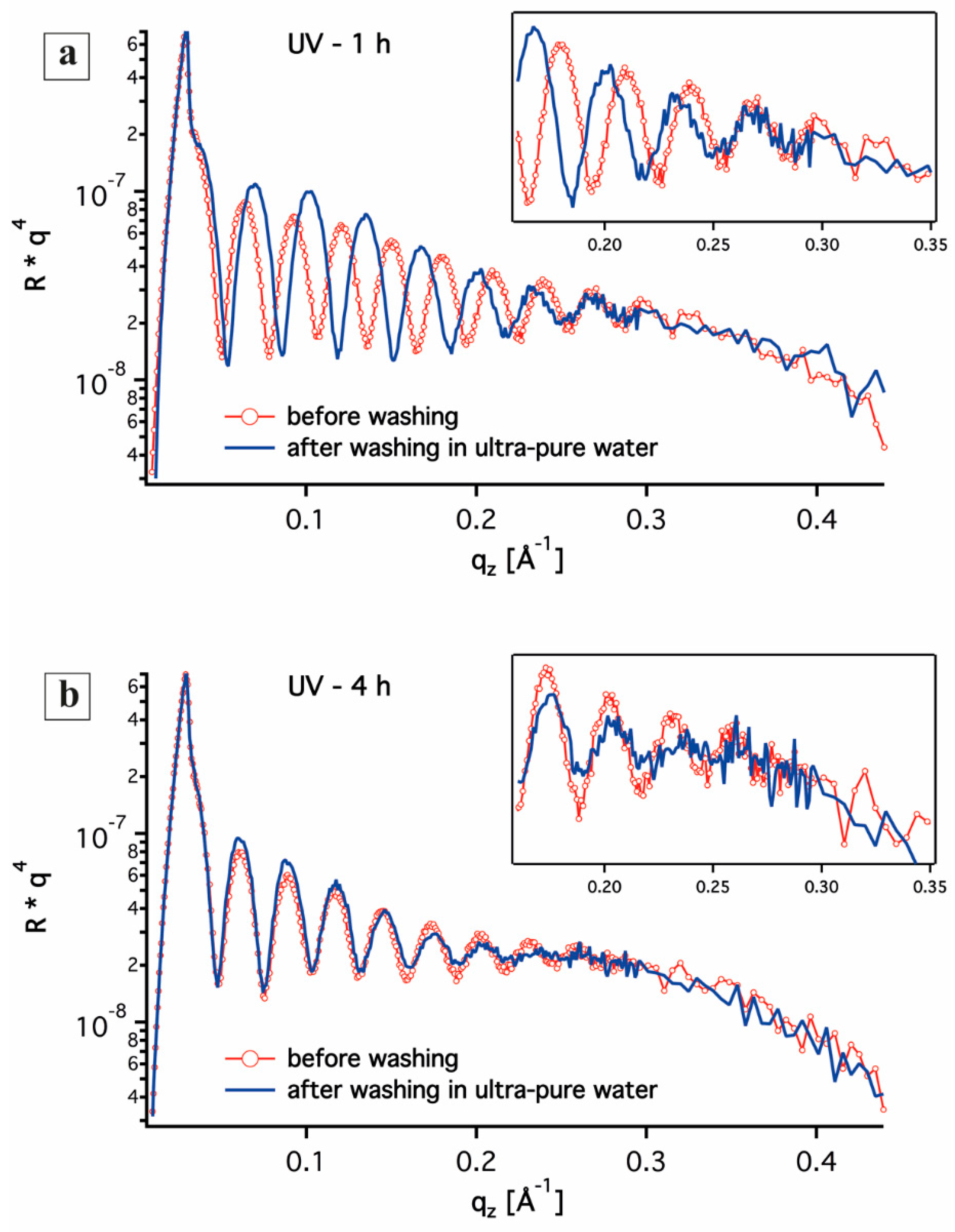
3.1. In-Situ X-ray Reflectivity
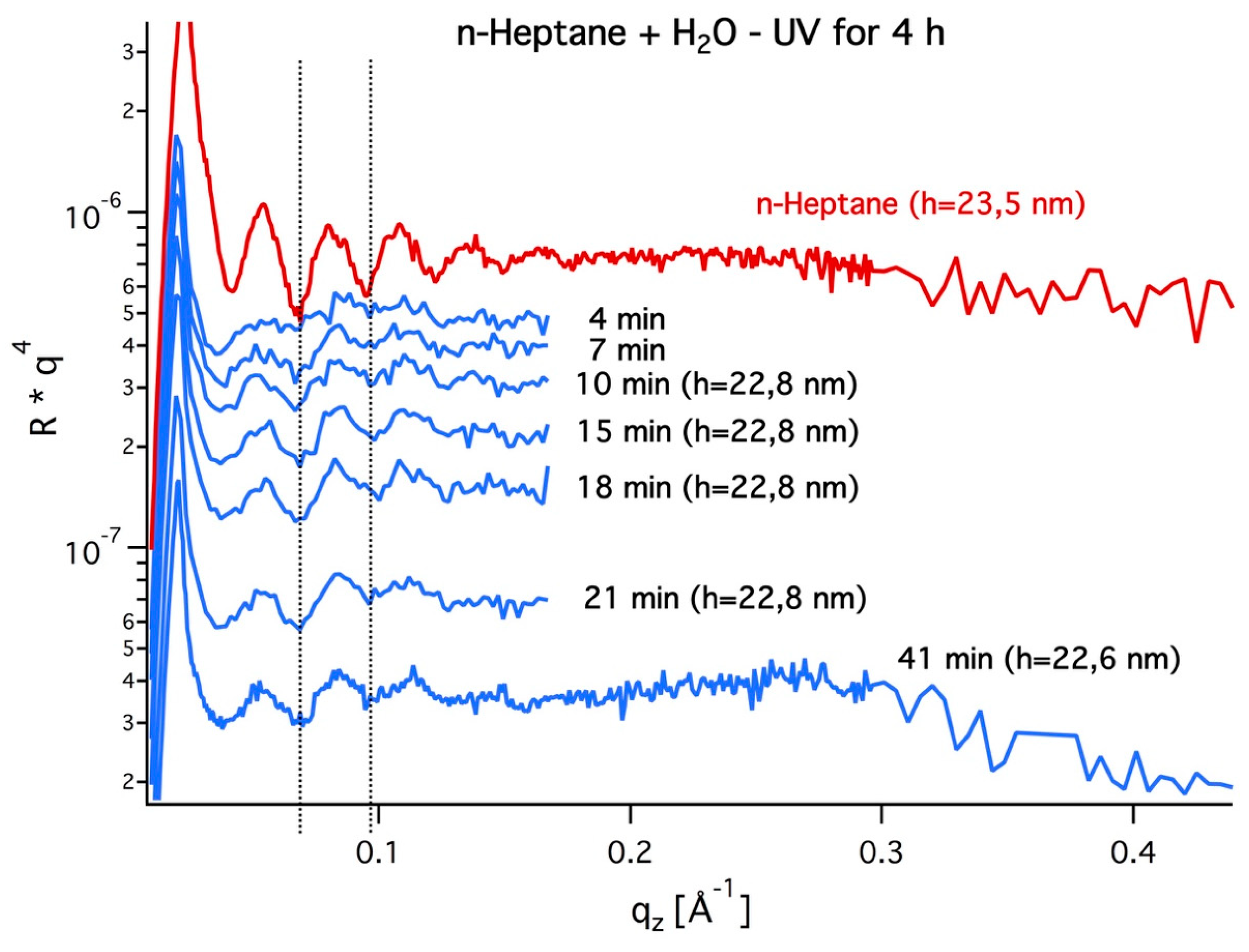
3.2. Grazing Incidence Small-Angle X-ray Scattering
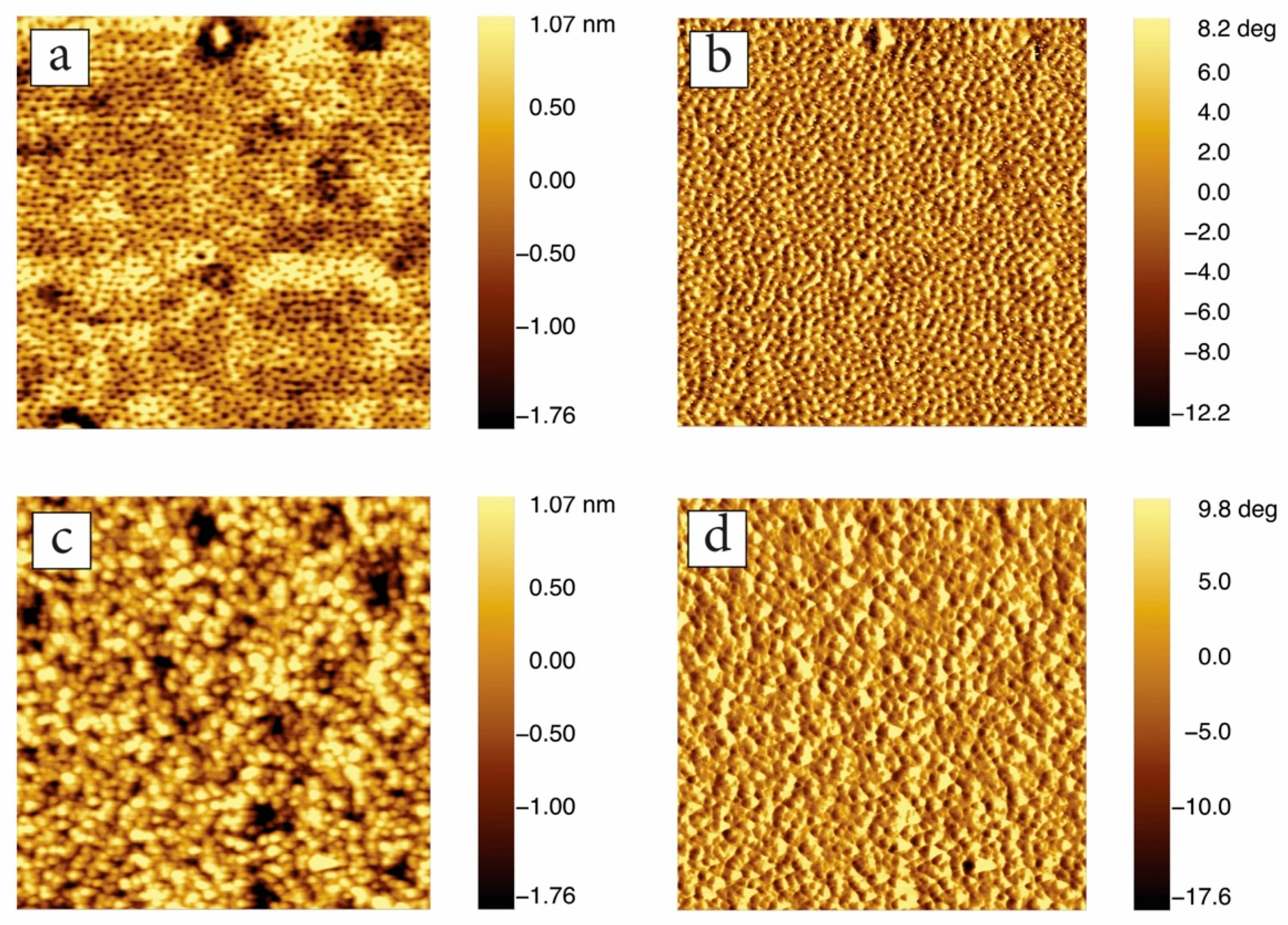
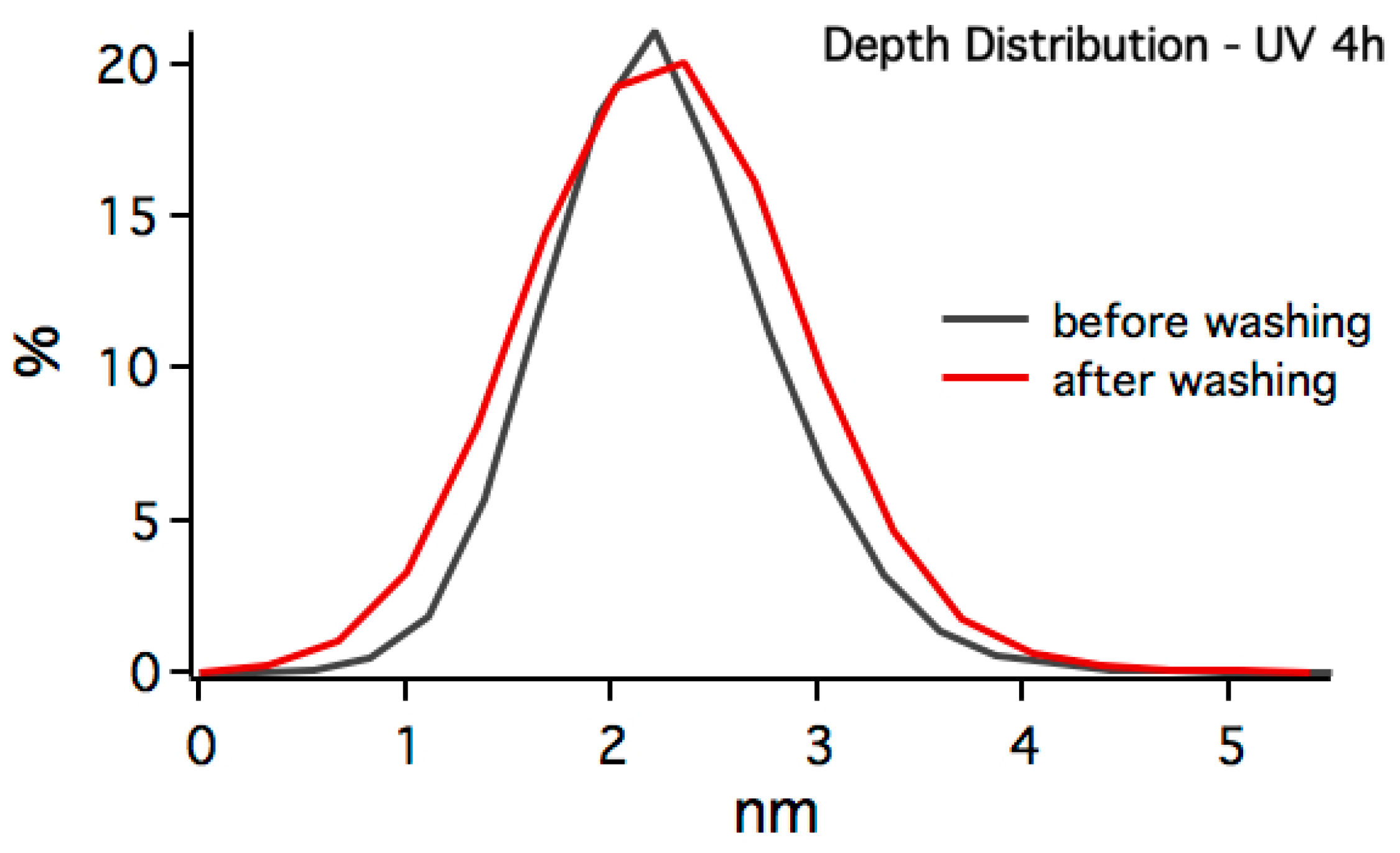
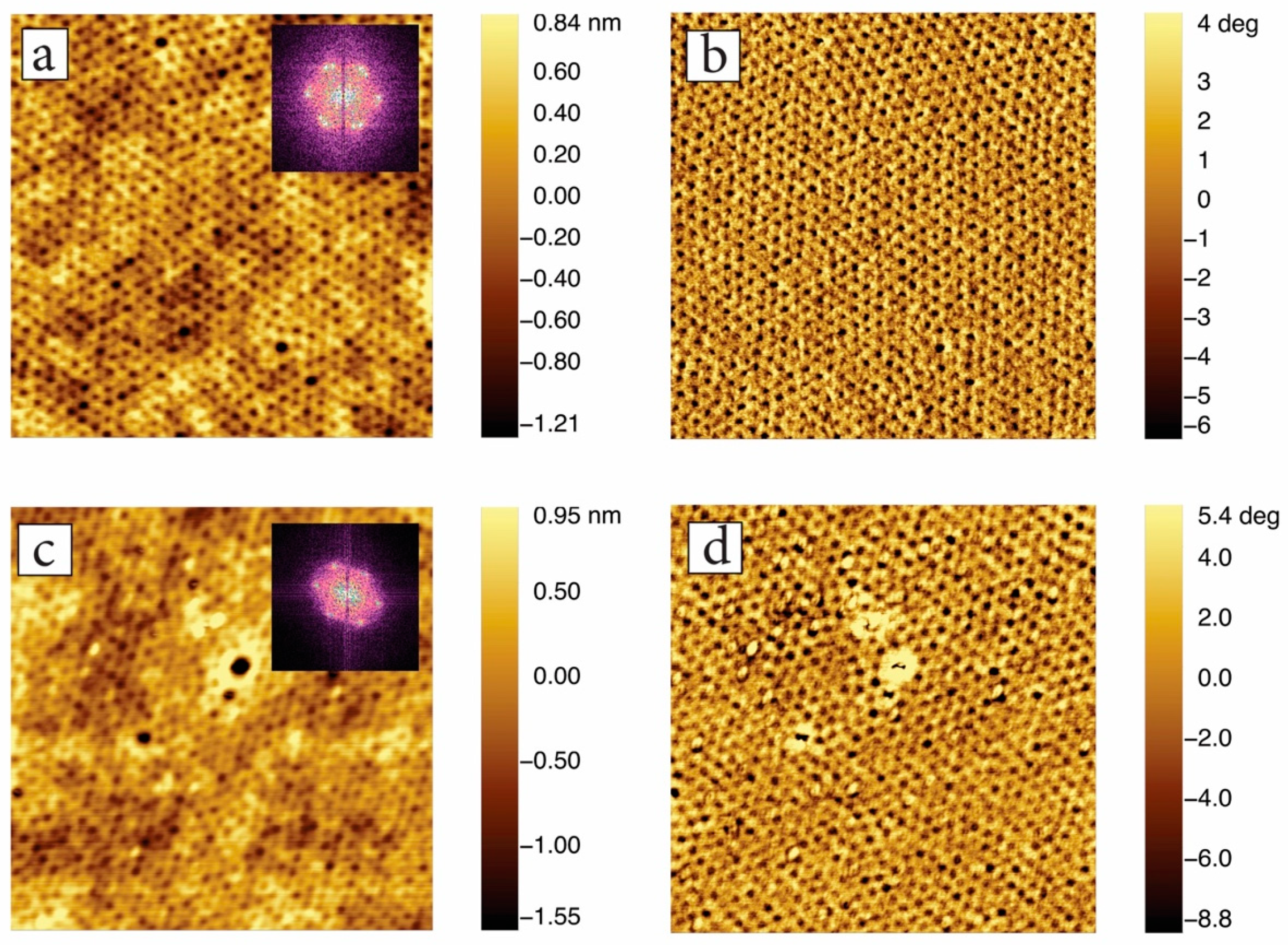
3.3. In-Situ Sacnning Force Microscopy
4. Summary
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gin, D.L.; Gu, W. Nanoporous Catalytic Materials with Organic Frameworks. Adv. Mater. 2001, 13, 1407. [Google Scholar] [CrossRef]
- Davis, M.E. Ordered porous materials for emerging applications. Nature 2002, 417, 813. [Google Scholar] [CrossRef] [PubMed]
- Lei, C.; Shin, Y.; Liu, J.; Ackerman, E.J. Entrapping Enzyme in a Functionalized Nanoporous Support. J. Am. Chem. Soc. 2002, 124, 11242. [Google Scholar] [CrossRef] [PubMed]
- Costeux, S. CO2-Blown Nanocellular Foams. J. Appl. Polym. Sci. 2014, 131, 41293. [Google Scholar] [CrossRef]
- Guo, H.; Nicolae, A.; Kumar, V. Solid-State Microcellular and Nanocellular Polysulfone Foams. J. Poly. Sci. Part B 2015, 53, 975–985. [Google Scholar] [CrossRef]
- Krause, B.; Koops, G.-H.; van der Vegt, N.; Wessling, M.; Wübbenhorst, M.; van Turnhout, J. Ultralow-k Dielectrics Made by Supercritical Foaming of Thin Polymer Films. Adv. Mater. 2002, 14, 1041–1046. [Google Scholar] [CrossRef]
