Comparative Investigation on the Soil Burial Degradation Behaviour of Polymer Films for Agriculture before and after Photo-Oxidation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
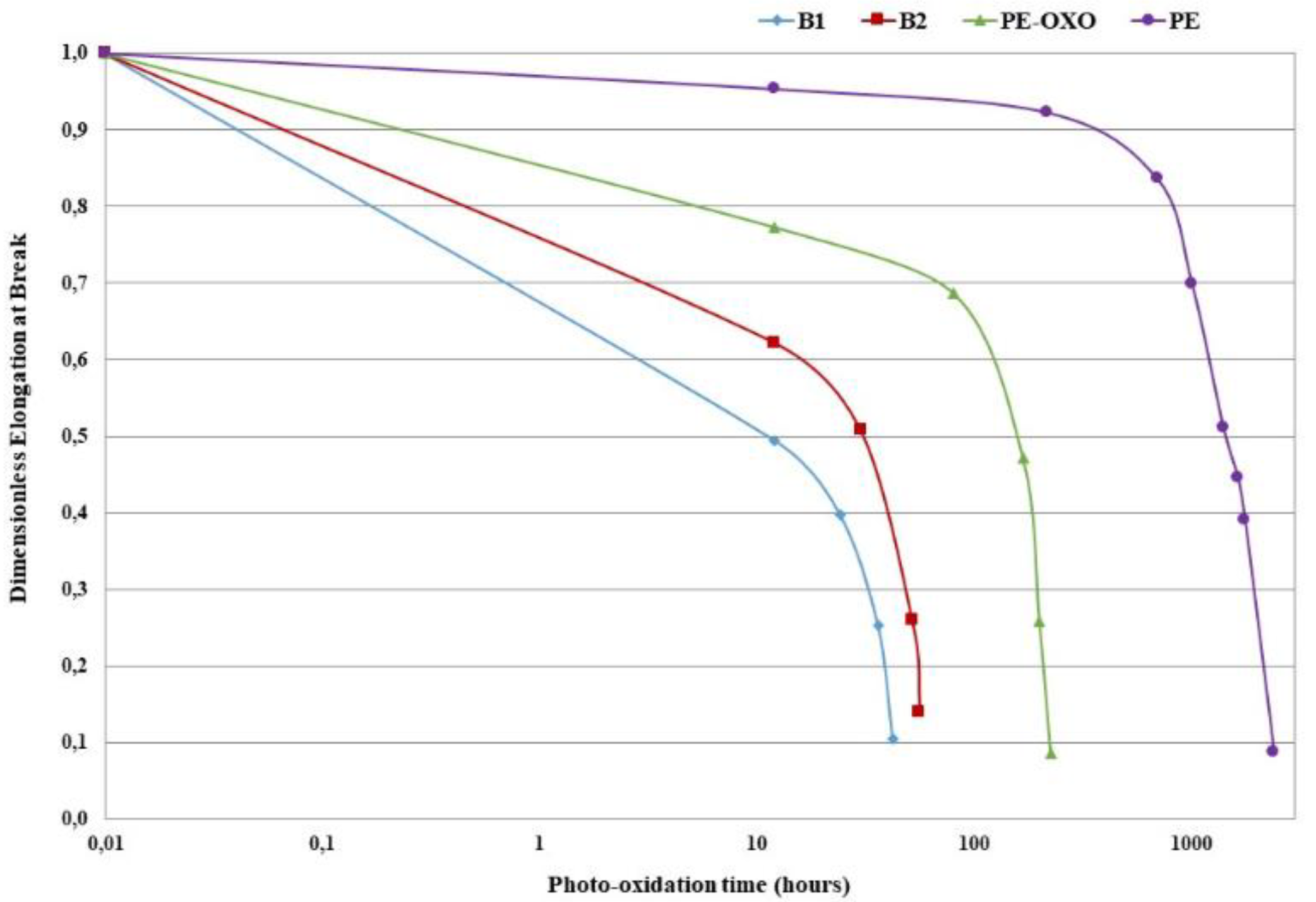
2.2.1. Accelerated Weathering Tests
2.2.2. Mechanical Properties
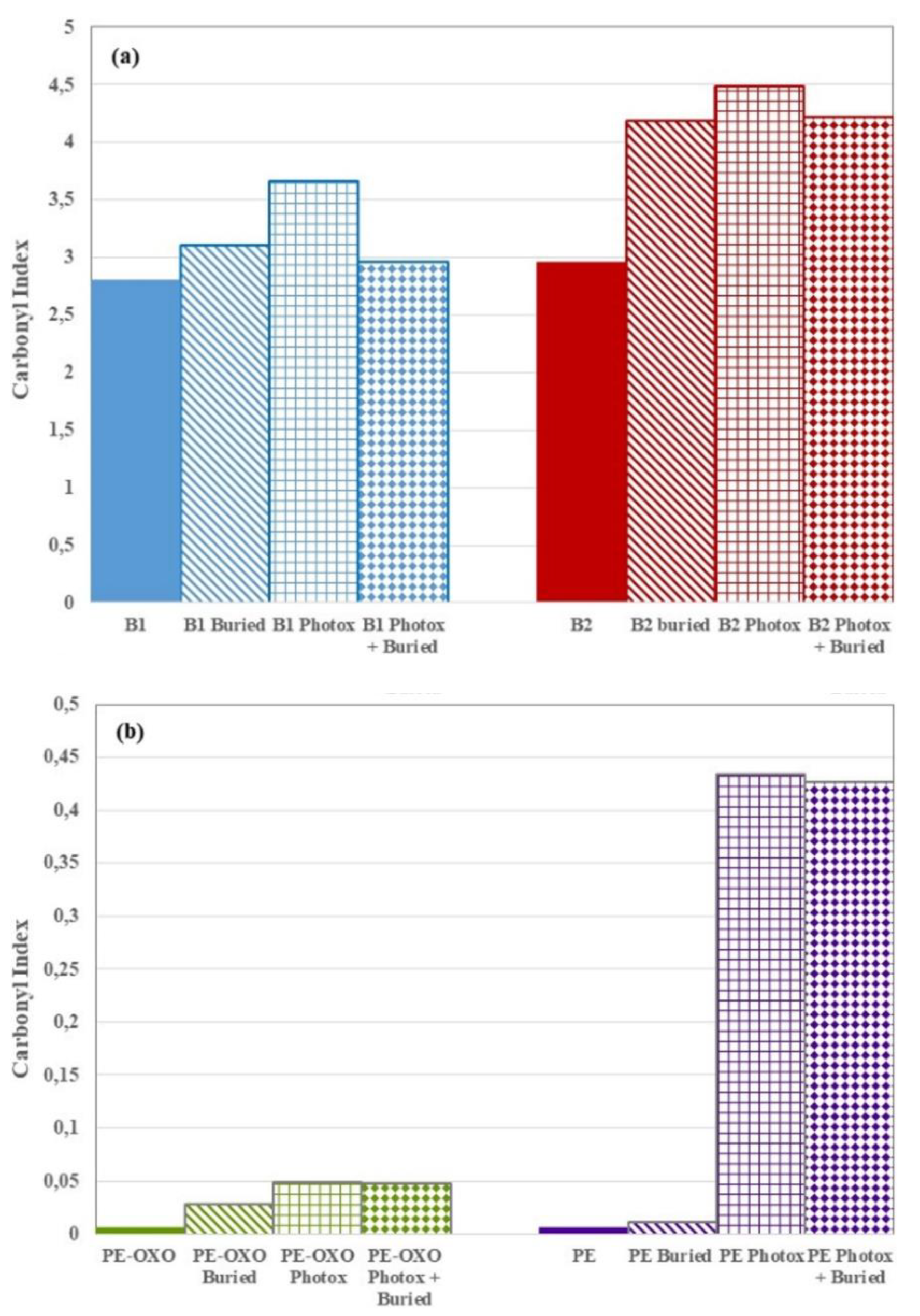
2.2.3. Attenuated Total Reflection-Fourier Transform Infra-Red (ATR-FTIR)
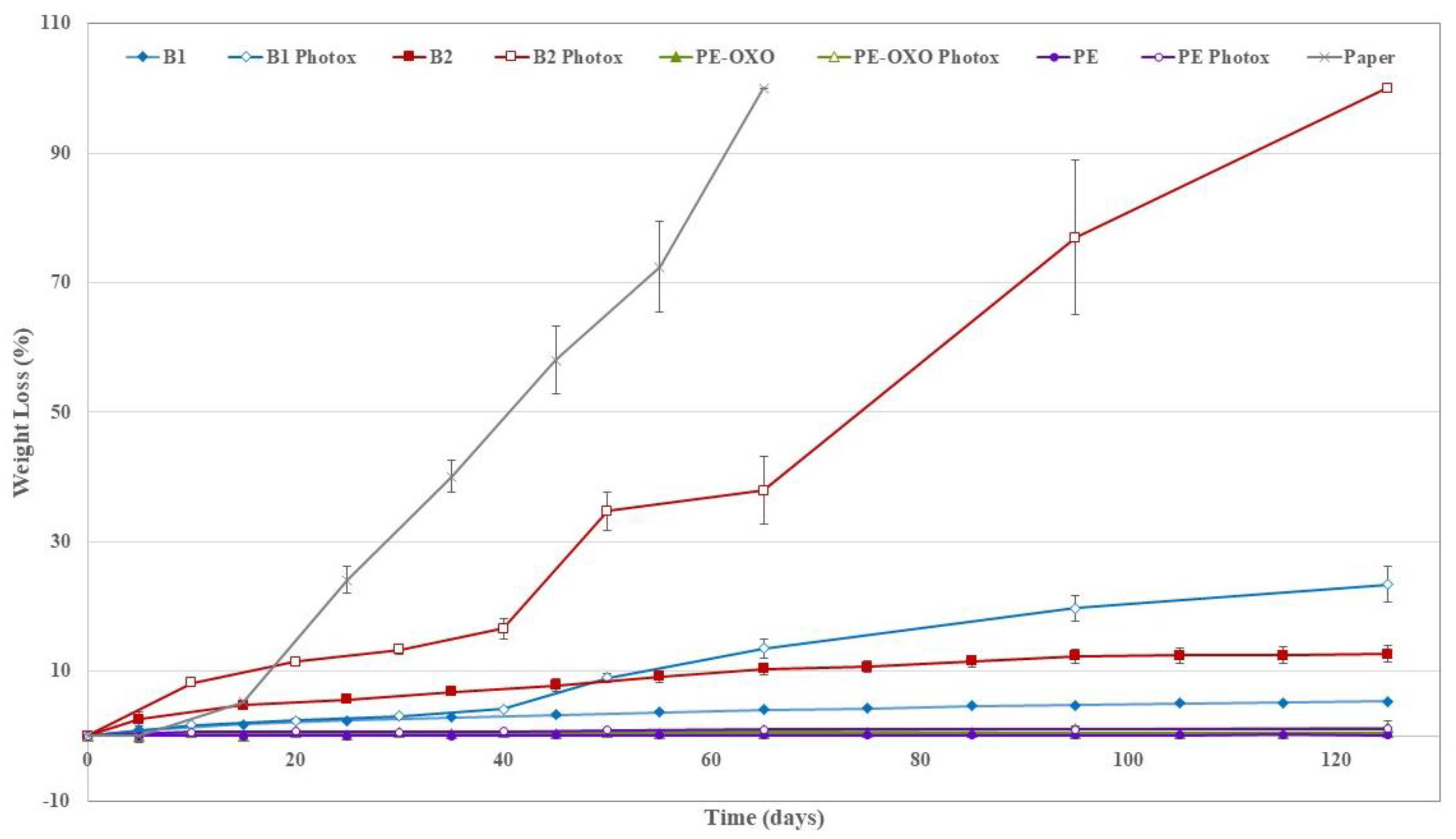
2.2.4. Soil Burial Test
2.2.5. Contact Angle
3. Results
3.1. Mechanical Properties
3.2. Soil Burial Degradation
3.3. Wettability of the Film Surfaces
3.4. UV and Soil Burial Induced Modifications
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dubois, P. Plastics in Agriculture; Brighton, C.A., Ed.; Applied Science Publisher Ltd.: London, UK, 1978; pp. 1–176. [Google Scholar]
- Briassoulis, D.; Babou, E.; Hiskakis, M.; Scarascia, P.P.; Guarde, D.; Dejean, C. Review, mapping and analysis of the agricultural plastic waste generation and consolidation in Europe. Waste Manag. Res. 2013, 31, 1262–1278. [Google Scholar] [CrossRef] [PubMed]
- Siwek, P.; Domagala-Swiatkiewicz, I.; Bucki, P.; Puchalski, M. Biodegradable agroplastics in 21st century horticulture. Polimery 2019, 64, 480–486. [Google Scholar] [CrossRef]
- Brodhagen, M.; Peyron, M.; Miles, C.; Inglis, D.A. Biodegradable plastic agricultural mulches and key features of microbial degradation. Appl. Microbiol. Biotechnol. 2015, 99, 1039–1056. [Google Scholar] [CrossRef] [PubMed]
