Preparation and Characterization of Waterborne UV Lacquer Product Modified by Zinc Oxide with Flower Shape
Abstract
1. Introduction
2. Experimental Part
2.1. Materials
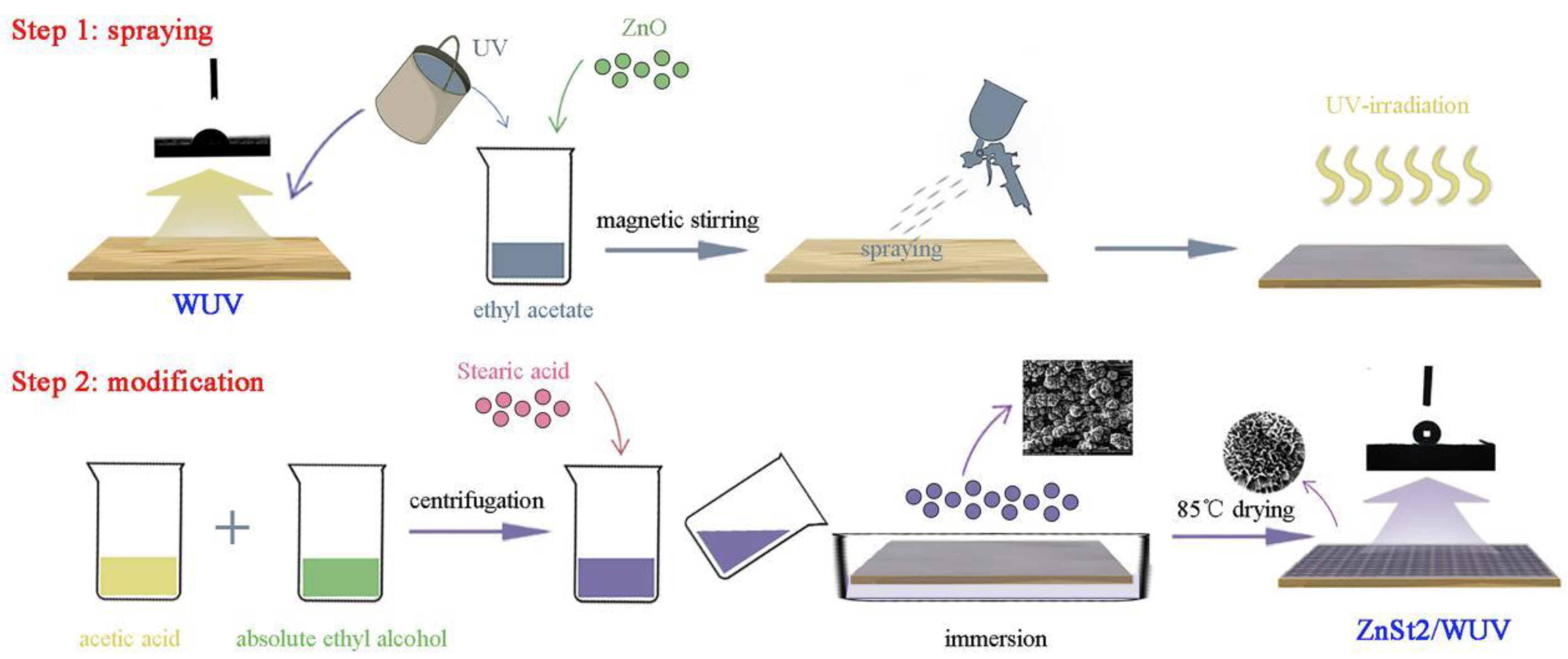
2.2. Experimental Method
2.3. Contact Angle (CA) Test
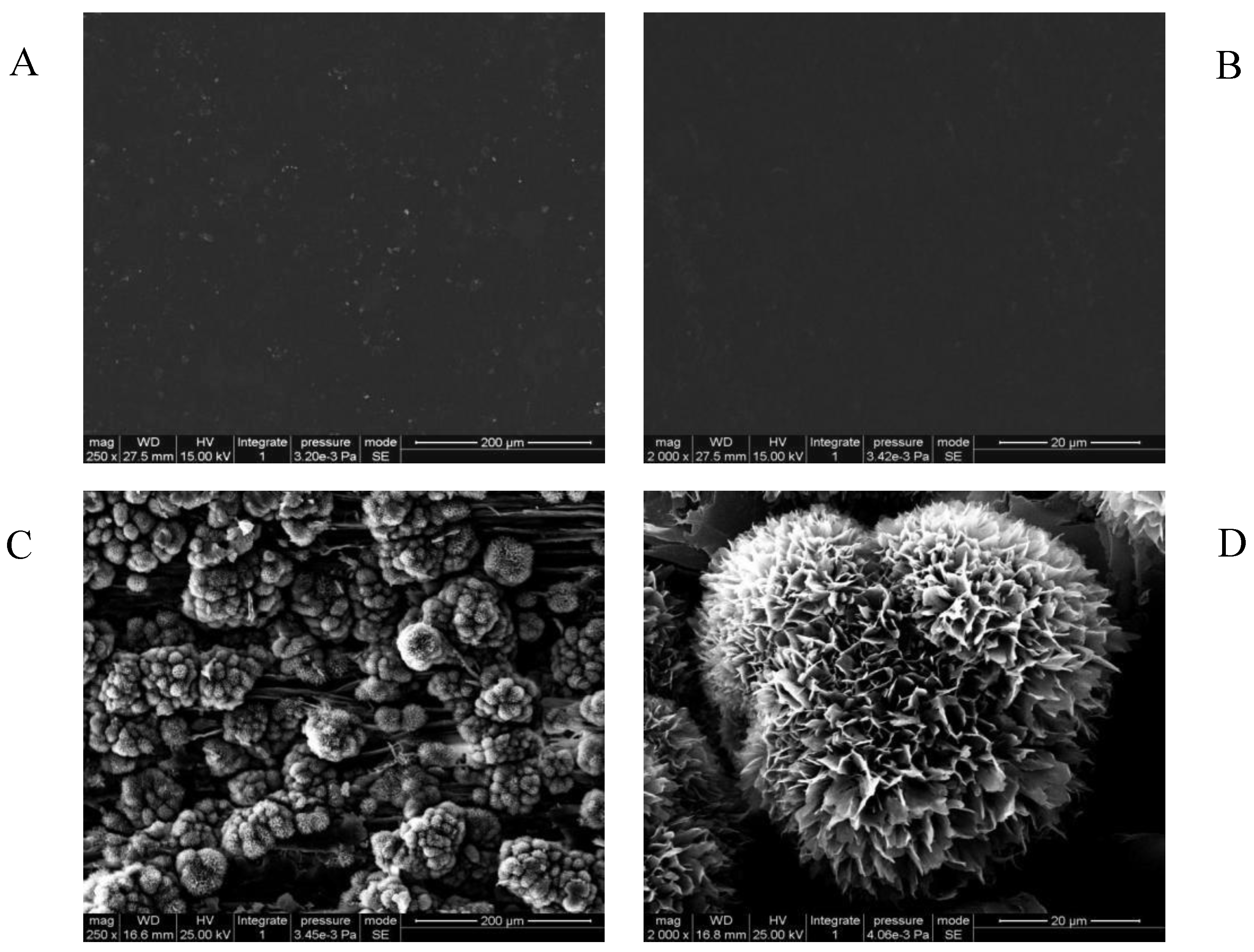
2.4. SEM
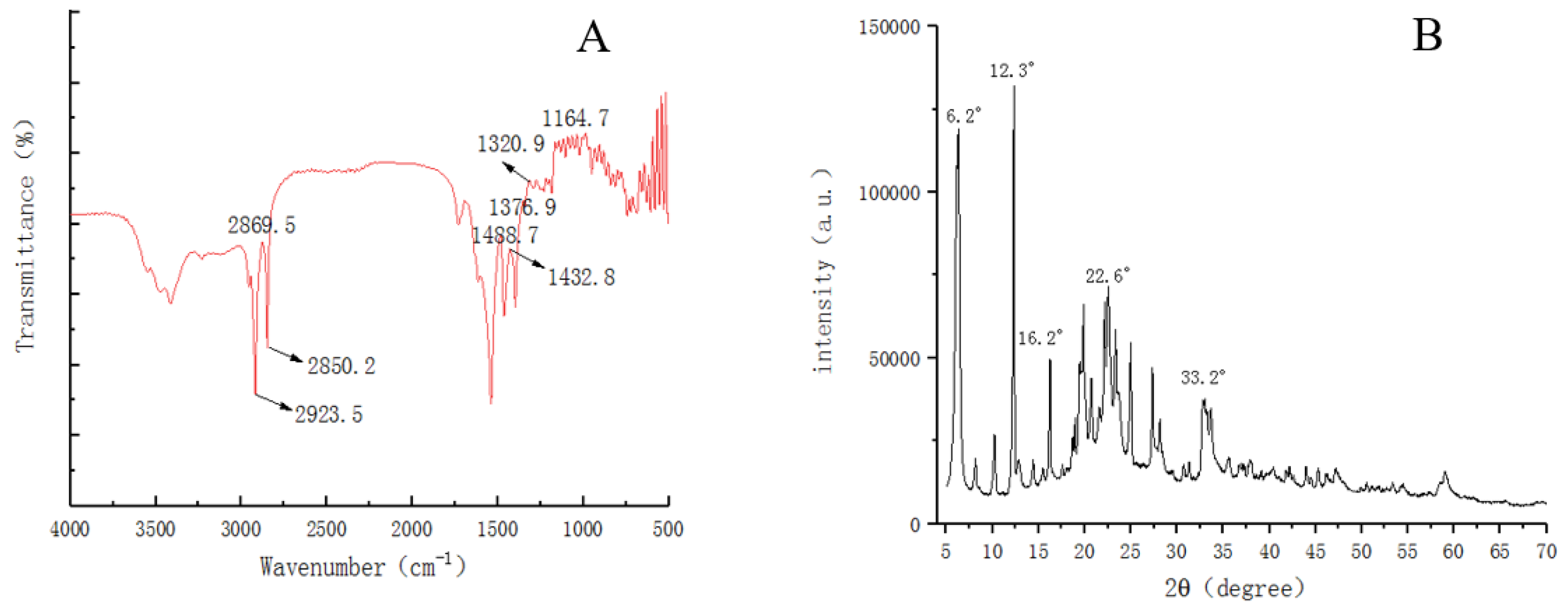
2.5. FTIR and XRD
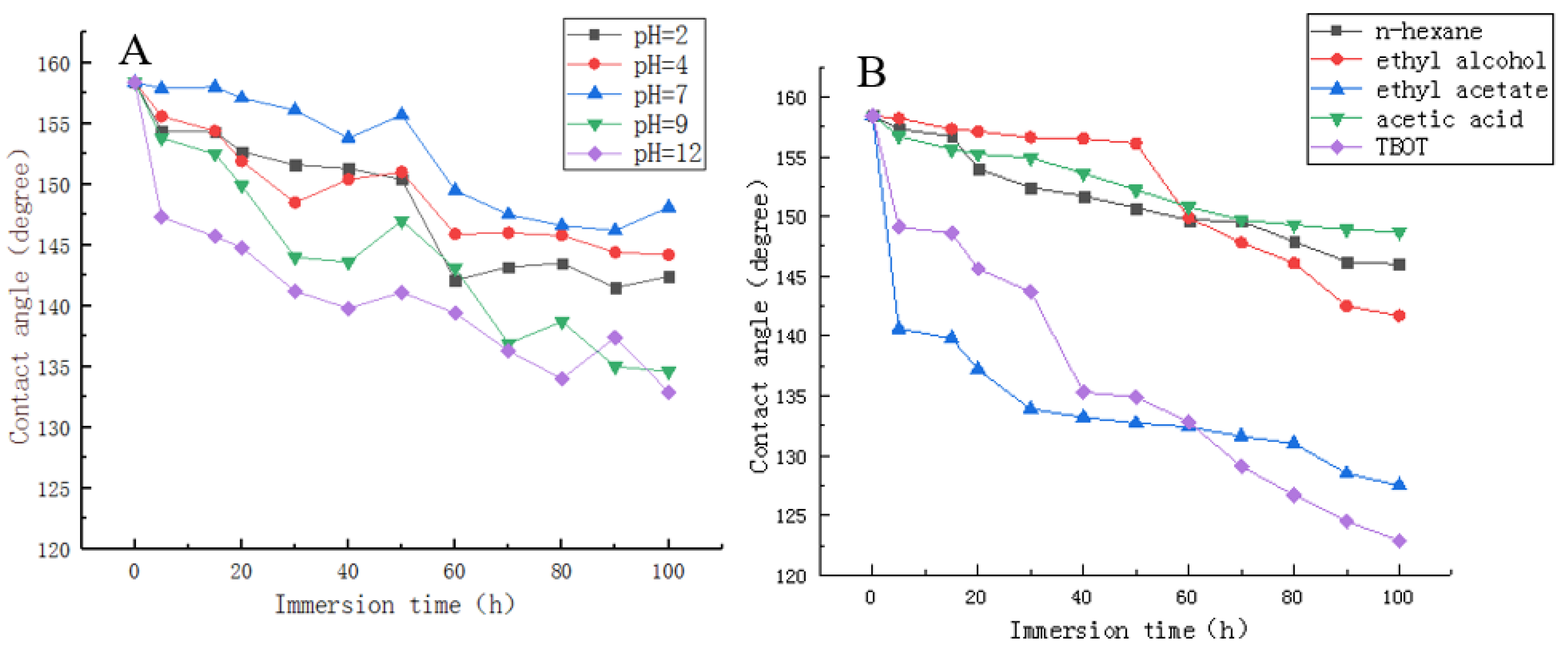
2.6. Different pH Values of Solution and Organic Solvent Immersion Test
2.7. Water Resistance Test
3. Results and Discussion
3.1. Contact Angle (CA) Test
3.2. SEM
3.3. FTIR and XRD
3.4. Different pH Values of Solution and Organic Solvent Immersion Test
3.5. Water Resistance Test
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yan, X.; Qian, X.; Chang, Y. Preparation and characterization of urea formaldehyde@ epoxy resin microcapsule on waterborne wood coatings. Coatings 2019, 9, 475. [Google Scholar] [CrossRef]
- Wang, N.; Zhang, Y.; Chen, J.; Zhang, J.; Fang, Q. Dopamine modified metal-organic frameworks on anti-corrosion properties of waterborne epoxy coatings. Prog. Org. Coat. 2017, 109, 126–134. [Google Scholar] [CrossRef]
- Xu, W.; Fang, X.Y.; Han, J.T.; Wu, Z.H.; Zhang, J.L. Effect of coating thickness on sound absorption property of four wood species commonly used for piano soundboards. Wood Fiber Sci. 2020, 52, 28–43. [Google Scholar] [CrossRef]
- Yan, X.; Qian, X.; Lu, R.; Miyakoshi, T. Comparison and optimization of reactive dyes and coating performance on fraxinus mandshurica veneer. Polymers 2018, 10, 1302. [Google Scholar] [CrossRef]
- Shahriari, L.; Mohseni, M.; Yahyaei, H. The effect of cross-linking density on water vapor and oxygen permeability of hybrid UV cured nano coatings. Prog. Org. Coat. 2019, 134, 66–77. [Google Scholar] [CrossRef]