- Jang, J.; Bae, J. Fabrication of mesoporous polymer using soft template method. Chem. Commun. 2005, 9, 1200–1202. [Google Scholar] [CrossRef] [PubMed]
- Segalman, R.A. Patterning with block copolymer thin films. Mater. Sci. Eng. 2005, 48, 191. [Google Scholar] [CrossRef]
- Bosworth, J.K.; Ober, C.K. Top-Down versus Bottom-Up Patterning of Polymers. Polym. Sci. A Compr. Ref. 2012, 8, 9. [Google Scholar]
- Bang, J.; Jeong, U.; Ryu, D.Y.; Russell, T.P.; Hawker, C.J. Block Copolymer Nanolithography: Translation of Molecular Level Control to Nanoscale Patterns. Adv. Mater. 2009, 21, 4769. [Google Scholar] [CrossRef]
- Wu, D.; Xu, F.; Sun, B.; Fu, R.; He, H.; Matyjaszewski, K. Design and Preparation of Porous Polymers. Chem. Rev. 2012, 112, 3959. [Google Scholar] [CrossRef] [PubMed]
- Ochsmann, J.W.; Lenz, S.; Emmerling, S.G.J.; Kappes, R.S.; Nett, S.K.; Lechmann, M.C.; Roth, S.V.; Gutmann, J.S. PS-b-PEO block copolymer thin films as structured reservoirs for nanoscale precipitation reactions. J. Polym. Sci. Part B Polym. Phys. 2010, 48, 1569. [Google Scholar] [CrossRef]