- Avérous, L.; Pollet, E. Biodegradable Polymers. In Environmental Silicate Nano-Biocomposites. Green Energy and Technology; Avérous, L., Pollet, E., Eds.; Springer: London, UK, 2012; pp. 13–39. [Google Scholar]
- Briassoulis, D. Analysis of the mechanical and degradation performances of optimised agricultural biodegradable films. Polym. Degrad. Stab. 2007, 92, 1115–1132. [Google Scholar] [CrossRef]
- Briassoulis, D.; Babou, M.; Hiskakis, M. Degradation behaviour and field performance of experimental biodegradable drip irrigation systems. J. Polym. Environ. 2011, 19, 341–361. [Google Scholar] [CrossRef]
- Wypych, G. Handbook of UV Degradation and Stabilization, 2nd ed.; ChemTec Publishing: Scarborough, ON, Canada, 2015; pp. 1–420. [Google Scholar]
- Rizzarelli, P.; Rapisarda, M.; Perna, S.; Mirabella, E.F.; La Carta, S.; Puglisi, C.; Valenti, G. Determination of polyethylene in biodegradable polymer blends and in compostable carrier bags by Py-GC/MS and TGA. J. Anal. Appl. Pyrol. 2016, 117, 72–81. [Google Scholar] [CrossRef]
- Solaro, R.; Corti, A.; Chiellini, E. A new respirometric test simulating soil burial conditions for the evaluation of polymer biodegradation. J. Envirom. Polym. Degrad. 1998, 6, 203–208. [Google Scholar] [CrossRef]
- Wu, H.; Wen, B.; Zhou, H.; Zhou, J.; Yu, Z.; Cui, L.; Huang, T.; Cao, F. Synthesis and degradability of copolyesters of 2,5-furandicarboxylic acid, lactic acid, and ethylene glycol. Polym. Degr. Stab. 2015, 121, 100–104. [Google Scholar] [CrossRef]