- Aizpurua, J.; Martin, L.; Fernández, M.; González, A.; Irusta, L. Recyclable, remendable and healing polyurethane/acrylic coatings from UV curable waterborne dispersions containing Diels-Alder moieties. Prog. Org. Coat. 2020, 139, 105460. [Google Scholar] [CrossRef]
- Wang, S.; Wu, Y.; Dai, J.; Teng, N.; Peng, Y.; Cao, L.; Liu, X. Making organic coatings greener: Renewable resource, solvent-free synthesis, UV curing and repairability. Eur. Polym. J. 2020, 123, 109439. [Google Scholar] [CrossRef]
- Yan, X.; Qian, X.; Chang, Y.; Lu, R.; Miyakoshi, T. The effect of glass fiber powder on the properties of waterborne coatings with thermochromic ink on a Chinese Fir surface. Polymers 2019, 11, 1733. [Google Scholar] [CrossRef]
- Rawat, R.S.; Chouhan, N.; Talwar, M.; Diwan, R.K.; Tyagi, A.K. UV coatings for wooden surfaces. Prog. Org. Coat. 2019, 135, 490–495. [Google Scholar] [CrossRef]
- Xiong, X.Q.; Yuan, Y.Y.; Niu, Y.T.; Zhang, L.T.; Wu, Z.H. Effects of different treatments on surface activity of rice straw particleboard. Sci. Adv. Mater. 2020, 12, 289–295. [Google Scholar] [CrossRef]
- Nosrati, R.; Olad, A.; Maryami, F. Visible-light induced anti-bacterial and self-cleaning waterborne polyacrylic coating modified with TiO2 /polypyrrole nanocomposite; preparation and characterization. J. Mol. Struct. 2018, 1163, 174–184. [Google Scholar] [CrossRef]
- Wu, Y.; Sun, Y.; Yang, F.; Zhang, H.; Wang, Y. The implication of benzene-ethanol extractive on mechanical properties of waterborne coating and wood cell wall by nanoindentation. Coatings 2019, 9, 449. [Google Scholar] [CrossRef]
- Hang, T.T.X.; Anh, N.T.; Truc, T.A.; Van Truoc, B.; Hoang, T.; Thanh, D.T.M.; Daopiset, S. Synthesis of 3-glycidoxypropyltrimethoxysilane modified hydrotalcite bearing molybdate as corrosion inhibitor for waterborne epoxy coating. J. Coat. Technol. Res. 2016, 13, 805–813. [Google Scholar] [CrossRef]