- Hong, S.W.; Russell, T.P. Block Copolymer Thin Films. Polym. Sci. Compr. Ref. 2012, 7, 45. [Google Scholar]
- Hamley, I.W. Ordering in thin films of block copolymers: Fundamentals to potential applications. Prog. Polym. Sci. 2009, 34, 1161. [Google Scholar] [CrossRef]
- Yang, S.Y.; Ryu, I.; Kim, H.Y.; Kim, J.K.; Jang, S.K.; Russell, T.P. Nanoporous Membranes with Ultrahigh Selectivity and Flux for the Filtration of Viruses. Adv. Mater. 2006, 18, 709. [Google Scholar] [CrossRef]
- She, M.-S.; Lo, T.-Y.; Hsueh, H.-Y.; Ho, R.-M. Nanostructured thin films of degradable block copolymers and their applications. NPG Asia Mater. 2013, 5, e42. [Google Scholar] [CrossRef]
- Hacking, S.A.; Du, Y. Patterning of Polymeric Materials for Biological Applications. Polym. Sci. Compr. Ref. 2012, 9, 439. [Google Scholar]
- Li, M.; Douki, K.; Goto, K.; Li, X.; Coenjarts, C.; Smilgies, D.-M.; Ober, C.K. Spatially Controlled Fabrication of Nanoporous Block Copolymers. Chem. Matter. 2004, 16, 3800. [Google Scholar] [CrossRef]
- Du, P.; Li, M.; Douki, K.; Li, X.; Garcia, C.B.W.; Jain, A.; Smilgies, D.-M.; Fitters, L.J.; Gruner, S.M.; Wiesner, U.; et al. Additive-Driven Phase-Selective Chemistry in Block Copolymer Thin Films: The Convergence of Top-Down and Bottom-Up Approaches. Adv. Matter 2004, 16, 953. [Google Scholar] [CrossRef]