- Rizzarelli, P.; Cirica, M.; Pastorelli, G.; Puglisi, C.; Valenti, G. Aliphatic poly(ester amide)s from sebacic acid and aminoalcohols of different chain length: Synthesis, characterization and soil burial degradation. Polym. Degrad. Stab. 2015, 121, 90–99. [Google Scholar] [CrossRef]
- Kijchavengkul, T.; Auras, R.; Rubino, M.; Alvarado, E.; Montero, J.R.C.; Rosales, J.M. Atmospheric and soil degradation of aliphatic-aromatic polyester films. Polym. Degrad. Stab. 2010, 95, 99–107. [Google Scholar] [CrossRef]
- Rapisarda, M.; La Mantia, F.P.; Ceraulo, M.; Mistretta, M.C.; Giuffrè, C.; Pellegrino, R.; Valenti, G.; Rizzarelli, P. Photo-oxidative and soil burial degradation of irrigation tubes based on biodegradable polymer blends. Polymers 2019, 11, 1489. [Google Scholar] [CrossRef] [PubMed]
- Koutny, M.; Lemaire, J.; Delort, A.M. Biodegradation of polyethylene films with prooxidant additives. Chemosphere 2006, 64, 1243–1252. [Google Scholar] [CrossRef] [PubMed]
- Stloukal, P.; Verney, V.; Commereuc, S.; Rychly, J.; Matisova-Rychlá, L.; Pis, V.; Koutny, M. Assessment of the interrelation between photooxidation and biodegradation of selected polyesters after artificial weathering. Chemosphere 2012, 88, 1214–1219. [Google Scholar] [CrossRef] [PubMed]
- Emadian, S.M.; Onay, T.T.; Demirel, B. Biodegradation of bioplastics in natural environments. Waste Manag. 2017, 59, 526–536. [Google Scholar] [CrossRef] [PubMed]
- Eubeler, J.P.; Zok, S.; Bernhard, M.; Knepper, T.P. Environmental biodegradation of synthetic polymers I. Test methodologies and procedures. Trends Anal. Chem. 2009, 28, 1057–1072. [Google Scholar] [CrossRef]
