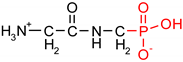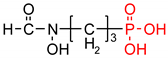Abstract
This research is focused on obtaining antimicrobial hybrid materials consisting of poly(lactide) nonwoven fabrics and using phosphoro-organic compound—fosfomycin—as a coating and modifying agent. Polylactide (PLA) presents biodegradable polymer with multifunctional application, widely engaged in medical related areas. Fosfomycin as functionalized phosphonates presents antibiotic properties expressed by broad spectrum of antimicrobial properties. The analysis of these biofunctionalized nonwoven fabrics processed by the melt-blown technique, included: scanning electron microscopy (SEM), UV/VIS transmittance, FTIR spectrometry, air permeability. The functionalized nonwovens were tested on microbial activity tests against colonies of gram-positive (Staphylococcus aureus) and gram-negative (Escherichia coli) bacteria.
1. Introduction
Polylactide (PLA) presents polymers of multifunctional application, widely used in medical related areas [1]. PLA hybrids with antibacterial additivities (bactericide agents) provide antiseptic properties, and therefore are applied in a variety of medical applications, namely, such as bioactive fibers for drug delivery [2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18], for tissue engineering applications [19,20,21,22], forwound healing materials [23,24,25,26] and membranes for efficient treatment of burns [27,28].
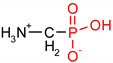
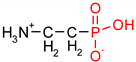
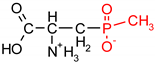
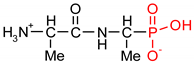
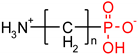
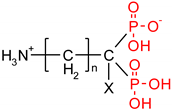
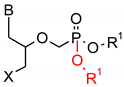
As one group of such potential additives/components, they can serve as functionalized phosphonates. Functionalized phosphonate compounds, especially amino-phosphonates, are known for their significant biological activity [29]. The list of representative biologically active phosphonates is presented in Table 1.

Table 1.
Structures, names, mode of actions and application area of representative biologically active functionalized phosphonic acid derivatives.
Fosfomycin (cis-1,2-epoxypropylphosphonic acid) exhibits broad spectrum activity against numerous bacterial, both gram-positive and gram-negative pathogens, including resistant and multi-resistant strains (Table 1).Fosfomycin acts as a bacterial cell wall inhibitor in the bacteria growth phase, interfering with the first committed step in peptidoglycan biosynthesis. Specifically, Fosfomycin irreversibly inhibits the UDP-N-acetylglucosamine enolpyruvyl transferase (MurA) enzyme by covalent modification of MurA (Figure 1), responsible for catalyzing the formation of N-acetylmuramic acid (precursor of peptidoglycan) [54].
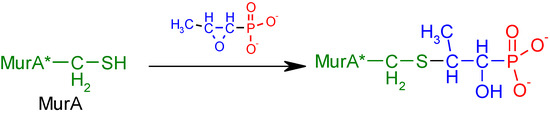
Figure 1.
Mechanism of action of fosfomycin on the UDP-N-acetylglucosamine enolpyruvyl transferase enzyme (MurA).
Due to the fact that Gram-positive and Gram-negative bacteria require the formation of N-acetylmuramic acid for the synthesis of peptidoglycan, fosfomycin’s antibacterial spectrum is very broad, and there is no possibility of crossed resistances with this compound. This antibiotic has therefore been employed for treating infections by multidrug-resistant pathogens such as methicillin-resistant Staphylococcus aureus (MRSA), methicillin-resistant coagulase-negative staphylococci (MRCNS), vancomycin-resistant enterococci (VRE), penicillin-resistant Streptococcus pneumoniae, extended-spectrum beta-lactamase (ESBL)-producing Enterobacterales, carbapenemase-producing Enterobacterales (CPE) and multidrug-resistant Pseudomonas aeruginosa [51].
Fosfomycin was accepted into clinical practice in the early 1970s [54,56]. Its use, however, has been limited for several years for treating mainly lower uncomplicated urinary tract infections (in the form of fosfomycin trometamol taken orally). Therefore, the application of fosfonomycin as an additive to various PLA medical utilities seems to be highly rational. However, the recent paper of Gulcuet al. revealed that the application of the PDL-fosfomycin hybrid used by authors in implant coating for the prevention of implant-related infections afforded worse results than corresponding hybrids based on PLA-gentamycin [58]. To dissolve these discrepancies, we undertook wide investigations on polymer hybrids, based on polylactide (PLA) nonwoven fabric modified on the surface by fosfomycin (FOSM).
As part of our research program directed on biologically active functionalized phosphonates [59,60,61,62,63], and their grafting on polymer matrix [64,65], we present the preparation, and physico-chemical and biological properties of PLA–FOS polymer hybrid.
2. Materials and Methods
2.1. Materials
2.1.1. Polymers
Poly(lactic acid) (PLA) granulate was purchased from NatureWorks LLC (Minnetonka, Minnesota, USA), type Ingeo™ Biopolymer 3251D, MFR = 30—40 g/10min (190 °C/2.16 kg), Tmp = 160—170 °C in the form of granulates, and was used for the fabrication of nonwoven samples.
2.1.2. Chemical Agents
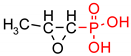
Fosfomycin[(−)-(1R,2S)-(1,2-Epoxypropyl)phosphonic acid,C3H5Na2O4P] from Sigma-Aldrich (St. Louis, MI, USA) was used for the surface modification of polymer nonwovens.
2.1.3. Finishing Agents
Lutexal Thickener HC (BASF, Ludwigshafen, Germany) – polyacrylate, ammonia salt as the thickening agent, ensures the viscosity and appropriate grip of product;
Pluriol 600 (BASF, Ludwigshafen, Germany) - poly(ethylene glycol) of molar mass 600 g/mol as the wetting agent, prevents the formation of agglomerates in coating paste and uniformity in coating dispersion;
Revacryl 247 (Synthomer, Essex, UK)- dispersion of low viscosity styrene-acrylic ester copolymer, combines all ingredients of coating paste with nonwoven fabric.
2.1.4. Bacterial Strains
Escherchia coli (ATCC 25922); Staphylococcus aureus (ATCC 6538) bacterial strains were purchased from Microbiologics (St. Cloud, MN, USA).
