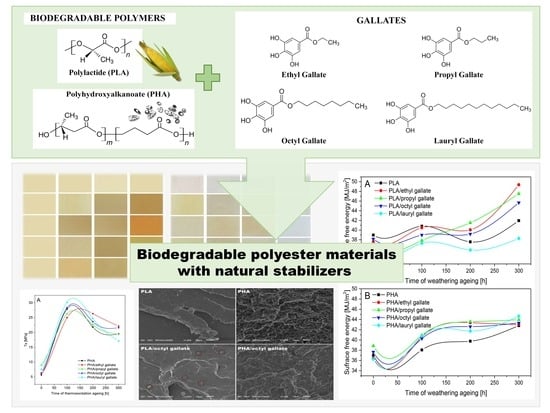
Biodegradable Polyester Materials Containing Gallates
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
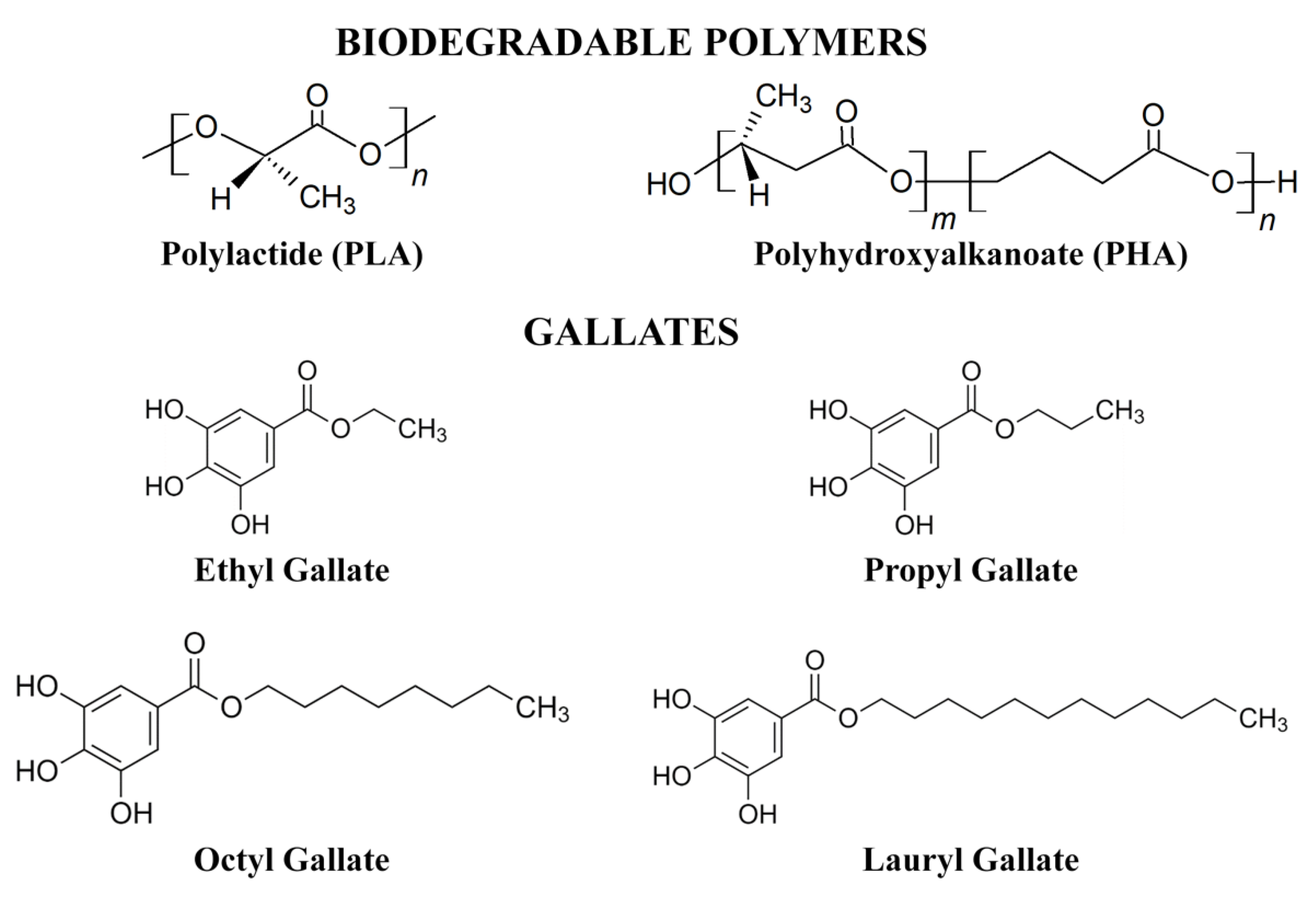
2.2. Preparation of PLA and PHA Samples Containing Gallates
2.3. Analysis of the Properties of Gallates
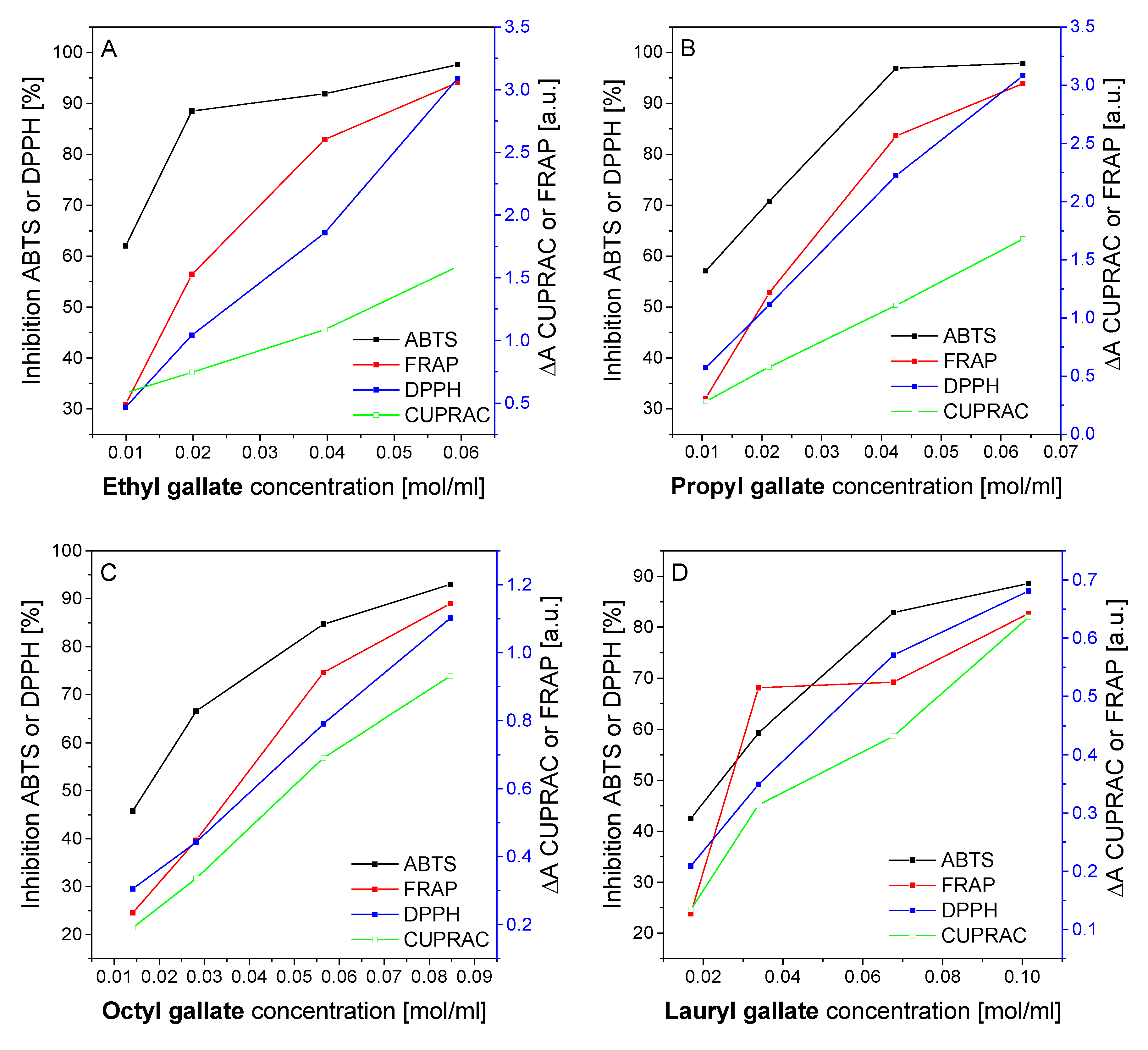
2.3.1. Antioxidant Activity Measured by the ABTS and DPPH Methods
2.3.2. Determination of Ion Reduction by the FRAP and CUPRAC Methods
2.3.3. Thermal Stability of Gallates
2.4. Analysis of PLA and PHA Samples Containing Gallates
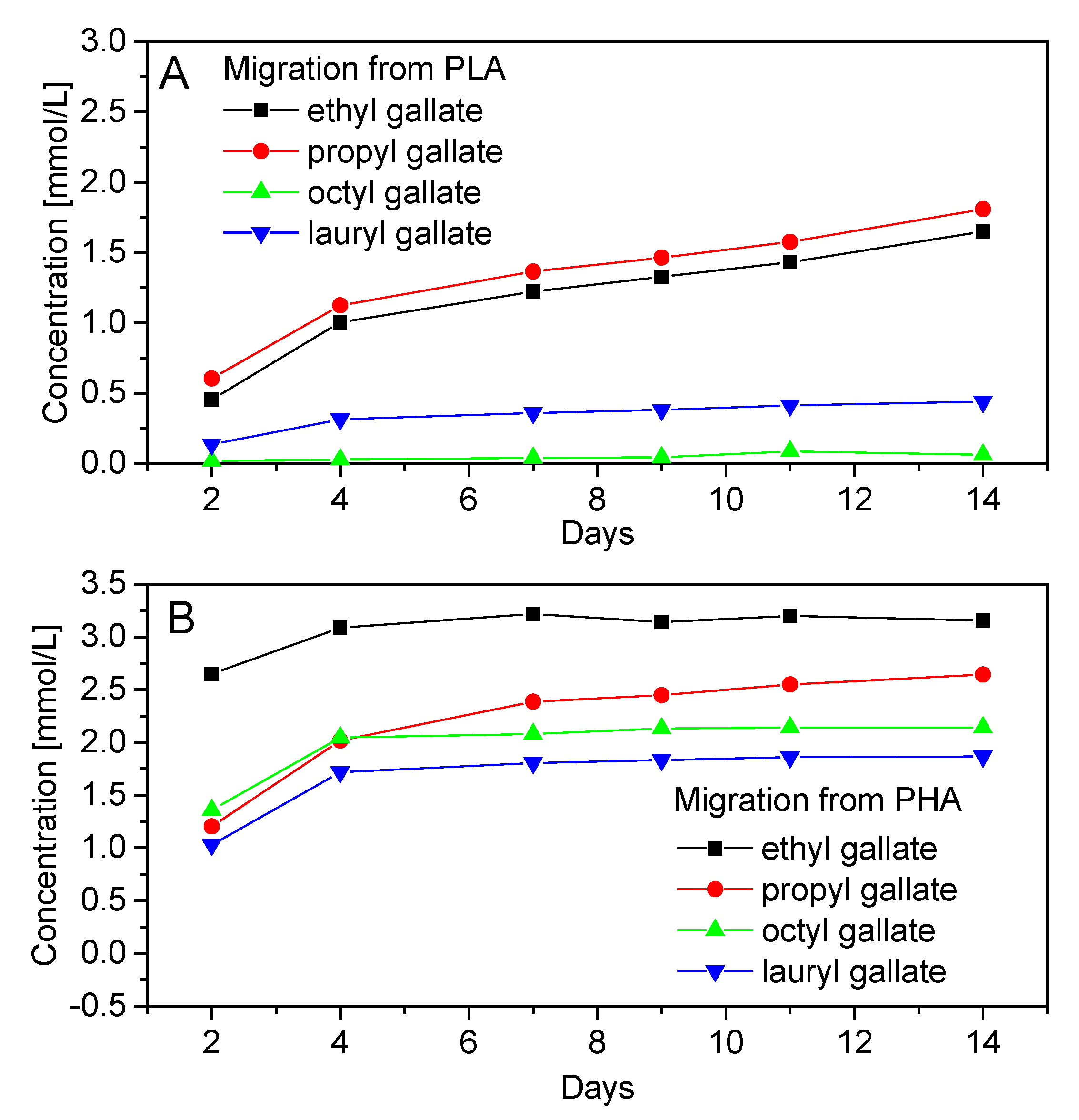
2.4.1. Specific Migration of Gallates from Polymers
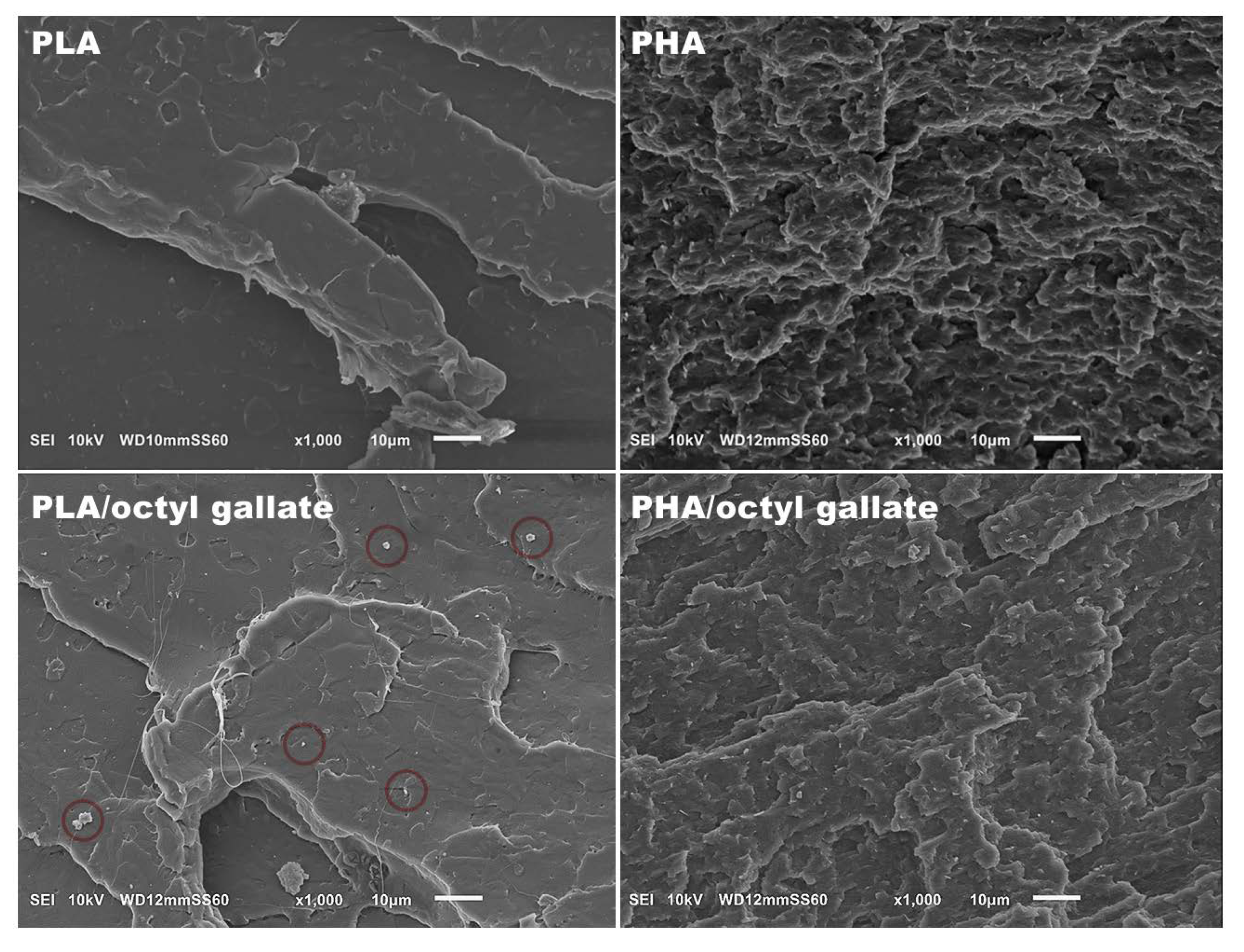
2.4.2. Scanning Electron Microscopy (SEM)
2.4.3. Differential Scanning Calorimetry (DSC)
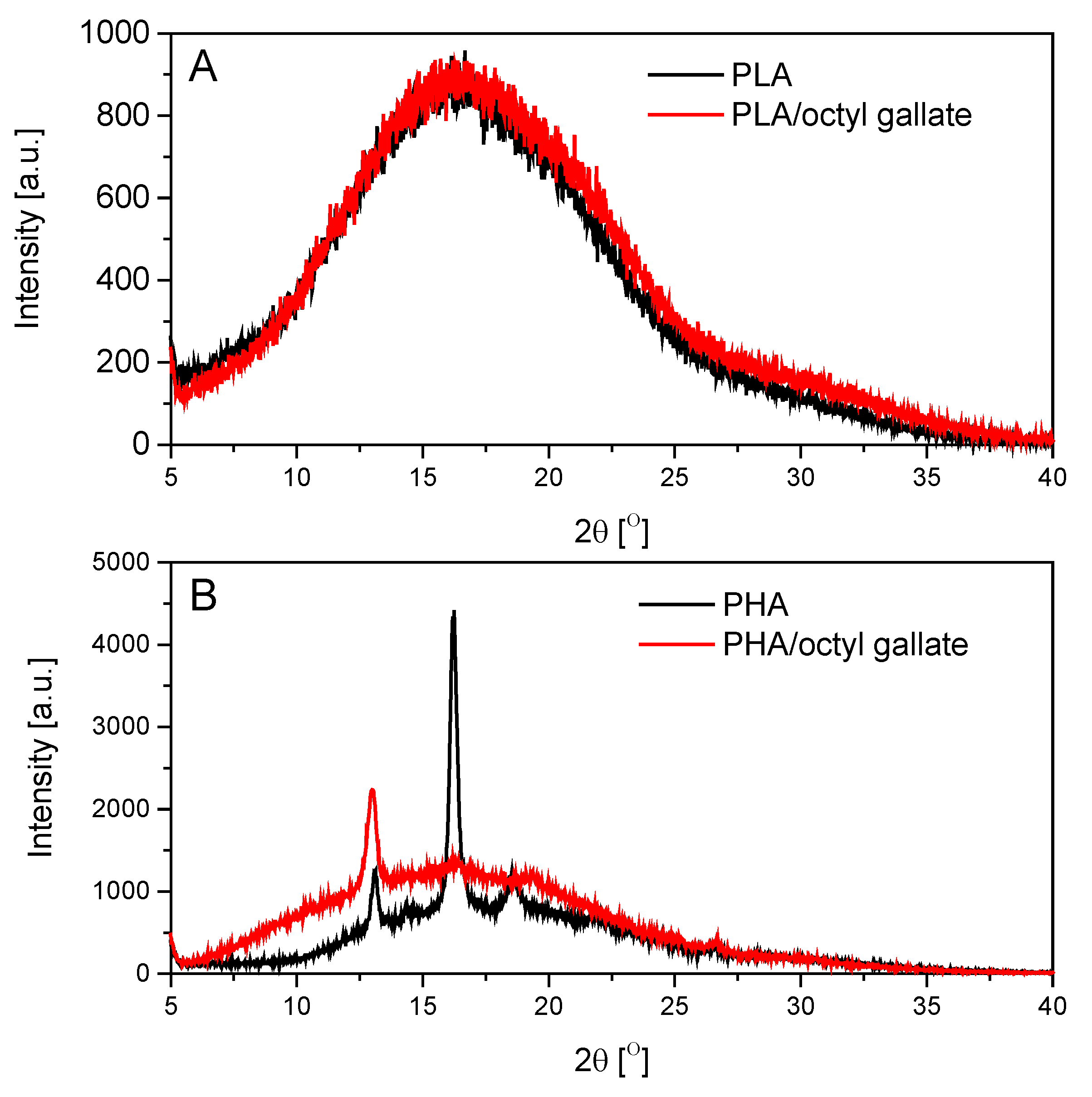
2.4.4. Wide-Angle X-ray Diffraction (WAXD)
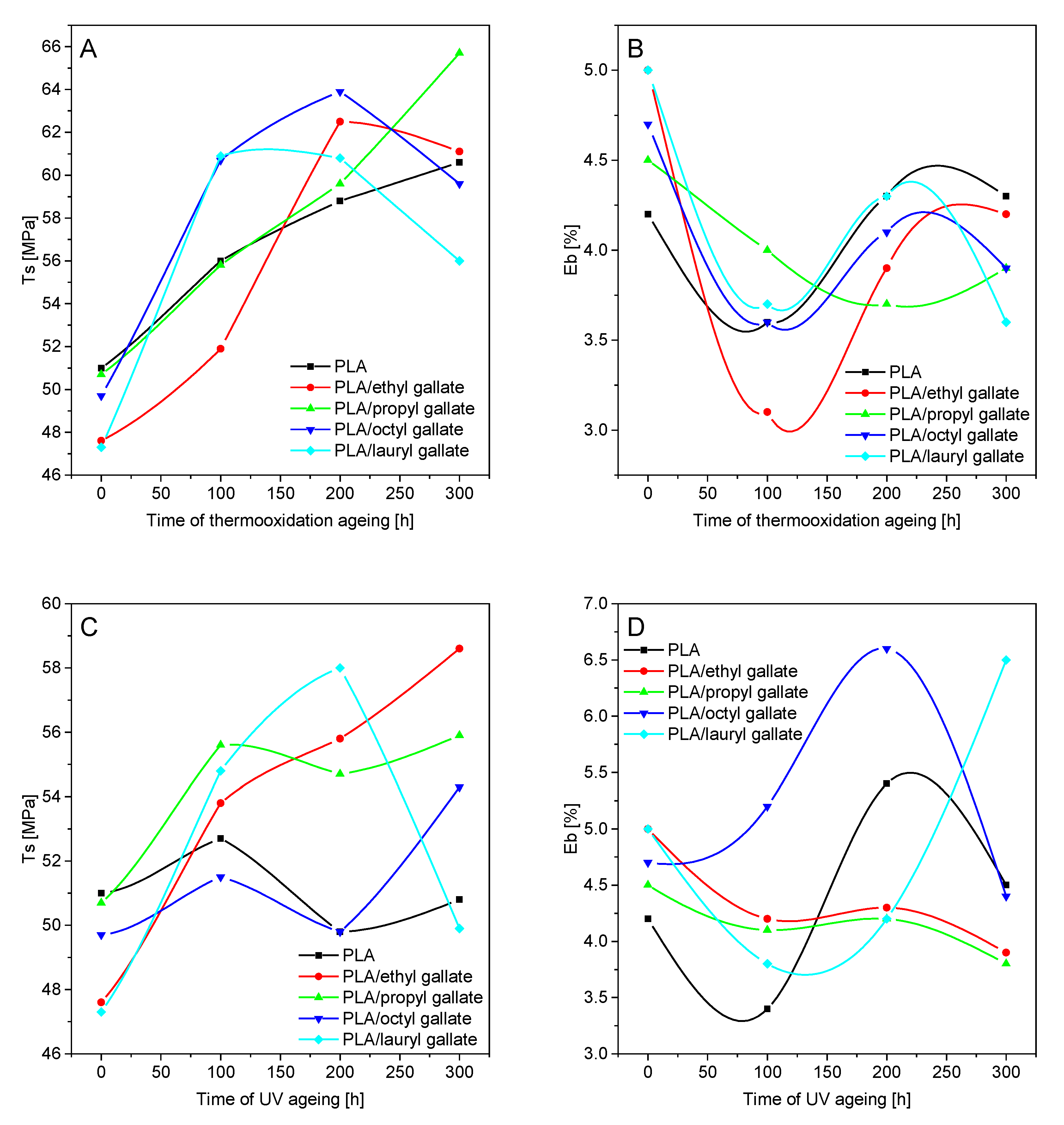
2.4.5. Mechanical properties
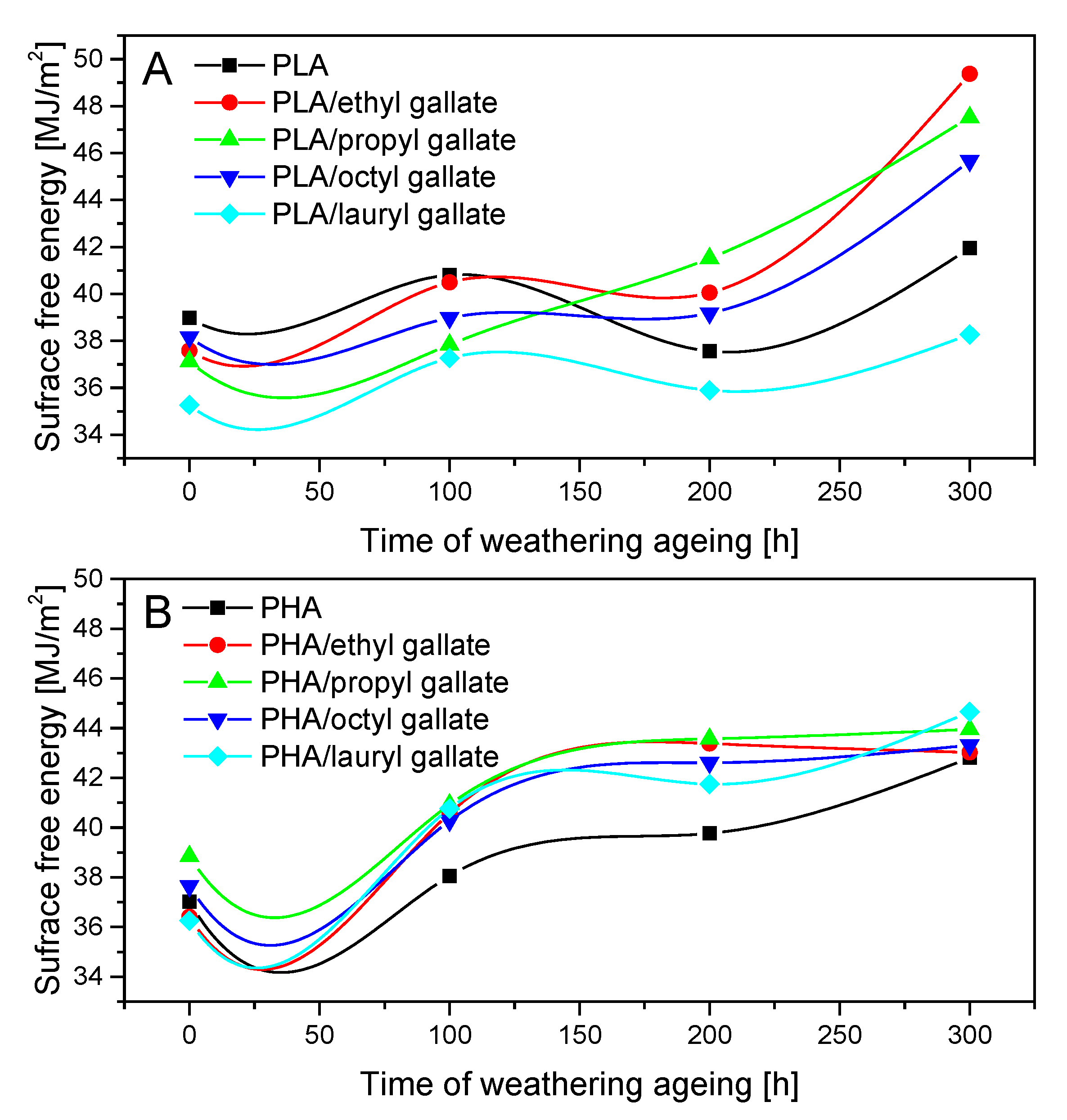
2.4.6. Surface Free Energy of Polyester Samples
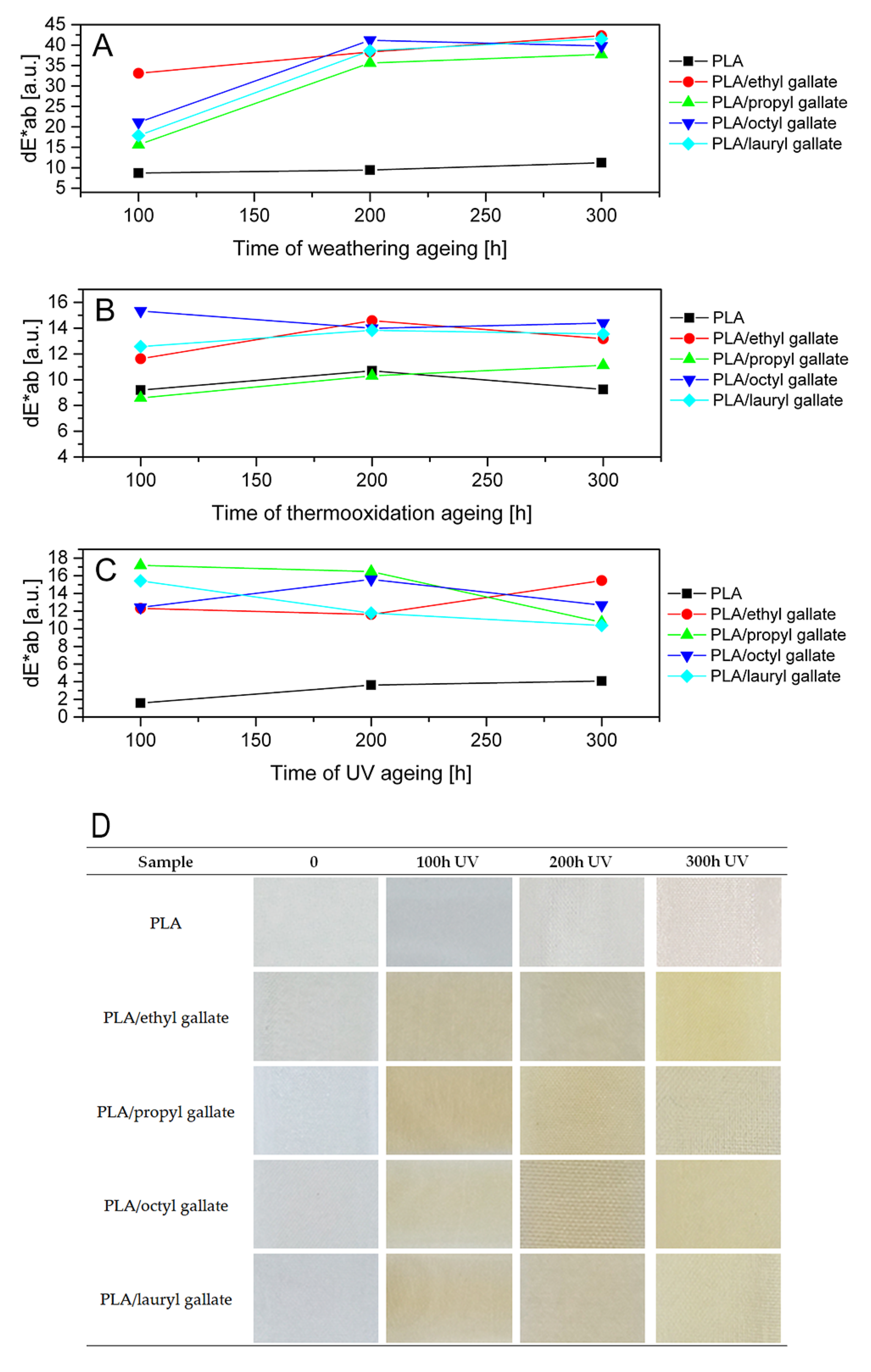
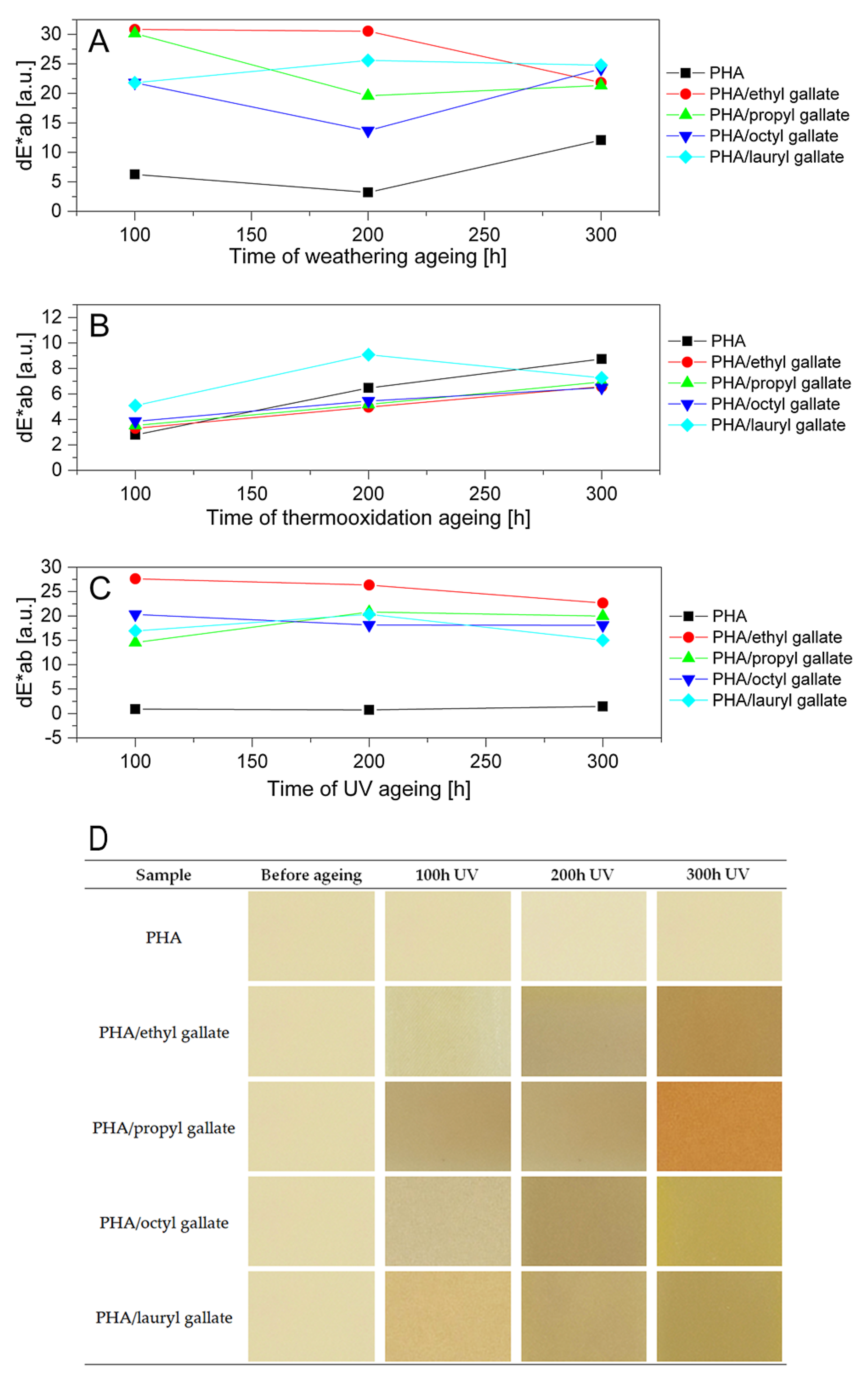
2.4.7. Change of Color After Ageing
2.5. Controlled Aging of Polyesters
2.5.1. UV Exposure
2.5.2. Thermooxidation Aging
2.5.3. Weathering Aging