- Rizzarelli, P.; Degli Innocenti, F.; Valenti, G.; Rapisarda, M. Biodegradation of green polymer composites: Laboratory procedures and standard test methods. In Advanced Applications of Bio-Degradable Green Composites; Inamuddin, M.P., Ed.; Materials Research Forum LLC: Millersville, PA, USA, 2020; pp. 1–44. ISBN 9781644900642. [Google Scholar] [CrossRef]
- EN17033. Plastics: Biodegradable Mulch Films for Use in Agriculture and Horticulture. Requirements and Test Methods; European Standard, European Committee for Standardization: Brussels, Belgium, 1 January 2018. [Google Scholar]
- Rizzarelli, P.; Puglisi, C.; Montaudo, G. Soil burial and enzymatic degradation in solution of aliphatic co-polyesters. Polym. Degrad. Stab. 2004, 85, 855–863. [Google Scholar] [CrossRef]
- Suresh, B.; Maruthamuthu, S.; Kannan, M.; Chandramohan, A. Mechanical and surface properties of low-density polyethylene film modified by photo-oxidation. Polym. J. 2011, 43, 398–406. [Google Scholar] [CrossRef]
- Gomes, L.B.; Klein, J.M.; Brandalise, R.N.; Zeni, M.; Zoppas, B.C.; Grisa, A.M.C. Study of Oxo-biodegradable Polyethylene Degradation in Simulated Soil. Mater. Res. 2014, 17 (Suppl. 1), 121–126. [Google Scholar] [CrossRef]
- Mistretta, M.C.; Botta, L.; Vinci, A.D.; Ceraulo, M.; La Mantia, F.P. Photooxidation of poypropylene/graphene nanoplatelets nanocomposites. Polym. Deg. Stab. 2019, 160, 35–43. [Google Scholar] [CrossRef]





| Sample Code | Film Thickness (μm) | Extrusion Temperature (°C) |
|---|---|---|
| PE | 20 | 180 |
| PE-OXO | 20 | 180 |
