Preparation and Characterization of Color Photocurable Resins for Full-Color Material Jetting Additive Manufacturing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Preparation of Resins
2.2. Characterization
3. Results and Discussion
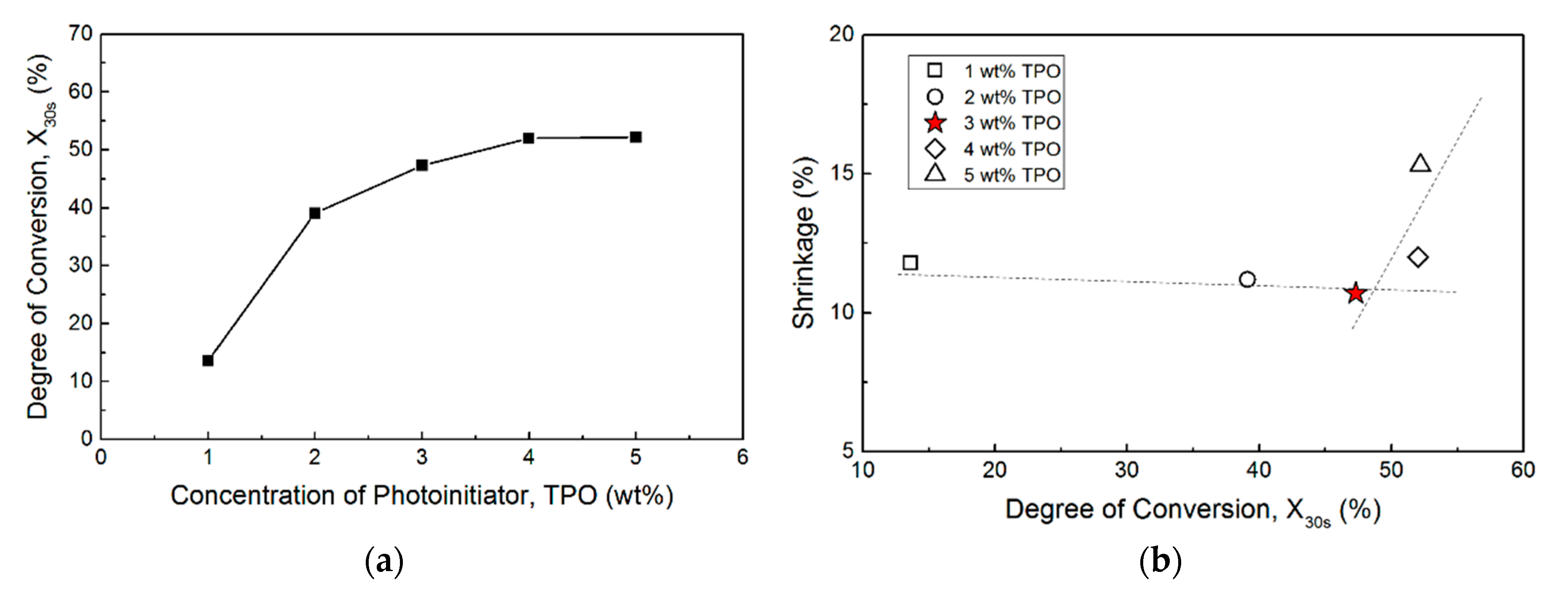
3.1. The Effects of the Concentration of Photo-Initiator (TPO)
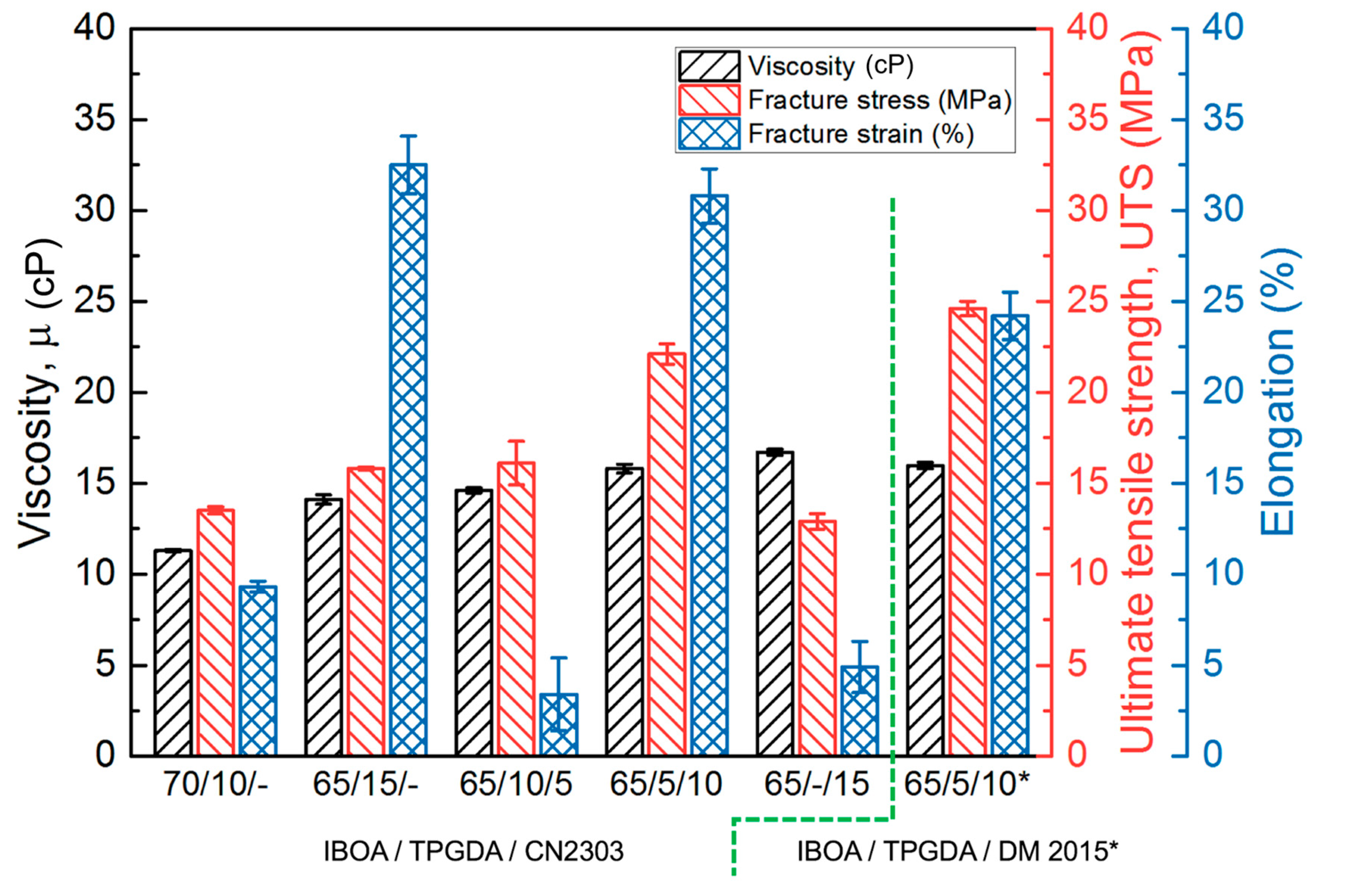
3.2. Aliphatic PUA Oligomer vs. Aromatic PUA Oligomer
3.3. Crosslinking
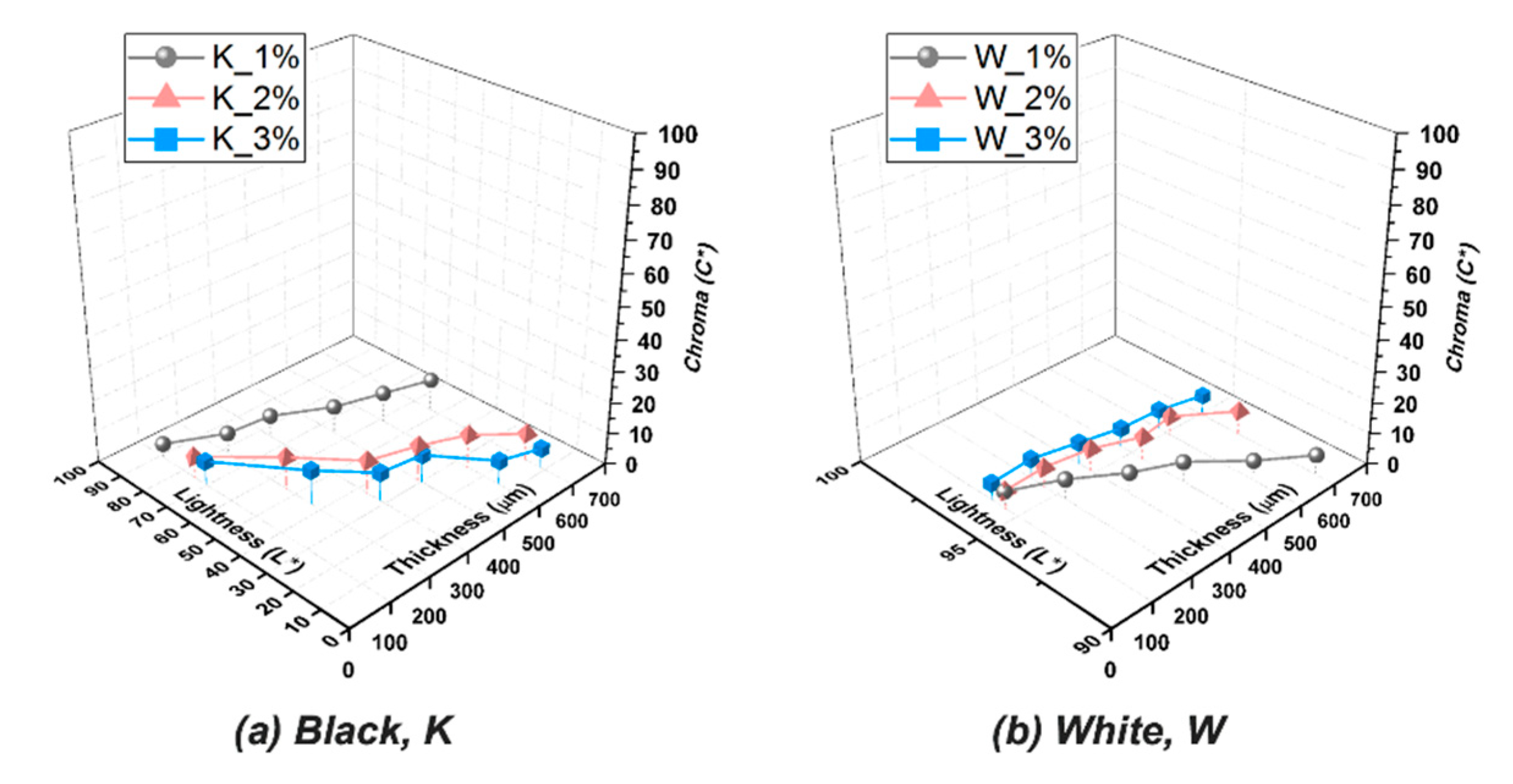
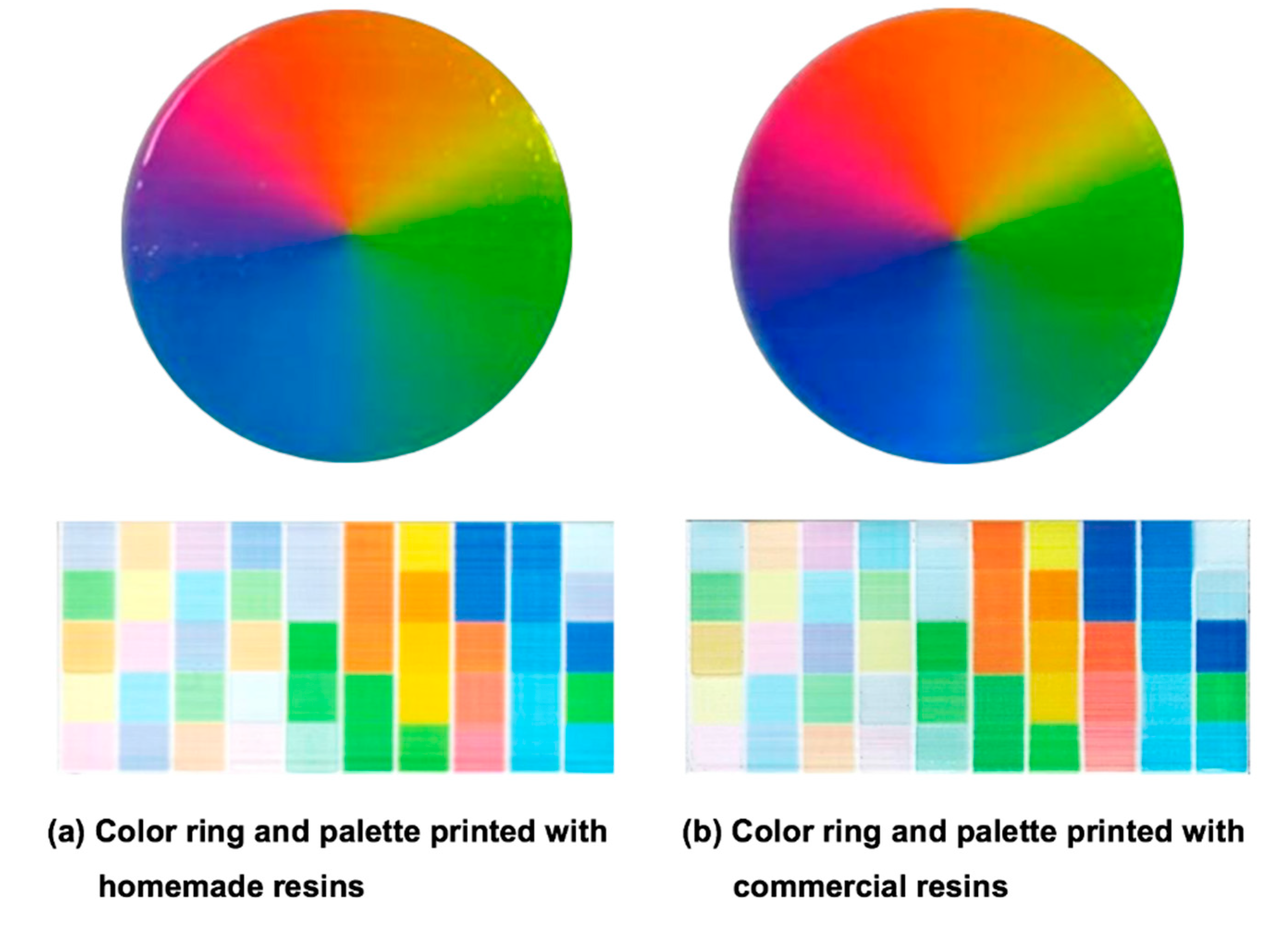
3.4. Color Appearances of Prepared Resins
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Berman, B. 3-D printing: The new industrial revolution. Bus. Horiz. 2012, 55, 155–162. [Google Scholar] [CrossRef]
- Huang, Y.; Leu, M.C.; Mazumder, J.; Donmez, A. Additive Manufacturing: Current State, Future Potential, Gaps and Needs, and Recommendations. J. Manuf. Sci. Eng. 2015, 137, 014001. [Google Scholar] [CrossRef] [Green Version]
- Caffrey, T.; Wohlers, T. Wohlers Report: 3D Printing and Additive Manufacturing State of the Industry; Wohlers Associates: Fort Collins, CO, USA, 2018. [Google Scholar]
- Ngo, T.D.; Kashani, A.; Imbalzano, G.; Nguyen, K.T.Q.; Hui, D. Additive manufacturing (3D printing): A review of materials, methods, applications and challenges. Compos. Part B Eng. 2018, 143, 172–196. [Google Scholar] [CrossRef]