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wróblewska-Krepsztul, J.; Rydzkowski, T.; Borowski, G.; Szczypiński, M.; Klepka, T.; Thakur, V.K. Recent progress in biodegradable polymers and nanocompositebased packaging materials for sustainable environment. Int. J. Polym. Anal. 2018, 23, 383–395. [Google Scholar] [CrossRef]
- Singh, S.; Sharma, S.; Umar, A.; Mehta, S.K.; Bhatti, M.S.; Kansal, S.K. Recycling of waste poly(ethylene terephthalate) bottles by alkaline hydrolysis and recovery of pure nanospindle-shaped terephthalic acid. J. Nanosci. Nanotechnol. 2018, 18, 5804–5809. [Google Scholar] [CrossRef] [PubMed]
- Prochon, M.; Marzec, A.; Szadkowski, B. Preparation and characterization of new environmentally friendly starch-cellulose materials modified with casein or gelatin for agricultural applications. Materials 2019, 12, 1684. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Perez, S.; Serrano, A.; Pantion, A.A.; Alonso-Farinas, B. Challenges of scaling-up PHA production from waste streams. A review. J. Environ. Manag. 2018, 205, 215–230. [Google Scholar] [CrossRef] [PubMed]
- Elvers, D.; Song, C.H.; Steinbüchel, A.; Leker, J. Technology trends in biodegradable polymers: evidence from patent analysis. Polym. Rev. 2016, 56, 584–606. [Google Scholar] [CrossRef]