- Xu, T.; Goldbach, J.T.; Misner, M.J.; Kim, S.; Gibaud, A.; Gang, O.; Ocko, B.; Guarini, K.W.; Black, C.T.; Hawker, C.J.; et al. Scattering Study on the Selective Solvent Swelling Induced Surfcae Reconstruction. Macromolecules 2004, 37, 2972. [Google Scholar] [CrossRef]
- Leiston-Belanger, J.M.; Russell, T.P.; Drockenmuller, E.; Hawker, C.J. A Thermal and Manufacturable Approach to Stabilized Diblock Copolymer Templates. Macromolecules 2005, 38, 7676–7683. [Google Scholar] [CrossRef]
- Lee, J.S.; Hirao, A.; Nakahama, S. Polymerization of monomers containing functional silyl groups. 5. Synthesis of new porous membranes with functional groups. Macromolecules 1988, 21, 274–276. [Google Scholar] [CrossRef]
- Bang, J.; Kim, S.H.; Drockenmuller, E.; Misner, M.J.; Russell, T.P.; Hawker, C.J. Defect-Free Nanoporous Thin Films from ABC Triblock Copolymers. J. Am. Chem. Soc. 2006, 128, 762–7629. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.; Moon, B. Synthesis of Photocleavable Poly(styrene-block-ethylene oxide) and Its Self-Assembly into Nanoporous Thin Films. Macromolecules 2009, 42, 455–458. [Google Scholar] [CrossRef]