| B1 | 13 | 190 |
| B2 | 15 | --- |
| Sample Code | Elastic Modulus, E (MPa) | Tensile Strength, TS (MPa) | Elongation at Break, EB0 (%) |
|---|---|---|---|
| PE | 206 ± 19 | 19.5 ± 1.1 | 586 ± 12 |
| PE-OXO | 183 ± 23 | 15.9 ± 1.4 | 582 ± 32 |
| B1 | 186 ± 16 | 8.6 ± 2.2 | 460 ± 11 |
| B2 | 129 ± 6 | 21.1 ± 2.0 | 392 ± 22 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
La Mantia, F.P.; Ascione, L.; Mistretta, M.C.; Rapisarda, M.; Rizzarelli, P. Comparative Investigation on the Soil Burial Degradation Behaviour of Polymer Films for Agriculture before and after Photo-Oxidation. Polymers 2020, 12, 753. https://doi.org/10.3390/polym12040753
La Mantia FP, Ascione L, Mistretta MC, Rapisarda M, Rizzarelli P. Comparative Investigation on the Soil Burial Degradation Behaviour of Polymer Films for Agriculture before and after Photo-Oxidation. Polymers. 2020; 12(4):753. https://doi.org/10.3390/polym12040753
Chicago/Turabian StyleLa Mantia, Francesco Paolo, Laura Ascione, Maria Chiara Mistretta, Marco Rapisarda, and Paola Rizzarelli. 2020. "Comparative Investigation on the Soil Burial Degradation Behaviour of Polymer Films for Agriculture before and after Photo-Oxidation" Polymers 12, no. 4: 753. https://doi.org/10.3390/polym12040753
APA StyleLa Mantia, F. P., Ascione, L., Mistretta, M. C., Rapisarda, M., & Rizzarelli, P. (2020). Comparative Investigation on the Soil Burial Degradation Behaviour of Polymer Films for Agriculture before and after Photo-Oxidation. Polymers, 12(4), 753. https://doi.org/10.3390/polym12040753