- Avinc, O.; Khoddami, A. Overview of poly(lactic acid) (PLA) fibre: Part I: production, properties, performance, environmental impact, and end-use applications of poly(lactic acid) fibres. Fibre Chem. 2009, 4, 391–401. [Google Scholar] [CrossRef]
- Bledzki, A.K.; Jaszkiewicz, A.; Urbaniak, M.; Stankowska-Walczak, D. Biocomposites in the past and in the future. Fibres Text East Eur. 2012, 20, 15–22. [Google Scholar]
- Rydz, J.; Sikorska, W.; Kyulavska, M.; Christova, D. Polyester-based (bio)degradable polymers as environmentally friendly materials for sustainable development. Int. J. Mol. Sci. 2015, 16, 564–596. [Google Scholar] [CrossRef]
- Nair, L.S.; Laurencin, C.T. Biodegradable polymers as biomaterials. Prog. Polym. Sci. 2007, 32, 762–798. [Google Scholar] [CrossRef]
- Middleton, J.C.; Tipton, A.J. Synthetic biodegradable polymers as orthopedic devices. Biomaterials 2000, 21, 2335–2346. [Google Scholar] [CrossRef]
- Tomizawa, S.; Hyakutake, M.; Saito, Y.; Agus, J.; Mizuno, K.; Abe, H.; Tsuge, T. Molecular weight change of polyhydroxyalkanoate (PHA) caused by the PhaC subunit of PHA synthase from bacillus cereus YB-4 in recombinant Escherichia coli. Biomacromolecules 2011, 12, 2660–2666. [Google Scholar] [CrossRef] [PubMed]