2.2. Methods
2.2.1. Nonwoven Fabrics
Poly(lactic acid) nonwovens were fabricated by the melt-blown technique, analogously as polypropylene nonwovens [65], using a one-screw laboratory extruder (Axon, Sweden) with a head with 30 holes of 0.35 mm diameter each, compressed air heater and collecting drum. Processing parameters for fabrication of poly(lactic acid) nonwoven are presented in Table 2.

Table 2.
Technique processing parameters applied for preparation of Poly(lactic acid) nonwoven.
2.2.2. Dip-Coating Modification of Poly(lactic acid) Nonwoven
Poly(lactic acid) nonwoven fabrics were modificated by the dip-coating method, analogously as polypropylene nonwovens [65]. The coating pastes of homogeneous dispersion and appropriate viscosity (70 dPas) were prepared based on styrene-acrylic resin, thickening agent, wetting agent and water. Component composition of used pastes is listed in Table 3.

Table 3.
Component composition of used pastes [%].
Fosfomycin powder was added into the paste in 3 variants of concentrations: 0.005%, 0.01%, 0.1%and then mixed for 10 min.
The poly(lactic acid) (PLA) nonwoven samples were impregnated with the paste, squeezed and dried to the constant weight for 5 h at a temperature of 50 °C. The increase of sample dry mass after modification was 15%.
2.2.3. SEM—Scanning Electron Microscopy
The scanning electron microscope analysis was performed on a TESCAN VEGA 3 SEM microscope (Czech Republic). The SEM microscopic examination of the surface topography was carried out in a high vacuum using the energy of the probe beam 20 ekV. The surface of each preparation was sprayed with a conductive substance (gold), using a Quorum Technologies Ltd. (UK) vacuum dust extractor. Magnification was 100×, 5000× and 20,000×.
2.2.4. ATR-FTIR—Attenuated Total Reflection Fourier Transform Infrared Spectroscopy
The chemical structure of poly(lactic acid) surface of nonwovens were assessed using ATR-FTIR spectroscopy in the range of 400–4000 cm−1 using a spectrometer Jasco’s 4200 (Tokyo, Japan) with ATR attachment Pike Gladi ATR (Cottonwood, AZ, USA).
2.2.5. UV-VIS Analysis
Changes of the physical properties as transmittance [%T] of poly(lactic acid) nonwoven fabrics, after coating modifications, were assessed using a double beam Jasco V-550 UV-VIS spectrophotometer (Tokyo, Japan) with integrating sphere attachment in the range: 200–800 nm.
2.2.6. Filtration Parameters
Air permeability of poly(lactic acid) nonwoven fabrics were determined for one layer of the nonwoven sample and the test, and were performed according to EN ISO 9237:1998 standard, analogously as polypropylene nonwovens [65]. An FX 3300 TEXTEST AG (Klimatest, Poland) permeability tester was used. Air at a pressure of 100 Pascal and 200 Pascal was passed through a fabric area of 20 cm2 diameter for testing. An average of 10 values was taken to be the final value of the sample.
2.2.7. Tensile Testing
Tensile testing of poly(lactic acid) nonwoven fabrics were carried out in accordance with the EN ISO 10319:2015-08 standard, analogously as polypropylene nonwovens [65]. A Tinius Olsen H50KS (Horsham, Pennsylvania, USA) tester was used. Stretching speed was 20 mm /min.
2.2.8. Microbial Activity
The antibacterial activity of resulting poly(lactic acid)nonwoven fabrics were tested according to PN-EN ISO 20645:2006 (Textile fabrics—Determination of antibacterial activity—Agar diffusion plate test) against a colony of gram-negative bacteria: Escherchia coli (ATCC 25922) and gram-positive bacteria: Staphylococcus aureus (ATCC 6538), analogously as polypropylene nonwovens [65].
Antibacterial activity of modified poly(lactic acid) nonwoven fabrics were tested by the agar diffusion method using Muller Hinton medium agar. The test was initiated by pouring each agar onto sterilized Petri dishes and it was allowed to solidify. The surfaces of agar media were inoculated by overnight broth cultures of bacteria (ATCC 25922: 2.2 × 108 CFU/mL, ATCC 6538: 1.9 × 108 CFU/Ml). Samples of sterile PLA discs (10 mm) were charged with coating pastes with various amounts of fosfomycin (Table 7) and then discs with hybrids PLA/Fosfomycin were placed onto the inoculated agar and incubated at 37 °C for 24 h. The diameter of the clear zone around the sample was measured as an indication of inhibition of the microbial species. All tests were carried out in duplicate. Simultaneously, the same tests were carried out for control samples—samples of unmodified nonwoven fabrics.
3. Resultsand Discussion
The analysis of the biofunctionalized poly(lactide) nonwoven fabrics covered: scanning electron microscopy (SEM), attenuated total reflection Fourier transform infrared spectroscopy (ATR-FTIR), UV/VIS transmittance, and technical parameters: filtration parameters, tensile properties.The activity against representative gram-negative bacteria (E. coli) and gram-positive bacteria (S. aureus) of nonwoven fabrics modified by fosfomycin has been performed.
3.1. Scanning Electron Microscopy
Scanning electron microscopy (SEM) presents the routine technique for morphological investigations of polylactide nonwovens, both electrospin PLA [66,67,68,69,70,71] as well as melt-blown PLA [72,73,74,75,76,77,78,79,80,81] fibers. Scanning electron microscopy spectra of polylactide nonwoven and polylactide nonwoven coated with Fosfomycin modifier are presented in Figure 2 and Figure 3, respectively.
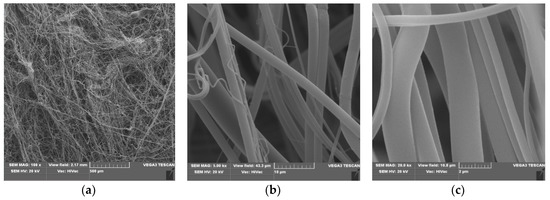
Figure 2.
SEM result of Polylactide nonwoven (PLA), magnification 100× (a), 5000× (b) and 20,000× (c).