- Schumers, J.M.; Vlad, A.; Huynen, I.; Gohy, J.F.; Fustin, C.A. Functionalized nanoporous thin films from photocleavable block copolymers. Macromol. Rapid Commun. 2012, 33, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Gu, W.; Sterner, E.; Russell, T.P.; Coughlin, E.B.; Theato, P. Highly Ordered Nanoporous Thin Films from Photocleavable Block Copolymers. Macromolecules 2011, 44, 6433–6440. [Google Scholar] [CrossRef]
- Müller-Buschbaum, P. Chapter 2: Structure Determination in thin Film Geometry Using Grazing Incidence Small-Angle Scattering. In Polymer Surfaces and Interfaces; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Müller-Buschbaum, P.; Hermsdorf, N.; Roth, S.V.; Wiedersich, J.; Cunise, S.; Gehrke, R. Comparative analysis of nanostructured diblock copolymer films. Spectrochim. Acta Part B 2004, 59, 1789. [Google Scholar] [CrossRef]
- Tolan, M. X-Ray Scattering from Soft-Matter Thin Films: Materials Science and Basic Research; Springer: Berlin/Heidelberg, Germany, 1999. [Google Scholar]
- Pietsch, U.; Holy, V.; Baumbach, T. High-Resolution X-Ray Scattering: From Thin Films to Lateral Nanostructures, 2nd ed.; Springer: New York, NY, USA, 2004. [Google Scholar]