- Kirschweng, B.; Tatraaljai, D.; Foldes, E.; Pukanszky, B. Natural antioxidants as stabilizers for polymers. Polym. Degrad. Stabil. 2017, 145, 25–40. [Google Scholar] [CrossRef]
- Hussain, T.; Tausif, M.; Ashraf, M. A review of progress in the dyeing of eco-friendly aliphatic polyester based polylactic acid fabrics. J. Clean Prod. 2015, 108, 476–483. [Google Scholar] [CrossRef]
- Singh, S.; Gaikwad, K.K.; Lee, J.S. Anthocyanin – A natural dye for smart food packaging systems. Korean J. Packag. Sci. Technol. 2018, 24, 167–180. [Google Scholar] [CrossRef]
- Tichoniuk, M.; Radomska, N.; Cierpiszewski, R. The application of natural dyes in food freshness indicators designed for intelligent packaging. Studia Oeconomica Posnaniensia 2017, 5, 19–34. [Google Scholar] [CrossRef]
- Hubert, B.; Eberl, L.; Feucht, W.; Polster, J. Influence of polyphenols on bacterial biofilm formation and quorum-sensing. Z. Naturforsch. 2003, 58, 879–884. [Google Scholar] [CrossRef]
- Appendini, P.; Hotchkiss, J.H. Review of antimicrobial food packaging. Innov. Food Sci. Emerg. 2002, 3, 113–126. [Google Scholar] [CrossRef]
- Latos-Brozio, M.; Masek, A. The application of (+)-catechin and polydatin as functional additives for biodegradable polyesters. Int. J. Mol. Sci. 2020, 21, 414. [Google Scholar] [CrossRef]