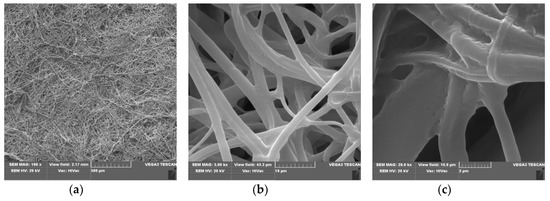
Figure 3.
The SEM result of Polylactide nonwoven with fosfomycincoating (PLA-MOD), magnification 100× (a), 5000× (b) and 20,000× (c).
SEM spectra of PLA nonwovens present uniform randomly oriented nanofibers, with interconnected pores (space) between the nanofibers and relatively smooth surface. The average diameters of PLA nanofibers range from 3 to 8 µm, (Figure 2b,c).
Morphological changes of PLA after a surface deposition of fosfomycin lead to more random orientation of the fibers, with a more rough surface, covered with dots of the modifier (Figure 3c). These are much more subtle than described for the modification of polypropylene nonwoven with Ala-AlaP [65].
3.2. ATR-FTIR Spectra
Comparison of ATR-FTIR spectra of fosfonomycin, polylactide nonwoven (PLA) and polylactide nonwoven charged with fosfonomycin (PLA-MOD) is presented in Figure 4, Figure 5 and Figure 6, respectively. Characteristic FTIR signals of fosfonomycin, and polylactide nonwoven (PLA)and polylactide–fosfonomycin nonwoven hybrid (PLA/fosfonomycin) are summarized in Table 4.
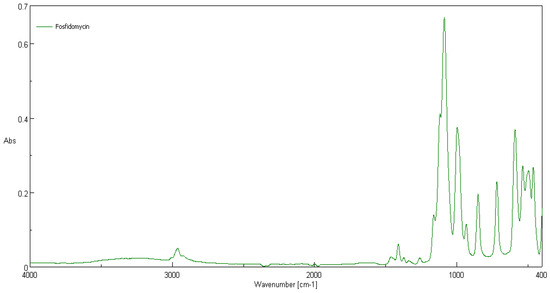
Figure 4.
ATR-FTIR spectrum of fosfonomycin.
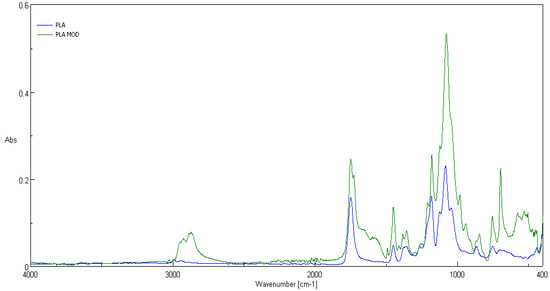
Figure 5.
ATR-FTIR spectrum of the polylactide nonwoven (PLA) and nonwoven sample coated by paste concentrations 0.1%fosfomycin (PLA-MOD).
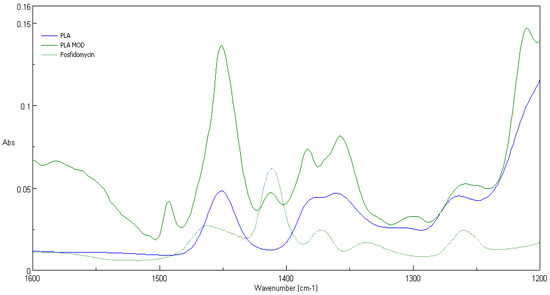
Figure 6.
FTIR bands determined forfosfonomycin in wave number: 1410 cm−1 in spectra of the nonwoven sample coated by paste with Fosfomycin (PLA-MOD).

Table 4.
Characteristic FTIR bands determined for fosfomycin (Fosfm), polylactide (PLA) and the fosfomycin–polylactide nonwoven hybrid (PLA/Fosfm).
The IR spectrum ofpolylactide nonwoven coated by coating paste with 0.1%fosfomycinconcentrations (PLA-MOD) reveals the band derived from representative band of herbicidal agent, namely at 1410 cm−1.
3.3. UV/VIS Transmittance Spectra
Transmittance spectra [%T] of polypropylene nonwoven samples (PLA) and coated nonwoven sample with fosfomycin modifier (PLA-MOD) in the range λ = 200–800 nm are presented in Figure 7.
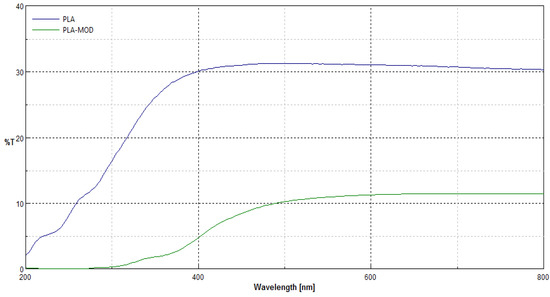
Figure 7.
Comparison of transmittance spectra [%T] of polylactide nonwoven samples without (PLA) and with Fosfomycin coating (PLA-MOD) in the range λ = 200–800 nm.
Transmittance (%T) spectra in the range λ = 200–800 nm of samples after modification revealed changes in macrostructure of the nonwoven after coating modification, expressed by the depress of transmittance ability in the all range of measuring spectra. Here, only the transmittance spectra of nonwoven coated by paste concentrations 0.1%fosfomycin (PLA-MOD) samples are shown, because the transmittance spectra’s nonwovens with different fosfomycin contents had the similar spectral characteristics and transmittance.
3.4. Technical Parameters
Technical parameters of coatedpolylactide nonwovens focused on filtration parameters and tensile strength. Filtration parameters expressed by the air permeability were detected for clean polylactide nonwoven and coatednonwovens. All these results are shown in Table 5 and indicated that modifications decreased, roughly two times (910 vs. 400–449 at 100 Pa and 880–890 at 200 Pa, respectively), the filtration properties of all nonwoven samples. Modified nonwoven samples with different fosfomycin contents had approximately a similar result for filtration properties. The fosfomycin content in the applied coating pastes on the polylactide nonwoven exhibit only slight effects on the filtration properties.

Table 5.
The air flow resistance of modified polylactide (PLA) nonwovens, according to: EN ISO 9237:1998.
The testing results of tensile strength durability for stretching [kN/m] and relative elongation at maximum load [%] of polylactidenonwoven fabrics and modified polylactide (PLA) nonwovensare listed in Table 6.