- Ligon, S.C.; Liska, R.; Stampfl, J.; Gurr, M.; Mülhaupt, R. Polymers for 3D Printing and Customized Additive Manufacturing. Chem. Rev. 2017, 117, 10212–10290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, G.; Chen, C.; Yu, Z.; Yin, H.; He, L.; Yuan, J. Color 3D Printing: Theory, Method, and Application. Intech Open 2018, 2, 25–51. [Google Scholar]
- Kaspar, A.; Babaei, V.; Didyk, P.; Foshey, M.; Matusik, W.; Vidimče, K. Color contoning for 3D printing. ACM Trans. Graph. 2017, 36, 1–15. [Google Scholar]
- Walters, P.; Huson, D.; Parraman, C.; Stanić, M. 3D printing in colour: Technical evaluation and creative applications. In Proceedings of the IMPACT 6 Multidisciplinary Printmaking Conference, Bristol, UK, 16–19 September 2009. [Google Scholar]
- ISO/ASTM International. Standard Terminology for Additive Manufacturing—General Principles—Terminology; ISO/ASTM 52900:2015(E); ISO/ASTM: Geneva, Switzerland; West Conshohocken, PA, USA, 2015. [Google Scholar]
- 3D Systems, Full Color 3D Printers. Available online: https://www.3dsystems.com/3d-printers/plastic (accessed on 5 March 2020).
- HP Development Company, HP Jet Fusion 580 Color 3D Printer. Available online: http://www8.hp.com/h20195/v2/GetPDF.aspx/4AA7-3728ENA.pdf (accessed on 12 February 2020).