- Masek, A.; Latos-Brozio, M. The effect of substances of plant origin on the thermal and thermo-oxidative ageing of aliphatic polyesters (PLA, PHA). Polymers 2018, 10, 1252. [Google Scholar] [CrossRef]
- Wang, F.; Zhou, S.; Yang, M.; Chen, Z.; Ran, S. Thermo-Mechanical Performance of Polylactide Composites Reinforced with Alkali-Treated Bamboo Fibers. Polymers 2018, 10, 401. [Google Scholar] [CrossRef]
- Kahkeshani, N.; Farzaei, F.; Fotouhi, M.; Alavi, S.S.H.; Bahramsoltani, R.; Naseri, R.; Momtaz, S.; Abbasabadi, Z.; Rahimi, R.; Farzaei, M.H.; et al. Pharmacological effects of gallic acid in health and diseases: A mechanistic review. Iran. J. Basic Med. Sci. 2019, 22, 225–237. [Google Scholar] [PubMed]
- Fernandes, F.; Salgado, H. Gallic acid: review of the methods of determination and quantification. Crit. Rev. Anal. Chem. 2016, 46, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Choubey, S.; Varughese, L.; Kumar, V.; Beniwal, V. Medicinal importance of gallic acid and its ester derivatives: a patent review. Pharm. Pat. Anal. 2015, 4, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Manuel, E.; Iugab, M.C.; Alvarez-Idaboy, J.R. Antioxidant activity of propyl gallate in aqueous and lipid media: a theoretical study. Phys. Chem. Chem. Phys. 2013, 15, 13137. [Google Scholar]