- Xu, R.; He, T. Corrosion of self-curing waterborne zinc oxide-potassium silicate coating modified with aluminium powder. J. Alloy. Compd. 2019, 811, 152008. [Google Scholar] [CrossRef]
- Zhong, S.; Li, J.; Yi, L.; Cai, Y.; Zhou, W. Cross-linked waterborne alkyd hybrid resin coatings modified by fluorinated acrylate-siloxane with high waterproof and anticorrosive performance. Polym. Adv. Technol. 2019, 30, 292–303. [Google Scholar] [CrossRef]
- Saladino, M.L.; Motaung, T.E.; Luyt, A.S.; Spinella, A.; Nasillo, G.; Caponetti, E. The effect of silica nanoparticles on the morphology, mechanical properties and thermal degradation kinetics of PMMA. Polym. Degrad. Stab. 2012, 97, 452–459. [Google Scholar] [CrossRef]
- Zhu, W.; Wu, Y.; Zhang, Y. Synthesis and characterisation of superhydrophobic CNC/ZnO nanocomposites by using stearic acid. Micro Nano Lett. 2019, 14, 1317–1321. [Google Scholar] [CrossRef]
- Zhong, W.L. Structure distortion, optical and electrical properties of ZnO thin films co-doped with Al and Sb by sol-gel spin coating. Chin. Geogr. 2010, 19, 515–519. [Google Scholar]
- Ismail, A.; Alahmad, M.; Alsabagh, M.; Abdallah, B. Effect of low dose-rate industrial Co-60 gamma irradiation on ZnO thin films: Structural and optical study. Microelectron. Reliab. 2020, 104, 113556. [Google Scholar] [CrossRef]
- Shalu, C.; Shukla, M.; Tiwari, A.; Agrawal, J.; Bilgaiyan, A.; Singh, V. Role of solvent used to cast P3HT thin films on the performance of ZnO/P3HT hybrid photo detector. Phys. E Low-Dimens. Syst. Nanostruct. 2020, 15, 113694. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, F.; Han, E.; Ke, W.; Luo, S. Effect of ZnO nanoparticles on anti-aging properties of polyurethane coating. Sci. Bull. 2009, 54, 3464–3472. [Google Scholar] [CrossRef]