- Wirkert, F.J.; Paulus, M.; Nase, J.; Möller, J.; Kujawski, S.; Sternemann, C.; Tolan, M. X-ray reflectivity measurements of luquid/solid interfaces under high hydrostatic pressure conditions. J. Synchrotron Radiat. 2014, 21, 76. [Google Scholar] [CrossRef]
- Kiesel, I.; Paulus, M.; Nase, J.; Tiemeyer, S.; Sternemann, C.; Rüster, K.; Wirkert, F.J.; Mende, K.; Büning, T.; Tolan, M. Temperature-driven Adsorption and Desorption of Proteins at Solid-Liquid Interfaces. Langmuir 2014, 30, 2077. [Google Scholar] [CrossRef]
- Wirkert, F.J.; Paulus, M.; Sternemann, C.; Nase, J.; Schroer, M.A.; Wieland, D.C.F.; Bieder, S.; Degen, P.; Rehage, H.; Tolan, M. Study of time and pressure dependent phenomena at the hard x-ray beamline BL9 of DELTA. J. Phys. Conf. Ser. 2013, 425, 202006. [Google Scholar] [CrossRef]
- Paulus, M.; Lietz, D.; Sternemann, C.; Shokuie, K.; Evers, F.; Tolan, M.; Czeslik, C.; Winter, R. An access to buried interfaces: The X-ray reflectivity set-up of BL9 at DELTA. J. Synchrotron Radiat. 2008, 15, 600. [Google Scholar] [CrossRef] [PubMed]
- Hexemer, A.; Bras, W.; Glossinger, J.; Schaible, E.; Gann, E.; Kirian, R.; MacDowell, A.; Church, M.; Rude, B.; Padmore, H. A SAXS/WAXS/GISAXS Beamline with Multilayer Monochromator. J. Phys. Conf. Ser. 2010, 247, 012007. [Google Scholar] [CrossRef]