- Travers, J.; Keenan, P.; MacCormack, F.; MacCormack, C. Colour 3-dimensional Printing With 3D Gamut Mapping. U.S. Patent 10071527 B2, 11 September 2018. [Google Scholar]
- Stratasys, J750™ Digital Anatomy™ 3D Printer. Available online: https://www.stratasys.com/3d-printers/j750-digital-anatomy (accessed on 5 March 2020).
- Stratasys, J8 Series 3D Printers. Available online: https://www.stratasys.com/3d-printers/j8-series (accessed on 5 March 2020).
- Le, H.P. Progress and Trends in Ink-jet Printing Technology. J. Imaging Sci. Technol. 1998, 42, 49–62. [Google Scholar]
- De Gans, B.J.; Duineveld, P.C.; Schubert, U.S. Inkjet printing of polymers: State of the art and future developments. Adv. Mater. 2004, 16, 203–213. [Google Scholar] [CrossRef]
- Tsai, M.-J.; Mei, C.W.; Cheng, Y.-L.; Chen, F.; Hu, Z.Y.; Huang, K.C. A study of a material jetting based color 3d printing system by using multiple piezoelectric heads. In Proceedings of the 2017 International Conference on Machine Learning and Cybernetics (ICMLC), Ningbo, China, 9–12 July 2017; pp. 664–669. [Google Scholar]
- Tsai, M.-J.; Hsu, Y.-S.; Cheng, Y.-L.; Chen, F.; Chang, C.-H. Development of a color photo-curable 3D printing mechatronics system by using multiple piezoelectric heads. In Proceedings of the 2016 International Conference on Machine Learning and Cybernetics (ICMLC), Jeju, Korea, 10–13 July 2016; pp. 709–714. [Google Scholar]