- Qu, J.E.; Ascencio, M.; Jiang, L.M.; Omanovic, S.; Yang, L.X. Improvement in corrosion resistance of WE43 magnesium alloy by the electrophoretic formation of a ZnO surface coating. J. Coat. Technol. Res. 2019, 16, 1559–1570. [Google Scholar] [CrossRef]
- Lin, J.; Chen, C.; Lin, C. Influence of sol–gel-derived ZnO:Al coating on luminescent properties of Y2O3:Eu3+ phosphor. J. Sol-Gel Sci. Technol. 2019, 92, 562–574. [Google Scholar] [CrossRef]
- Yadav, A.B.; Pandey, A.; Jit, S. Effects of annealing temperature on the structural, optical, and electrical properties of ZnO thin films grown on n-Si〈100〉substrates by the sol–gel spin coating method. Acta Metall. Sin. 2014, 27, 682–688. [Google Scholar] [CrossRef]
- Hussain, F.; Imran, M.; Khalil, R.M.A.; Niaz, N.A.; Rana, A.M.; Sattar, M.A.; Ismail, M.; Majid, A.; Kim, S.; Iqbal, F.; et al. An insight of Mg doped ZnO thin films: A comparative experimental and first-principle investigations. Phys. E Low-Dimens. Syst. Nanostruct. 2020, 115, 113658. [Google Scholar] [CrossRef]
- Saleem, M.; Farooq, W.A.; Khan, M.I.; Akhtar, M.N.; Rehman, S.U.; Ahmad, N.; Khalid, M.; Atif, M.; AlMutairi, M.A.; Irfan, M. Effect of ZnO nanoparticles coating layers on top of ZnO Nanowires for morphological, optical, and photovoltaic properties of dye-sensitized solar cells. Micromachines 2019, 10, 819. [Google Scholar] [CrossRef]
- Kumar, V.; Ntwaeaborwa, O.M.; Swart, H.C. Effect of oxygen partial pressure during pulsed laser deposition on the emission of Eu doped ZnO thin films. Phys. B Condens. Matter 2020, 576, 411713. [Google Scholar] [CrossRef]
- Srisuai, N.; Boonruang, S.; Horprathum, M.; Sarapukdee, P.; Denchitcharoen, S. Growth of highly uniform size-distribution ZnO NR arrays on sputtered ZnO thin film via hydrothermal with PMMA template assisted. Mater. Sci. Semicond. Process. 2020, 105, 104736. [Google Scholar] [CrossRef]