Table 6.
Results of tensile strength test of modified polylactide (PLA) nonwovens.
The results show increased oftensile strength for modified PLA nonwovens (0.120 [kN/m]) in comparison with unmodified nonwoven (0.032 [kN/m]). There are no significant effects of modification on relative elongation of tested samples.
3.5. Antimicrobial Activity
The polylactide nonwoven (PLA), and polylactide nonwoven charged with fosfonomycin (PLA-MOD) were subjected to antimicrobial activity tests against gram-negative E. coli (ATCC11229) and gram-positive S. aureus (ATCC 6538) [84,85,86] (Table 7).

Table 7.
Results of tests on the antibacterial activity of fosfomycin modified nonwovens.
The results of these studies prove antimicrobial protection against different bacterial microorganisms of biofunctionalized materials. Even only a 0.005% concentration of Fosfomycin coating paste applied on polylactic acidnonwovens provides antimicrobial properties for E. coli and against S. aureus (Table 7), expressed by bold visible inhibition zones of bacterial growthin Petri dishes (Figure 8 and Figure 9).
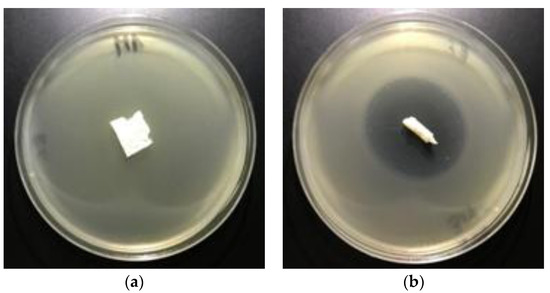
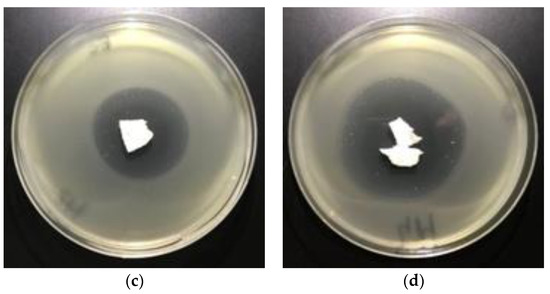
Figure 8.
The nonwovens antimicrobial activity tests against E. coli. Inhibition zones of bacterial growth on Petri dishes, modified PLA nonwoven with fosfomycin coating pastes concentrations: a—0%; b—0.005%; c—0.01%; d—0.1%.
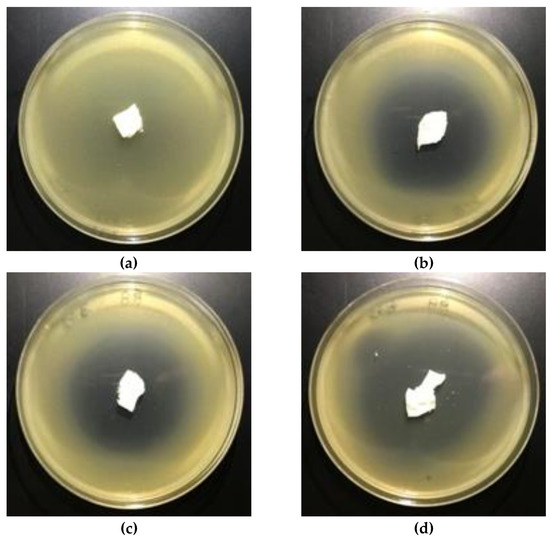
Figure 9.
The nonwovens antimicrobial activity tests against S. aureus. Inhibition zones of bacterial growth on Petri dishes, modified PLA nonwoven with fosfomycin coating pastes concentrations: a—0%, b—0.005%; c—0.01%; d—0.1%.
4. Conclusions
As the consumption of disposable nonwoven products with short life increases, biodegradable polymers have great potential in use. On the other hand, demand for nonwovens is continually increasing. Polylactic acid (PLA) brings environmental benefits and can be used for the production of nonwoven fabrics.
This study focused on the functionalization of PLA nonwoven by the coating of bioactive compounds—Fosfomycin, into/on to the surface of their fibers. Fosfomycin is a low cost commercial antibiotic of natural occurrence, originally produced by certain types of Streptomyces, characterized by safety of use. The structure and mechanical properties of the obtained new biofunctionalized PLA nonwoven products were characterized by scanning electron microscopy (SEM), UV/VIS transmittance, FTIR spectrometry, and air permeability. This work has shown that our coating technology can be used to prepare new biodegradable or, “eco-friendly” biocomposite products with good mechanical properties. A significant attribute of the described process is uncomplicated, safe of implementation on an industrial scale and low production costs. All of the chemical agents and finishing agents which were used in this work are popular and easily available. The specific advantage of using hybrid materials based on polylactic acid (PLA) is the selected application in biomedical areas as an antibacterial material.