- Tsai, M.-J.; Li, C.-R.; Huang, Y.-K. A vision-based automatic alignment technology for color 3D additive manufacturing system with multiple print head. In Proceedings of the 2018 International Conference on Image and Video Processing, and Artificial Intelligence IVPAI, Shanghai, China, 29 October 2018; SPIE: Bellingham, WA, USA. [Google Scholar]
- Cheng, Y.-L.; Chang, C.-H.; Kuo, C. Experimental study on leveling mechanism for material-jetting-type color 3D printing. Rapid Prototyp. J. 2020, 26, 11–20. [Google Scholar] [CrossRef]
- Sun, P.-L.; Sie, Y.-P. Color Uniformity Improvement for an Inkjet Color 3D Printing System. In Proceedings of the IS&T International Symposium on Electron. Imaging 2016 Color Imaging XXI Displaying, Processing, Hardcopy, and Applications, San Francisco, CA, USA, 14–18 February 2016; COLOR-342. pp. 1–6. [Google Scholar]
- Hull, C. Apparatus for Production of Three-Dimensional Objects by Stereolithography. U.S. Patent 4575330, 11 March 1986. [Google Scholar]
- Murphy, E.J.; Ansel, R.E.; Krajewski, J.J. Method of Forming a Three-Dimensional Object by Stereolithography and Composition Therefore. U.S. Patent 4942001A, 17 July 1990. [Google Scholar]