- Garrido, J.; Garrido, E.M.; Borges, F. Studies on the food additive propyl gallate: synthesis, structural characterization, and evaluation of the antioxidant activity. J. Chem. Educ. 2012, 89, 130–133. [Google Scholar] [CrossRef]
- Gunckel, S.; Santander, P.; Cordano, G.; Ferreira, J.; Munoz, S.; Nunez-Vergara, L.J.; Squella, J.A. Antioxidant activity of gallates: an electrochemical study in aqueous media. Chem.-Biol. Interact. 1998, 114, 45–59. [Google Scholar] [CrossRef]
- Leclercq, C.; Arcella, D.; Turrini, A. Estimates of the theoretical maximum daily intake of erythorbic acid, gallates, butylated hydroxyanisole (BHA) and butylated hydroxytoluene (BHT) in Italy: a stepwise approach. Food Chem. Toxicol. 2000, 38, 1075–1084. [Google Scholar] [CrossRef]
- Kubo, I.; Masuoka, N.; Xiao, P.; Haraguchi, H. Antioxidant Activity of Dodecyl Gallate. J. Agric. Food Chem. 2002, 50, 3533–3539. [Google Scholar] [CrossRef]
- Masek, A.; Chrzescijanska, E.; Latos, M.; Kosmalska, A. Electrochemical and spectrophotometric characterization of the propolis antioxidants properties. Int. J. Electrochem. Sci. 2019, 14, 1231–1247. [Google Scholar] [CrossRef]
- Masek, A.; Latos, M.; Chrzescijanska, E.; Zaborski, M. Antioxidant properties of rose extract (Rosa villosa L.) measured using electrochemical and UV/Vis spectrophotometric methods. Int. J. Electrochem. Sci. 2017, 12, 10994–11005. [Google Scholar] [CrossRef]