Author Contributions
M.H.K. designed the research study, contributed to the data interpretation and to the manuscript drafting and revisions, analyzed the data, and contributed to writing the manuscript. Z.M. analyzed the data and participated in the publication preparation. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Polish Ministry of Science and Higher Education within statutory research work carried out at the Lukasiewicz Research Network -Textile Research Institute, Lodz, Poland.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Auras, R.; Lim, L.T.; Selke, S.E.M.; Tsuji, H. (Eds.) Poly(Lactic Acid): Synthesis, Structures, Properties, Processing, and Applications; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010. [Google Scholar]
- Gollwitzer, H.; Ibrahim, K.; Meyer, H.; Mittelmeier, W.; Busch, R.; Stemberger, A. Antibacterial poly(D,L-lactic acid) coating of medical implants using a biodegradable drug delivery technology. J. Antimicrob. Chemother. 2003, 51, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Del Valle, L.J.; Camps, R.; Díaz, A.; Alfonso, L.F.; Rodríguez-Galán, A.; Puiggalí, J. Electrospinning of polylactide and polycaprolactone mixtures for preparation of materials with tunable drug release properties. J. Polym. Res. 2011, 18, 1903–1917. [Google Scholar] [CrossRef]
- Reise, M.; Wyrwa, R.; Müller, U.; Zylinski, M.; Voelpel, A.; Schnabelrauch, M.; Berg, A.; Jandt, K.D.; Watts, D.C.; Sigusch, B.W. Release of metronidazole from electrospun poly(l-lactide-co-d/l-lactide) fibers for local periodontitis treatment. Dent. Mater. 2012, 28, 179–188. [Google Scholar] [CrossRef]
- Qin, Y.; Yuan, M.; Li, L.; Li, W.; Xue, J. Formulation and evaluation of in situ forming PLA implant containing tinidazole for the treatment of periodontitis. J. Biomed. Mater. Res. B Appl. Biomater. 2012, 100, 2197–2202. [Google Scholar] [CrossRef]
- Torres-Giner, S.; Martinez-Abad, A.; Gimeno-Alcañiz, J.V.; Ocio, M.J.; Lagaron, J.M. Controlled delivery of gentamicin antibiotic from bioactive electrospun polylactide-based ultrathin fibers. Adv. Eng. Mater. 2012, 14, B112–B122. [Google Scholar] [CrossRef]
- Kau, Y.C.; Chen, D.W.C.; Hsieh, Y.T.; Lee, F.Y.; Liu, S.J. Compression molding of biodegradable drug-eluting implants for sustained release of metronidazole and doxycycline. J. Appl. Polym. Sci. 2013, 127, 554–560. [Google Scholar] [CrossRef]
- Zhou, J.; Han, S.; Dou, Y.; Lu, J.; Wang, C.; He, H.; Li, X.; Zhang, J. Nanostructured poly(l-lactide) matrix as novel platform for drug delivery. Int. J. Pharm. 2013, 448, 175–188. [Google Scholar] [CrossRef]
- Chen, S.; Wang, G.; Wu, T.; Zhao, X.; Liu, S.; Li, G.; Cui, W.; Fan, C. Silver nanoparticles/ibuprofen-loaded poly(L-lactide) fibrous membrane: Anti-infection and anti-adhesion effects. Int. J. Mol. Sci. 2014, 15, 14014–14025. [Google Scholar] [CrossRef]
- Pérez-Madrigal, M.M.; Llorens, E.; Del Valle, L.J.; Puiggali, J.; Armelin, E.; Alemán, C. Semiconducting, biodegradable and bioactive fibers for drug delivery. Express Polym. Lett. 2016, 10, 628–646. [Google Scholar] [CrossRef]
- Schkarpetkin, D.; Reise, M.; Wyrwa, R.; Völpel, A.; Berg, A.; Schweder, M.; Schnabelrauch, M.; Watts, D.C.; Sigusch, B.W. Development of novel electrospun dual-drug fiber mats loaded with a combination of ampicillin and metronidazole. Dent. Mater. 2016, 32, 951–960. [Google Scholar] [CrossRef]
- Yakub, G.; Toncheva, A.; Manolova, N.; Rashkov, I.; Danchev, D.; Kussovski, V. Electrospun polylactide-based materials for curcumin release: Photostability, antimicrobial activity, and anticoagulant effect. J. Appl. Polym. Sci. 2016, 133, 42940. [Google Scholar] [CrossRef]
- Herrera, M.T.; Artunduaga, J.J.; Ortiz, C.C.; Torres, R.G. Synthesis of antibiotic loaded polylactic acid nanoparticles and their antibacterial activity against Escherichia coli O157:H7 and methicillin-resistant Staphylococcus aureus. Biomedica 2017, 37, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Karaszewska, A.; Kamińska, I.; Kiwała, M.; Gadzinowski, M.; Gosecki, M.; Słomkowski, S. Preparation and properties of textile materials modified with triclosan-loaded polylactide microparticles. Polym. Adv. Technol. 2017, 28, 1185–1193. [Google Scholar] [CrossRef]
- Shahi, R.G.; Albuquerque, M.T.P.; Münchow, E.A.; Blanchard, S.B.; Gregory, R.L.; Bottino, M.C. Novel bioactive tetracycline-containing electrospun polymer fibers as a potential antibacterial dental implant coating. Odontology 2017, 105, 354–363. [Google Scholar] [CrossRef]
- Han, C.; Cai, N.; Chan, V.; Liu, M.; Feng, X.; Yu, F. Enhanced drug delivery, mechanical properties and antimicrobial activities in poly(lactic acid) nanofiber with mesoporous Fe3O4-COOH nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2018, 559, 104–114. [Google Scholar] [CrossRef]
- Cui, S.; Sun, X.; Li, K.; Gou, D.; Zhou, Y.; Hu, J.; Liu, Y. Polylactide nanofibers delivering doxycycline for chronic wound treatment. Mat. Sci. Eng. C 2019, 104, 109745. [Google Scholar] [CrossRef]
- Zhao, X.; Han, Y.; Zhu, T.; Feng, N.; Sun, Y.; Song, Z.; Li, S.; Liu, J.; Ding, J. Electrospun polylactide-nano-hydroxyapatite-vancomycin composite scaffolds for advanced osteomyelitis therapy. J. Biomed. Nanotechnol. 2019, 16, 1213–1222. [Google Scholar] [CrossRef]