- Zhu, W.; Wu, Y.; Zhang, Y. Fabrication and characterization of superhydrophobicity ZnO nanoparticles with two morphologies by using stearic acid. Mater. Res. Express 2019, 6, 1150d1. [Google Scholar] [CrossRef]
- Sharifalhoseini, Z.; Entezari, M.H.; Davoodi, A.; Shahidi, M. Surface modification of mild steel before acrylic resin coating by hybrid ZnO/GO nanostructures to improve the corrosion protection. J. Ind. Eng. Chem. 2020, 83, 333–342. [Google Scholar] [CrossRef]
- Zhou, J.; Li, K.; Wang, B.; Ai, F. Nano-hydroxyapatite/ZnO coating prepared on a biodegradable Mg–Zn–Ca bulk metallic glass by one-step hydrothermal method in acid situation. Ceram. Int. 2020, 46, 6958–6964. [Google Scholar] [CrossRef]
- Guo, H.; Michen, B.; Burgert, I. Estudios reales en banco de pruebas de la Casa ETH de Recursos Naturales – protección superficial de la madera para aplicaciones exteriores. Inf. Constr. 2017, 69, 220. [Google Scholar] [CrossRef]
- Nair, S.; Nagarajappa, G.B.; Pandey, K.K. UV stabilization of wood by nano metal oxides dispersed in propylene glycol. J. Photochem. Photobiol. B Biol. 2018, 183, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.K.; Giagli, K.; Tsalagkas, D.; Mishra, H.; Talegaonkar, S.; Gryc, V.; Wimmer, R. Changing Face of Wood Science in Modern Era: Contribution of Nanotechnology. Recent Pat. Nanotechnol. 2018, 12, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Tuong, V.M.; Chu, T.V. Improvement of color stability of acacia hybrid wood by TiO2 nano sol impregnation. BioResources 2015, 10, 5417–5425. [Google Scholar] [CrossRef]
- Yu, Y.; Jiang, Z.; Wang, G.; Song, Y. Growth of ZnO nanofilms on wood with improved photostability. Holzforschung 2010, 64, 385–390. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, W.; Lu, J.; Zhu, C.; Lin, W.; Feng, J. Aqueous epoxy-based superhydrophobic coatings: Fabrication and stability in water. Prog. Org. Coat. 2018, 121, 201–208. [Google Scholar] [CrossRef]