- Farah, S.; Anderson, D.G.; Langer, R. Physical and mechanical properties of PLA and their functions in widespread applications—A comprehensive review. Adv. Drug Deliv. Rev. 2016, 107, 367–392. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; McDonald, A.G.; Coats, E.R. Characterization of polyhydroxybutyrate biosynthesized from crude glycerol waste using mixed microbial consortia. J. Appl. Polym. Sci. 2013, 129, 1314–1321. [Google Scholar] [CrossRef]
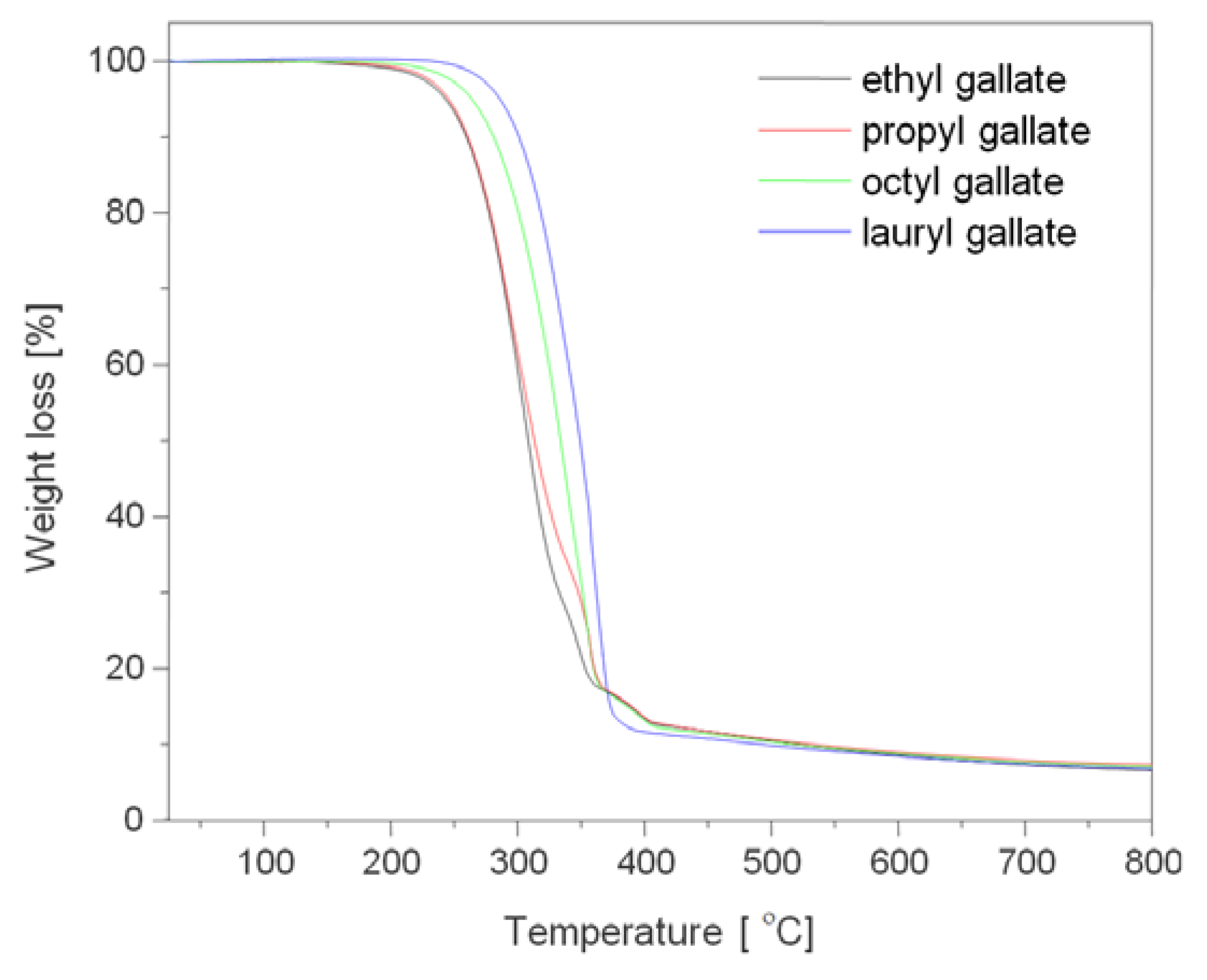

| Sample | T20 | T50 | T90 |
|---|---|---|---|
| ethyl gallate | 277 | 308 | 478 |
| propyl gallate | 273 | 309 | 483 |
| octyl gallate | 300 | 333 | 468 |
| lauryl gallate | 318 | 349 | 433 |
| Sample | Tg/°C | ΔHcc/J/g | Tcc/°C | ΔHm/J/g | Tm/°C | ΔHo/J/g | To/°C | Xc/% |
|---|---|---|---|---|---|---|---|---|
| PLA | 58.4 | 4.6 | 108.6 | 3.1 | 145.6 | 22.6 | 226.2 295.7 | 0 |
| PLA/ethyl gallate | 57.0 | 5.1 | 105.8 | 2.1 | 145.0 | 5.3 | 276.0 305.7 | 0 |
| PLA/propyl gallate | 57.3 | 4.4 | 110.0 | 2.8 | 144.7 | 11.4 | 287.2 302.7 | 0 |
| PLA/octyl gallate | 59.9 | 7.8 | 105.2 | 5.4 | 144.1 | 57.9 | 270.8 315.4 | 0 |