- Davachi, S.M.; Kaffashi, B.; Zamanian, A.; Torabinejad, B.; Ziaeirad, Z. Investigating composite systems based on poly L-lactide and poly L-lactide/triclosan nanoparticles for tissue engineering and medical applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 58, 294–309. [Google Scholar] [CrossRef]
- Bertuoli, P.T.; Ordoño, J.; Armelin, E.; Pérez-Amodio, S.; Baldissera, A.F.; Ferreira, C.A.; Puiggalí, J.; Engel, E.; del Valle, L.J.; Alemán, C. Electrospun conducting and biocompatible uniaxial and core–shell fibers having poly(lactic acid), poly(ethylene glycol), and polyaniline for cardiac tissue engineering. ACS Omega 2019, 4, 3660–3672. [Google Scholar] [CrossRef]
- Chen, X.; Gao, C.; Jiang, J.; Wu, Y.; Zhu, P.; Chen, G. 3D printed porous PLA/nHA composite scaffolds with enhanced osteogenesis and osteoconductivity in vivo for bone regeneration. Biomed. Mater. 2019, 14, 065003. [Google Scholar] [CrossRef]
- Farzamfar, S.; Naseri-Nosar, M.; Sahrapeyma, H.; Ehterami, A.; Goodarzi, A.; Rahmati, M.; Lakalayeh, G.A.; Ghorbani, S.; Vaez, A.; Salehi, M. Tetracycline hydrochloride-containing poly (ε-caprolactone)/poly lactic acid scaffold for bone tissue engineering application: In vitro and in vivo study. Int. J. Polym. Mater. Polym. 2019, 68, 472–479. [Google Scholar] [CrossRef]
- Wang, H.; Wei, Q.; Wang, X.; Gao, W.; Zhao, X. Antibacterial properties of PLA nonwoven medical dressings coated with nanostructured silver. Fiber. Polym. 2008, 9, 556–560. [Google Scholar] [CrossRef]
- Liu, Y.; Liang, X.; Zhang, R.; Lan, W.; Qin, W. Fabrication of electrospun polylactic acid/ Cinnamaldehyde/β-cyclodextrin fibers as an antimicrobial wound dressing. Polymers 2017, 9, 464. [Google Scholar] [CrossRef]
- Foong, C.Y.; Hamzah, M.S.A.; Razak, S.I.A.; Saidin, S.; Nayan, N.H.M. Influence of poly(lactic acid) layer on the physical and antibacterial properties of dry bacterial cellulose sheet for potential acute wound healing materials. Fiber. Polym. 2018, 19, 263–271. [Google Scholar] [CrossRef]
- Vakilian, S.; Norouzi, M.; Soufi-Zomorrod, M.; Shabani, I.; Hosseinzadeh, S.; Soleimani, M.L. Inermis-loaded nanofibrous scaffolds for wound dressing applications. Tissue Cell 2018, 51, 32–38. [Google Scholar] [CrossRef]
- Moslem, Z.; Sadri, M.; Pebdeni, A.B. Antimicrobial and cellular activity of poly(L-lactide)/ chitosan/Imipenem antibiotic composite nanofibers. Fiber. Polym. 2016, 17, 1336–1342. [Google Scholar] [CrossRef]
- Li, W.; Yu, Q.; Yao, H.; Zhu, Y.; Topham, P.D.; Yue, K.; Ren, L.; Wang, L. Superhydrophobic hierarchical fiber/bead composite membranes for efficient treatment of burns. Acta Biomater. 2019, 92, 60–70. [Google Scholar] [CrossRef]
- Kukhar, V.P.; Hudson, H.R. (Eds.) Aminophosphonic and Aminophosphinic Acids. Chemistry and Biological Activity; Wiley&Sons Ltd.: New York, NY, USA, 2000. [Google Scholar]
- Parajuli, K.R.; Zhang, Q.; Liu, S.; You, Z. Aminomethylphosphonic acid inhibits growth and metastasis of human prostate cancer in an orthotopic xenograft mouse model. Oncotarget 2016, 7, 10616–10626. [Google Scholar] [CrossRef][Green Version]
- Parajuli, K.R.; Zhang, Q.; Liu, S.; You, Z. Aminomethyl phosphonic acid and methoxyacetic acid induce apoptosis in prostate cancer cells. Int. J. Mol. Sci. 2015, 16, 11750–11765. [Google Scholar] [CrossRef]
- Horiguchi, M.; Kandatsu, M. Isolation of 2-aminoethane phosphonic acid from rumen protozoa. Nature 1959, 184 (Suppl. 12), 901–902. [Google Scholar] [CrossRef]
- Tan, S.A.; Tan, L.G. Distribution of ciliatine (2-aminoethylphosphonic acid) and phosphonoalanine (2-amino-3-phosphonopropionic acid) in human tissues. Clin. Physiol. Biochem. 1989, 7, 303–309. [Google Scholar] [PubMed]
- Watts, M. Glufosinate-Ammonium Monograph. 2008. Available online: http://www.pananz.net/wp-content/uploads/2013/04/Glufosinate-monograph-12-Dec-2008.pdf (accessed on 18 June 2019).
- Franz, J.E.; Mao, M.K.; Sikorski, J.A. Glyphosate: A Unique Global Herbicide; American Chemical Society: Washington, DC, USA, 1997. [Google Scholar]
- Dill, G.M.; Sammons, R.D.; Feng, P.C.C.; Kohn, F.; Kretzmer, K.; Mehrsheikh, A.; Bleeke, M.; Honegger, J.L.; Farmer, D.; Wright, D.; et al. Glyphosate: Discovery, development, applications, and properties. In Glyphosate Resistance in Crops and Weeds. History, Development and Management; Nandula, V.K., Ed.; John Wiley& Sons, Inc.: Hoboken, NJ, USA, 2010. [Google Scholar] [CrossRef]
- Allen, J.G.; Atherton, F.R.; Hall, M.J.; Hassal, C.H.; Holmes, S.W.; Lambert, R.W.; Nisbet, L.J.; Ringrose, P.S. Phosphonopeptides, a new class of synthetic antibacterial agents. Nature 1978, 272, 56–58. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.G.; Atherton, F.R.; Hall, M.J.; Hassall, C.H.; Holmes, S.W.; Lambert, R.W.; Nisbet, L.J.; Ringrose, P.S. Phosphonopeptides as antibacterial agents: Alaphosphin and related phosphono-peptides. Antimicrob. Agents Chemother. 1979, 15, 684–695. [Google Scholar] [CrossRef]