- Babonneau, D. Software package for modelling and analysis of GISAXS data using IGOR Pro. J. Appl. Crystallogr. 2010, 43, 929–936. [Google Scholar] [CrossRef]
- Hansen, C.M. Hansen Solubility Parameters: A User’s Handbook, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Altinpinar, S.; Zhao, H.; Ali, W.; Kappes, R.; Schuchardt, P.; Salehi, S.; Santoro, G.; Theato, P.; Roth, S.; Gutmann, J.S. Distortion of Ultrathin Photocleavable Block Copolymer Films during Photocleavage and Nanopore Formation. Langmuir 2015, 31, 8947. [Google Scholar] [CrossRef] [PubMed]
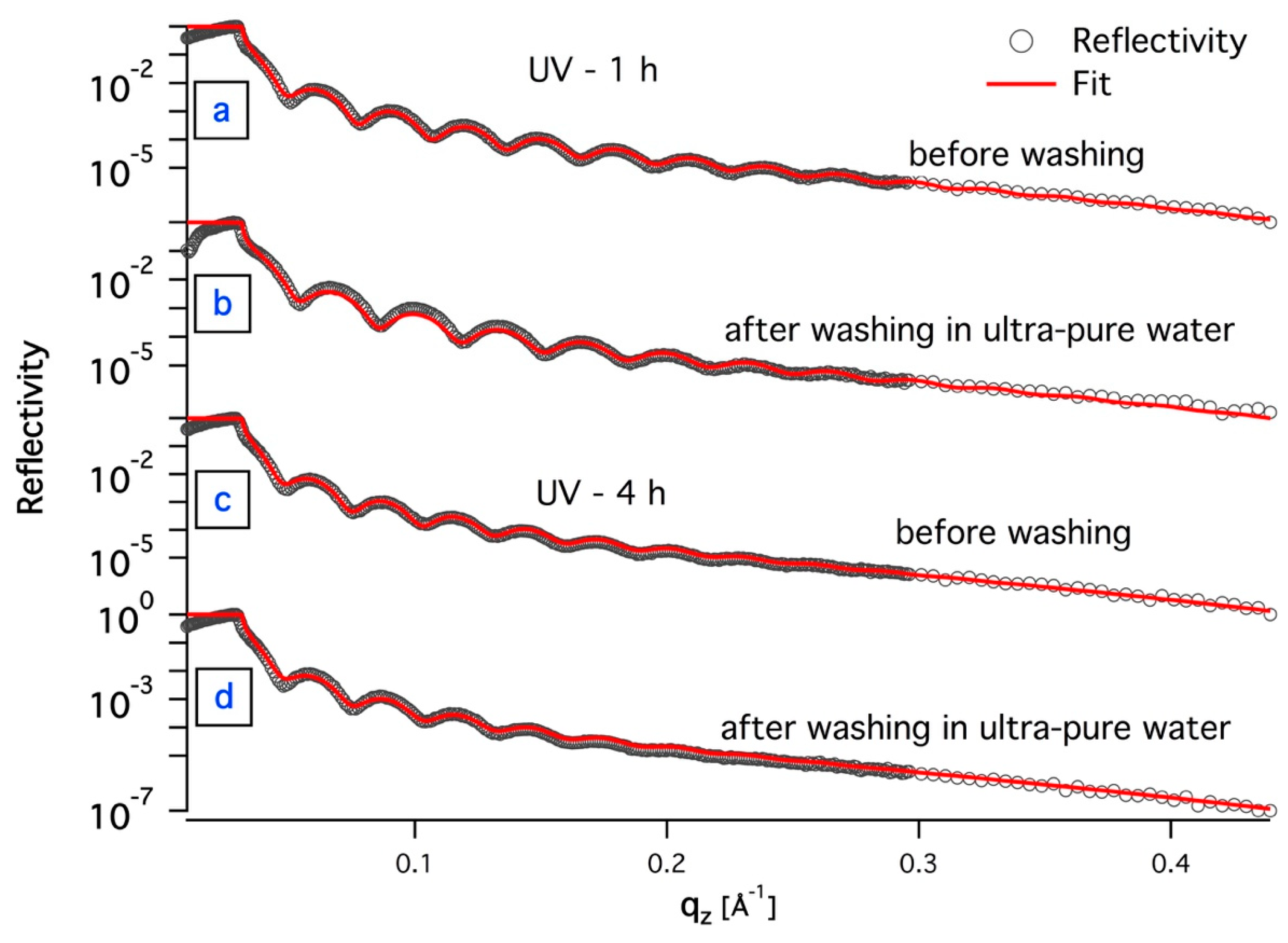

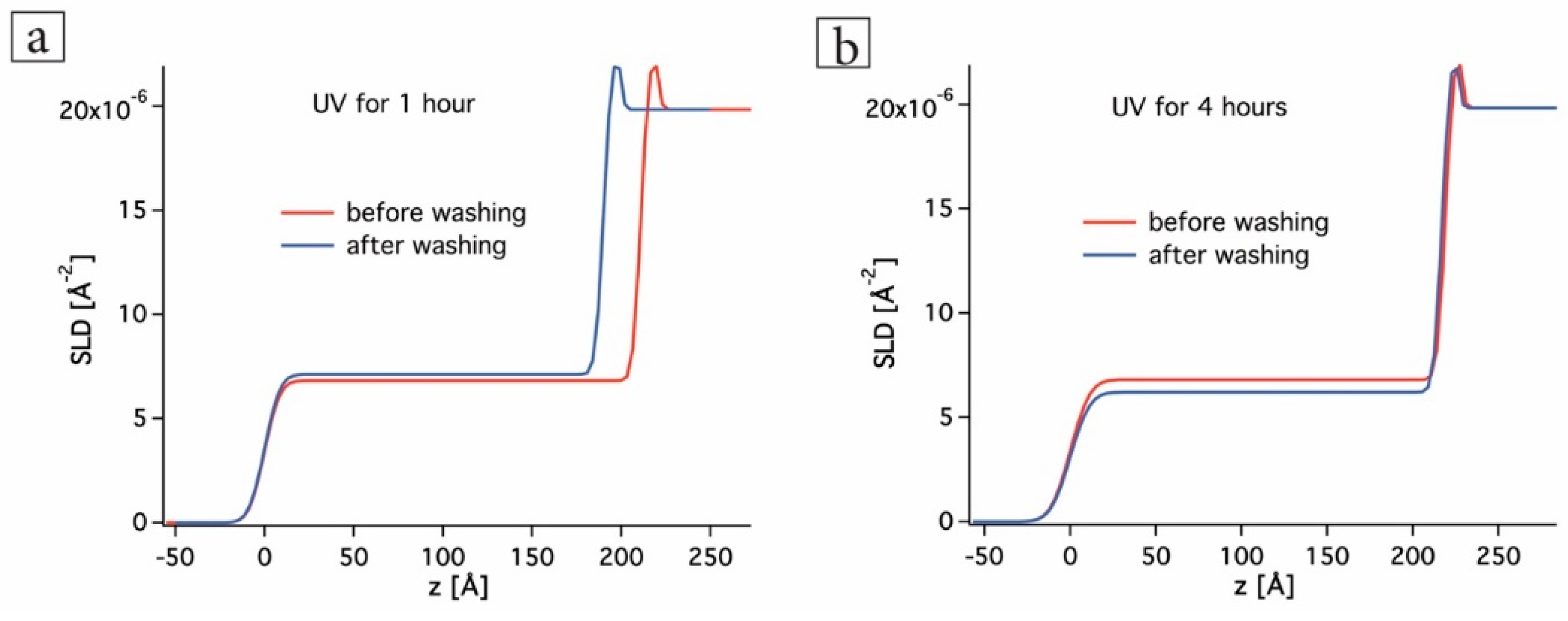
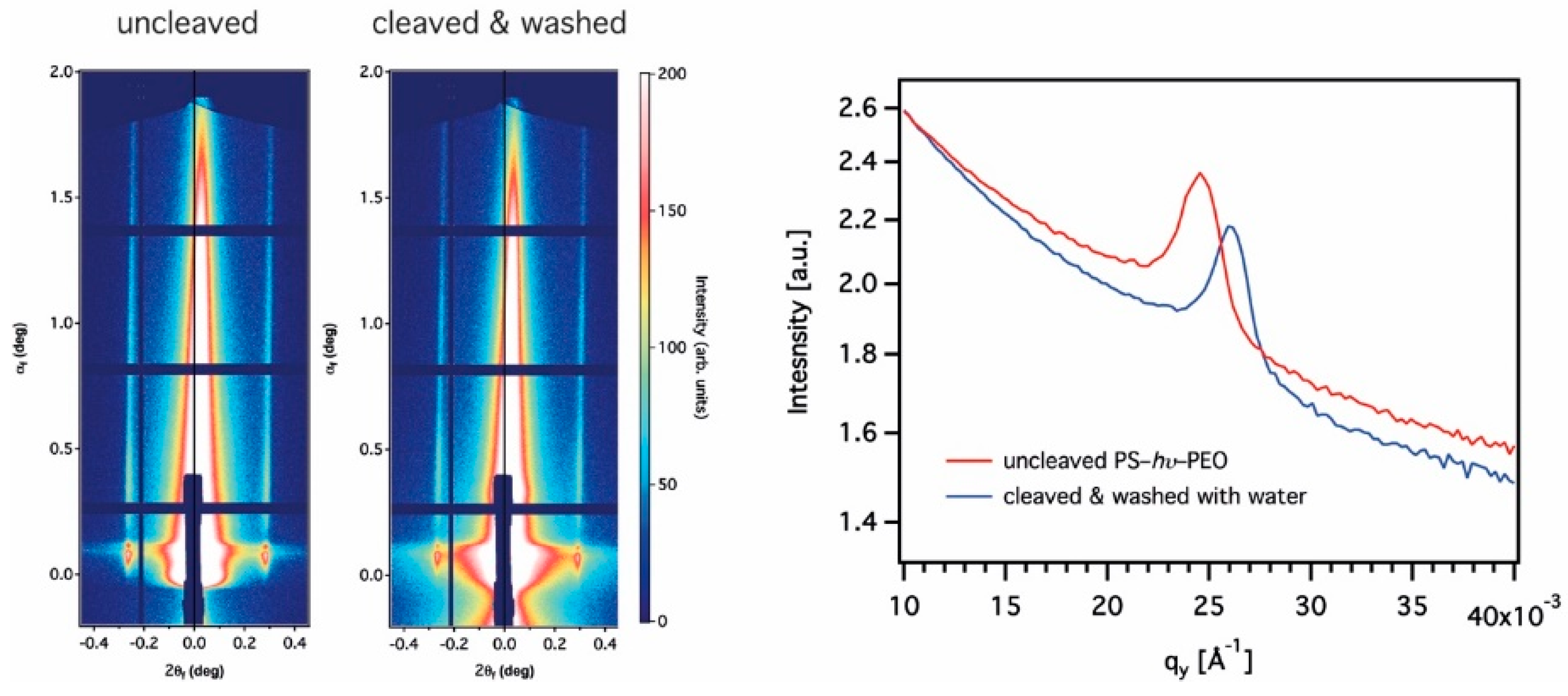
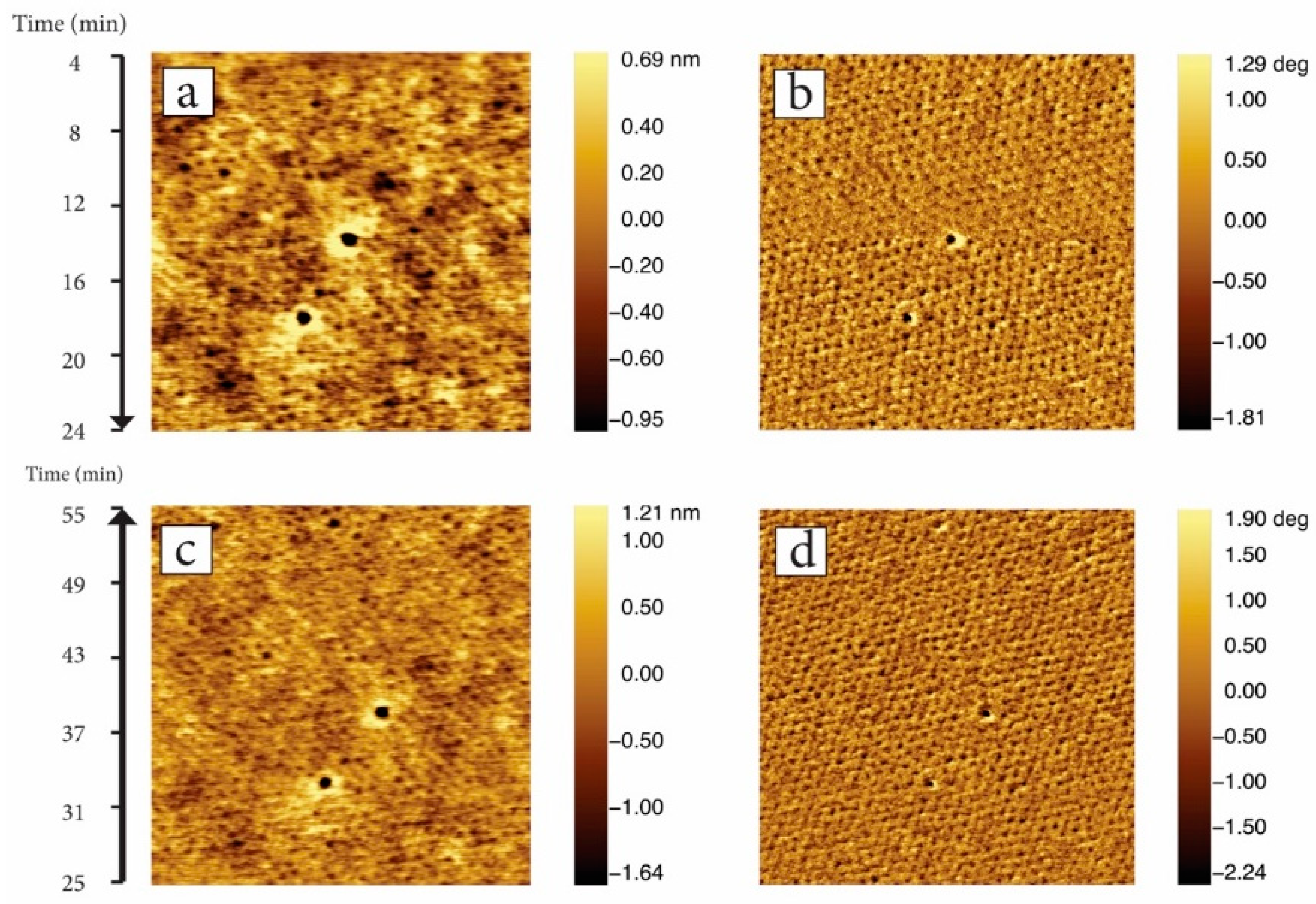
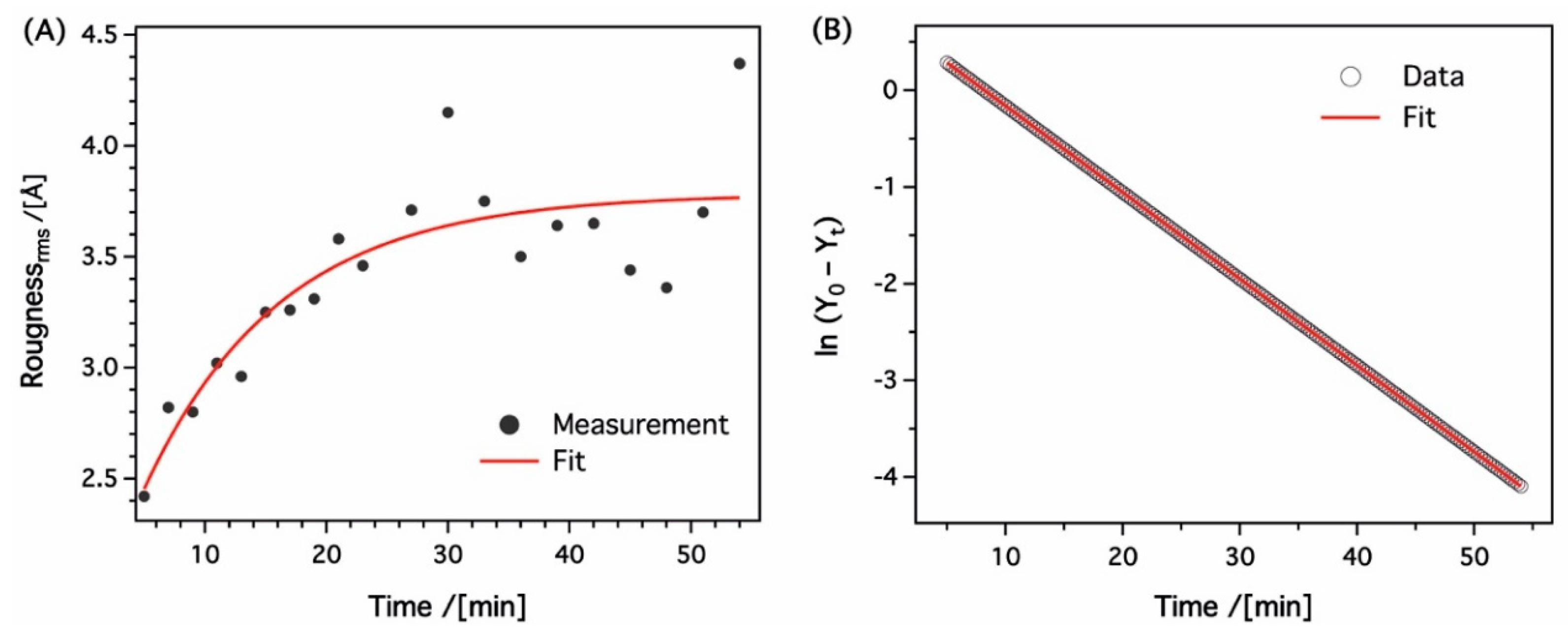
| Sample | Roughness [Å] | Film Thickness [nm] | Electron Density [Å−2] |
|---|---|---|---|
| Uncleaved | 6.7 | 21.9 | 6.802 × 10−6 |
| PS-hν-PEO | |||
| Cleaved PS-hν-PEO | 6.8 | 19.0 | 7.102 × 10−6 |
| 41 min. H2O washing |
| Sample | Roughness [Å] | Film Thickness [nm] | Electron Density [Å−2] |
|---|---|---|---|
| Uncleaved | 9 | 21.9 | 6.802 × 10−6 |
| PS-hν-PEO | |||
| Cleaved PS-hν-PEO | 10 | 21.7 | 6.202 × 10−6 |