| PLA/lauryl gallate | 59.6 | 16.3 | 101.0 | 14.0 | 141.9 | 17.5 | 238.8 316.5 | 0 |
| PHA | 36.7 | 15.2 | 76.5 | (1) 6.5 (2) 33.9 | (1) 127.9 (2) 156. 4 | 9.7 | 199.1 259.3 | 17.1 |
| PHA/ethyl gallate | 39.9 | 14.5 | 80.2 | (1) 5.2 (2) 31.6 | (1) 127.9 (2) 156.1 | 8.5 | 216.9 263.5 | 15.2 |
| PHA/propyl gallate | 38.4 | 13.5 | 80.0 | (1) 6.3 (2) 33.1 | (1) 127.7 (2) 156.7 | 11.4 | 225.0 267.4 | 17.7 |
| PHA/octyl gallate | 39.9 | 13.5 | 80.7 | (1) 5.1 (2) 32.3 | (1) 127.5 (2) 156.5 | 25.4 | 225.0 274.1 | 16.3 |
| PHA/lauryl gallate | 39.9 | 16.6 | 81.5 | (1) 6.2 (2) 33.8 | (1) 123.1 (2) 156.1 | 22.0 | 210.2 269.9 | 16.1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Latos-Brozio, M.; Masek, A. Biodegradable Polyester Materials Containing Gallates. Polymers 2020, 12, 677. https://doi.org/10.3390/polym12030677
Latos-Brozio M, Masek A. Biodegradable Polyester Materials Containing Gallates. Polymers. 2020; 12(3):677. https://doi.org/10.3390/polym12030677
Chicago/Turabian StyleLatos-Brozio, Malgorzata, and Anna Masek. 2020. "Biodegradable Polyester Materials Containing Gallates" Polymers 12, no. 3: 677. https://doi.org/10.3390/polym12030677
APA StyleLatos-Brozio, M., & Masek, A. (2020). Biodegradable Polyester Materials Containing Gallates. Polymers, 12(3), 677. https://doi.org/10.3390/polym12030677