- Coats, A.L.; Harrison, J.P.; Hay, J.S.; Ramos, M.J. Stereolithography Resin and Methods. U.S. Patent 7211368, 1 May 2007. [Google Scholar]
- Steinmann, B.; Schulthess, A. Liquid, Radiation-Curable Composition, Especially for Stereolithography. U.S. Patent 5972563, 26 October 1999. [Google Scholar]
- Guo, Y.; Ji, Z.; Zhang, Y.; Wang, X.; Zhou, F. Solvent-free and photocurable polyimide inks for 3D printing. J. Mater. Chem. A 2017, 5, 16307–16314. [Google Scholar] [CrossRef]
- Steinmann, B.; Wolf, J.-P.; Schulthess, A.; Hunziker, M. Photosensitive Compositions. U.S. Patent 5476748, 19 December 1995. [Google Scholar]
- Ueda, M.; Takase, K.; Kurosawa, T. Stereolithography Resin Compositions and Three-Dimensional Objects Made Therefrom. U.S. Patent 8980971, 17 March 2015. [Google Scholar]
- Hutchings, L.R.; Dodds, J.M.; Roberts-Bleming, S.J. HyperMacs: Highly branched polymers prepared by the polycondensation of AB2 macromonomers, synthesis and characterization. Macromolecules 2005, 38, 5970–5980. [Google Scholar] [CrossRef]
- Hutchings, L.R.; Dodds, J.M.; Rees, D.; Kimani, S.M.; Wu, J.J.; Smith, E. HyperMacs to HyperBlocks: A novel class of branched thermoplastic elastomer. Macromolecules 2009, 42, 8675–8687. [Google Scholar] [CrossRef] [Green Version]