| 41 min. H2O washing |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Altinpinar, S.; Ali, W.; Schuchardt, P.; Yildiz, P.; Zhao, H.; Theato, P.; Gutmann, J.S. Porous Ultra-Thin Films from Photocleavable Block Copolymers: In-Situ Degradation Kinetics Study of Pore Material. Polymers 2020, 12, 781. https://doi.org/10.3390/polym12040781
Altinpinar S, Ali W, Schuchardt P, Yildiz P, Zhao H, Theato P, Gutmann JS. Porous Ultra-Thin Films from Photocleavable Block Copolymers: In-Situ Degradation Kinetics Study of Pore Material. Polymers. 2020; 12(4):781. https://doi.org/10.3390/polym12040781
Chicago/Turabian StyleAltinpinar, Sedakat, Wael Ali, Patrick Schuchardt, Pinar Yildiz, Hui Zhao, Patrick Theato, and Jochen S. Gutmann. 2020. "Porous Ultra-Thin Films from Photocleavable Block Copolymers: In-Situ Degradation Kinetics Study of Pore Material" Polymers 12, no. 4: 781. https://doi.org/10.3390/polym12040781
APA StyleAltinpinar, S., Ali, W., Schuchardt, P., Yildiz, P., Zhao, H., Theato, P., & Gutmann, J. S. (2020). Porous Ultra-Thin Films from Photocleavable Block Copolymers: In-Situ Degradation Kinetics Study of Pore Material. Polymers, 12(4), 781. https://doi.org/10.3390/polym12040781