- Atherton, F.R.; Hall, M.J.; Hassall, C.H.; Lambert, R.W.; Lloyd, W.J.; Ringrose, P.S. Phosphonopeptides as antibacterial agents: Mechanism of action of alaphosphin. Antimicrob. Agents Chemother. 1979, 15, 696–705. [Google Scholar] [CrossRef]
- Atherton, F.R.; Hassall, C.H.; Lambert, R.W. Synthesis and structure-activity relationships of antibacterial phosphonopeptides incorporating (1-aminoethyl)phosphonic acid and (aminomethyl)-phosphonic acid. J. Med. Chem. 1986, 29, 29–40. [Google Scholar] [CrossRef]
- Lejczak, B.; Kafarski, P.; Sztajer, H.; Mastalerz, P. Antibacterial activity of phosphono dipeptides related to alafosfalin. J. Med. Chem. 1986, 29, 2212–2217. [Google Scholar] [CrossRef]
- Neu, H.C.; Kamimura, T. In vitro and in vivo antibacterial activity of FR-31564, a phosphonic acid antimicrobial agent. Antimicrob.Agents Chemother. 1981, 19, 1013–1023. [Google Scholar] [CrossRef]
- Jomaa, H.; Wiesner, J.; Sanderbrand, S.; Altincicek, B.; Weidemeyer, C.; Hintz, M.; Türbachova, I.; Eberl, M.; Zeidler, J.; Lichtenthaler, H.K.; et al. Inhibitors of the nonmevalonate pathway of isoprenoid biosynthesis as antimalarial drugs. Science 1999, 285, 1573–1576. [Google Scholar] [CrossRef]
- Wiesner, J.; Borrmann, S.; Jomaa, H. Fosmidomycin for the treatment of malaria. Parasitol. Res. 2003, 90 (Suppl. 2), S71–S76. [Google Scholar] [CrossRef]
- Zhang, B.; Watts, K.M.; Hodge, D.; Kemp, L.M.; Hunstad, D.A.; Hicks, L.M.; Odom, A.R. A second target of the antimalarial and antibacterial agent fosmidomycin revealed by cellular metabolic profiling. Biochemistry 2011, 50, 3570–3577. [Google Scholar] [CrossRef]
- Krishna, S.; Staines, H.M. Non-Antifolate Antibiotics: Clindamycin, Doxycycline, Azithromycin and Fosmidomycin. In Treatment and Prevention of Malaria. Milestones in Drug Therapy; Staines, H., Krishna, S., Eds.; Springer: Basel, Switzerland, 2011; Volume 41, pp. 141–156. [Google Scholar] [CrossRef]
- Jane, D. Neuroactive aminophosphonic and aminophosphinic acid derivatives. In Aminophosphonic and Aminophosphinic Acids. Chemistry and Biological Activity; Kukhar, V.P., Hudson, H.R., Eds.; Wiley& Sons Ltd.: New York, NY, USA, 2000; Chpt. 14; pp. 483–536. [Google Scholar]
- Cremers, S.; Drake, M.T.; Ebetino, F.H.; Bilezikian, J.P.; Russell, R.G.G. Pharmacology of bisphosphonates. Br. J. Clin. Pharmacol. 2019, 85, 1052–1062. [Google Scholar] [CrossRef] [PubMed]
- Cremers, S.; Papapoulos, S. Pharmacology of bisphosphonates. Bone 2011, 49, 42–49. [Google Scholar] [CrossRef]
- Rogers, M.J.; Gordon, S.; Benford, H.L.; Coxon, F.P.; Luckman, S.P.; Monkkonen, J.; Frith, J.C. Cellular and molecular mechanisms of action of bisphosphonates. Cancer 2000, 88 (Suppl. 12), 2961–2978. [Google Scholar] [CrossRef]
- Russell, R.G.G.; Croucher, P.I.; Rogers, M.J. Bisphosphonates: Pharmacology, mechanisms of action and clinical uses. Osteoporos. Int. 1999, 9 (Suppl. 2), S66–S80. [Google Scholar] [CrossRef]
- De Clercq, E.; Holý, A. Acyclic nucleoside phosphonates: A key class of antiviral drugs. Nat. Rev. Drug Discov. 2005, 4, 928–940. [Google Scholar] [CrossRef] [PubMed]
- De Clercq, E. A 40-year journey in search of selective antiviral chemotherapy. Annu. Rev. Pharmacol. Toxicol. 2011, 51, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.S.; Balfour, J.A.; Bryson, H.M. Fosfomycin Tromethamine. A Review of its antibacterial activity, pharmacokinetic properties and therapeutic efficacy as a single-dose oral treatment for acute uncomplicated lower urinary tract infections. Drugs 1997, 53, 537–656. [Google Scholar] [CrossRef]
- Falagas, M.E.; Giannopoulou, K.P.; Kokolakis, G.N.; Rafailidis, P.I. Fosfomycin: Use beyond urinary tract and gastrointestinal infections. Clin. Infect. Dis. 2008, 46, 1069–1077. [Google Scholar] [CrossRef] [PubMed]
- Falagas, M.E.; Vouloumanou, E.K.; Samonis, G.; Vardakas, K.Z. Fosfomycin. Clin. Microbiol. Rev. 2016, 29, 321–347. [Google Scholar] [CrossRef]
- Díez-Aguilar, M.; Cantón, R. New microbiological aspects of fosfomycin. Rev. Esp. Quimioter. 2019, 32 (Suppl. 1), 8–18. [Google Scholar]
- Gulcu, A.; Akman, A.; Demirkan, A.F.; Yorukoglu, A.C.; Kaleli, I.; Bir, F. Fosfomycin Addition to Poly(D,L-Lactide) Coating does not affect prophylaxis efficacy in rat implant-related infection model, but that of gentamicin does. PLoS ONE 2016, 11, e0165544. [Google Scholar] [CrossRef]
- Drabowicz, J.; Jakubowski, H.; Kudzin, M.H.; Kudzin, Z.H. Nomenclature of aminoalkylphosphonic acids and derivatives. Evolution of the code system. Acta Biochim. Pol. 2015, 62, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Kudzin, Z.H.; Kudzin, M.H.; Drabowicz, J.; Stevens, C.V. Aminophosphonic acids-phosphorus analogues of natural amino acids. Part 1: Syntheses of α-aminophosphonic acids. Curr. Org. Chem. 2011, 15, 2015–2071. [Google Scholar] [CrossRef]
- Kudzin, Z.H.; Depczyński, R.; Kudzin, M.H.; Drabowicz, J. 1-(N-chloroacetylamino)-alkylphosphonic acids - Synthetic precursors of phosphonopeptides. Amino Acids 2008, 34, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Kudzin, Z.H.; Kudzin, M.H.; Drabowicz, J. Thioureidoalkylphosphonates in the synthesis of 1-aminoalkylphosphonic acids. The Ptc-aminophosphonate method. Arkivoc 2011, 2011, 227–269. [Google Scholar] [CrossRef]