- Jia, S.; Lu, X.; Luo, S.; Qing, Y.; Yan, N.; Wu, Y. Efficiently texturing hierarchical epoxy layer for smart superhydrophobic surfaces with excellent durability and exceptional stability exposed to fire. Chem. Eng. J. 2018, 348, 212–223. [Google Scholar] [CrossRef]
- Jeong, J.H.; Han, Y.C.; Yang, J.H.; Kwak, D.S.; Jeong, H.M. Waterborne polyurethane modified with poly(ethylene glycol) macromer for waterproof breathable coating. Prog. Org. Coat. 2017, 103, 69–75. [Google Scholar] [CrossRef]
- Grüneberger, F.; Künniger, T.; Huch, A.; Zimmermann, T.; Arnold, M. Nanofibrillated cellulose in wood coatings: Dispersion and stabilization of ZnO as UV absorber. Prog. Org. Coat. 2015, 87, 112–121. [Google Scholar] [CrossRef]
- Miklečić, J.; Turkulin, H.; Jirouš-Rajković, V. Weathering performance of surface of thermally modified wood finished with nanoparticles-modified waterborne polyacrylate coatings. Appl. Surf. Sci. 2017, 408, 103–109. [Google Scholar] [CrossRef]
- Yu, D.; Wen, S.; Yang, J.; Wang, J.; Chen, Y.; Luo, J.; Wu, Y. RGO modified ZnAl-LDH as epoxy nanostructure filler: A novel synthetic approach to anticorrosive waterborne coating. Surf. Coat. Technol. 2017, 326, 207–215. [Google Scholar] [CrossRef]
- Wang, S.; Hu, Z.; Shi, J.; Chen, G.; Zhang, Q.; Weng, Z.; Wu, K.; Lu, M. Green synthesis of graphene with the assistance of modified lignin and its application in anticorrosive waterborne epoxy coatings. Appl. Surf. Sci. 2019, 484, 759–770. [Google Scholar] [CrossRef]
- Dong, H.; Zheng, L.; Yu, P.; Jiang, Q.; Wu, Y.; Huang, C.; Yin, B. Characterization and Application of Lignin–Carbohydrate Complexes from Lignocellulosic Materials as Antioxidants for Scavenging In Vitro and In Vivo Reactive Oxygen Species. ACS Sustain. Chem. Eng. 2020, 8, 256–266. [Google Scholar] [CrossRef]