- Drabowicz, J.; Jordan, F.; Kudzin, M.H.; Kudzin, Z.H.; Stevens, C.V.; Urbaniak, P. Reactivity of aminophosphonic acids. Oxidative dephosphonylation of 1-aminoalkylphosphonic acids by aqueous halogens. Dalton Trans. 2016, 45, 2308–2317. [Google Scholar] [CrossRef]
- Sójka-Ledakowicz, J.; Chruściel, J.J.; Kudzin, M.H.; Łatwińska, M.; Kiwała, M. Antimicrobial functionalization of textile materials with copper silicate. Fibres Text. East. Eur. 2016, 24, 151–156. [Google Scholar] [CrossRef]
- Kudzin, M.H.; Mrozińska, Z.; Walawska, A.; Sójka-Ledakowicz, J. Biofunctionalization of textile materials.1. Biofunctionalization of poly(propylene) (PP) nonwovens fabrics by alafosfalin. Coatings 2019, 9, 412. [Google Scholar] [CrossRef]
- Jia, L.; Prabhakaran, M.P.; Qin, X.; Ramakrishna, S. Stem cell differentiation on electrospun nanofibrous substrates for vascular tissue engineering. Mater. Sci. Eng. C 2013, 33, 4640–4650. [Google Scholar] [CrossRef]
- Casasola, R.; Thomas, N.L.; Trybala, A.; Georgiadou, S. Electrospun poly lactic acid (PLA) fibres: Effect of different solvent systems on fibre morphology and diameter. Polymer 2014, 55, 4728–4737. [Google Scholar] [CrossRef]
- Gómez-Pachón, E.Y.; Vera-Graziano, R.; Campos, R.M. Structure of poly(lactic-acid) PLA nanofibers scaffolds prepared by electrospinning. IOP Conf. Ser. Mater. Sci. Eng. 2014, 59, 012003. [Google Scholar] [CrossRef]
- Piccirillo, G.; Bochicchio, B.; Pepe, A.; Schenke-Layland, K.; Hinderer, S. Electrospun poly-L-lactide scaffold for the controlled and targeted delivery of a synthetically obtained Diclofenac prodrug to treat actinic keratosis. Acta Biomater. 2017, 52, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Wang, L.; Xiang, C.; Li, L. Electrospun porous PLLA and poly(LLA-co-CL) fibers by phase separation. New J. Chem. 2018, 42, 5102. [Google Scholar] [CrossRef]
- Matysiak, W.; Kapica, A.; Tański, T.; Dubiel, A. Analysis of the influence of electro spinning process parameters on the morphology of poly(Lactic acid) fibres. Arch. Mater. Sci. Eng. 2019, 96, 73–78. [Google Scholar] [CrossRef]
- Krucińska, I.; Surma, B.; Chrzanowski, M.; Skrzetuska, E.; Puchalski, M. Application of melt-blown technology in the manufacturing of a solvent vapor-sensitive, non-woven fabric composed of poly(lactic acid) loaded with multi-walled carbon nanotubes. Text. Res. J. 2013, 83, 859–870. [Google Scholar] [CrossRef]
- Chrzanowska, O.; Struszczyk, M.H.; Krucinska, I. Small diameter tubular structure design using solvent-free textile techniques. J. Appl. Polym. Sci. 2014, 131, 40147. [Google Scholar] [CrossRef]
- Majchrzycka, K.; Brochocka, A.; Brycki, B. Biocidal agent for modification of poly(lactic acid) high-efficiency filtering nonwovens. Fibres Text. East. Eur. 2015, 23, 88–95. [Google Scholar] [CrossRef]
- Łatwińska, M.; Sójka-Ledakowicz, J.; Chruściel, J.; Piórkowski, M. PLA and PP composite nonwoven with antimicrobial activity for filtration applications. Int. J. Polym. Sci. 2016, 2016, 2510372. [Google Scholar] [CrossRef]
- Szuman, K.; Krucińska, I.; Boguń, M.; Draczyński, Z. PLA/PHA- biodegradable blends for pneumothermic fabrication of nonwovens. Autex Res. J. 2016, 16, 119–127. [Google Scholar] [CrossRef]
- Feng, J. Preparation and properties of poly(lactic acid) fiber melt blown non-woven disordered mats. Mater. Lett. 2017, 189, 180–183. [Google Scholar] [CrossRef]
- Yu, B.; Wang, M.; Sun, H.; Zhu, F.; Han, J.; Bhat, G. Preparation and properties of poly (lactic acid)/magnetic Fe3O4 composites and nonwovens. RSC Adv. 2017, 7, 41929–41935. [Google Scholar] [CrossRef]
- Vadas, D.; Kmetykó, D.; Marosi, G.; Bocz, K. Application of melt-blown Poly(lactic acid) fibres in self-reinforced composites. Polymers 2018, 10, 766. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, G.; Bhat, G.S.; Azari, H.; Pen, H. Electret characteristics of melt-blown polylactic acid fabrics for air filtration application. J. Appl. Polym. Sci. 2020, 137, 48309. [Google Scholar] [CrossRef]
- Zhu, F.; Yu, B.; Su, J.; Han, J. Study on PLA/PA11 bio-based toughening melt-blown nonwovens. Autex Res. J. 2020, 20. in press. [Google Scholar] [CrossRef]
- Kister, G.; Cassanas, G.; Vert, M. Effects of morphology, conformation and configuration on the IR and Raman spectra of various poly( lactic acid)s. Polymer 1998, 39, 267–273. [Google Scholar] [CrossRef]
- Carstenn-Lichterfelde, C.; Fernandez-Ibanez, M.; Gálvez-Ruano, E.; Bellanato, J. Structural study of fosfomycin [(–)-cis-1,2-epoxypropylphosphonic acid] salts and related compounds. J. Chem. Soc. Perkin Trans. 2 1983, 943–947. [Google Scholar] [CrossRef]
- ENISO 20645. 2006–Textile Fabrics–Determination of Antibacterial Activity—Agar Diffusion Plate Test; International Organization for Standardization: Geneva, Switzerland, 2006. [Google Scholar]
- Jiang, L.; Wang, F.; Han, F.; Prinyawiwatkul, W.; No, H.K.; Ge, B. Evaluation of diffusion and dilution methods to determine the antimicrobial activity of water-soluble chitosan derivatives. J. Appl. Microbiol. 2012, 114, 956–963. [Google Scholar] [CrossRef]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).