- Sun, S.; Zhao, Z.; Umemura, K. Further Exploration of Sucrose-Citric Acid Adhesive: Synthesis and Application on Plywood. Polymers 2019, 11, 1875. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Sun, S.; Wu, D.; Zhang, M.; Huang, C.; Umemura, K.; Yong, Q. Synthesis and characterization of sucrose and ammonium dihydrogen phosphate (SADP) adhesive for plywood. Polymers 2019, 11, 1909. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Chen, X.; Lin, H.; Han, C.; Zhang, S. A simple fabrication of superhydrophobic wood surface by natural rosin based compound via impregnation at room temperature. Eur. J. Wood Wood Prod. 2018, 76, 1417–1425. [Google Scholar] [CrossRef]
- Gao, H.; Duan, Y.; Yuan, Z. Preparation and oil–water separation performance of ultra-hydrophilic subaqueous ultra-hydrophobic pvdf-g-paa porous membrane. Acta Chem. Sin. 2016, 37, 1208–1215. [Google Scholar]
- Wang, S.; Shi, J.; Liu, C.; Xie, C.; Wang, C. Fabrication of a superhydrophobic surface on a wood substrate. Appl. Surf. Sci. 2011, 257, 9362–9365. [Google Scholar] [CrossRef]
- Zhao, Z.; Sakai, S.; Wu, D.; Chen, Z.; Zhu, N.; Gui, C.; Zhang, M.; Umemura, K.; Yong, Q. Investigation of synthesis mechanism, optimal hot-pressing conditions, and curing behavior of sucrose and ammonium dihydrogen phosphate adhesive. Polymers 2020, 12, 216. [Google Scholar] [CrossRef]
- Wei, X.; Li, Q.; Hao, H.; Yang, H.; Li, Y.; Sun, T.; Li, X. Preparation, physicochemical and preservation properties of Ti/ZnO in situ SiOx chitosan composite coatings. J. Sci. Food Agric. 2019, 100, 570–577. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wan, M.; Wang, Z.; Zhang, X.; Zhao, Y.; Sun, L. Fabrication and characterization of degradable and durable fluoride-free super-hydrophobic cotton fabrics for oil/water separation. Surf. Coat. Technol. 2019, 378, 125079. [Google Scholar] [CrossRef]
- Pandey, S.; Singh, S.P. Organic solvent tolerance of an α-Amylase from haloalkaliphilic bacteria as a function of pH, temperature, and salt concentrations. Appl. Biochem. Biotechnol. 2012, 166, 1747–1757. [Google Scholar] [CrossRef]
- Wang, C.; Tzeng, F.; Chen, H.; Chang, C. Ultraviolet-durable superhydrophobic zinc oxide-coated mesh films for surface and underwater–oil capture and transportation. Langmuir 2012, 28, 10015–10019. [Google Scholar] [CrossRef] [PubMed]
- Cai, P.; Bai, N.; Xu, L.; Tan, C.; Li, Q. Fabrication of superhydrophobic wood surface with enhanced environmental adaptability through a solution-immersion process. Surf. Coat. Technol. 2015, 277, 262–269. [Google Scholar] [CrossRef]

| Name of the Coating | Contact Angle of Water on the Coating Surface (°) | Image of Contact Angle |
|---|---|---|
| WUV | 68.2 |  |
| ZnSt2/WUV | 158.4 | 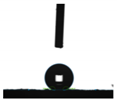 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Wu, X.; Yang, F.; Ye, J. Preparation and Characterization of Waterborne UV Lacquer Product Modified by Zinc Oxide with Flower Shape. Polymers 2020, 12, 668. https://doi.org/10.3390/polym12030668
Wu Y, Wu X, Yang F, Ye J. Preparation and Characterization of Waterborne UV Lacquer Product Modified by Zinc Oxide with Flower Shape. Polymers. 2020; 12(3):668. https://doi.org/10.3390/polym12030668
Chicago/Turabian StyleWu, Yan, Xinyu Wu, Feng Yang, and Jiaoyou Ye. 2020. "Preparation and Characterization of Waterborne UV Lacquer Product Modified by Zinc Oxide with Flower Shape" Polymers 12, no. 3: 668. https://doi.org/10.3390/polym12030668
APA StyleWu, Y., Wu, X., Yang, F., & Ye, J. (2020). Preparation and Characterization of Waterborne UV Lacquer Product Modified by Zinc Oxide with Flower Shape. Polymers, 12(3), 668. https://doi.org/10.3390/polym12030668