- Nugay, N.; Nugay, T.; Deodhar, T.; Keszler, B.L.; Kennedy, J.P. Low cost bifunctional initiators for bidirectional living cationic polymerization of olefins. II. hyperbranched styrene–isobutylene–styrene triblocks with superior combination of properties. J. Polym. Sci. Part A Polym. Chem. 2018, 56, 705–713. [Google Scholar] [CrossRef]
- Wang, W.; Lu, W.; Goodwin, A.; Wang, H.; Yin, P.; Kang, N.G.; Hong, K.; Mays, J.W. Recent advances in thermoplastic elastomers from living polymerizations: Macromolecular architectures and supramolecular chemistry. Prog. Polym. Sci. 2019, 95, 1–31. [Google Scholar] [CrossRef]
- Leppard, D.G.; Kohler, M.; Misev, L. Photopolymerizable Compositions Containing an Alkylbisacylphosphine Oxide. U.S. Patent 5472992, 5 December 1995. [Google Scholar]
- Pekarovicova, A.; Husovska, V. Printing Ink Formulations. In Printing on Polymers: Fundamentals and Applications; Izdebska, J., Thomas, S., Eds.; William Andrew Publishing: Waltham, MA, USA, 2016; pp. 41–55. [Google Scholar]
- Tauber, G.; Batz-Sohn, C.; Nelli, L.; McIntosh, R.; Kalbitz, W. Improved dispersibility of surface oxidized carbon black pigments for inkjet ink formulation. In Proceedings of the NIP & Digital Fabrication Conference, Austin, TX, USA, 1 January 2010; pp. 181–184. [Google Scholar]




| Chemicals | Abbreviation | Function | Functionality | Suppliers | Trade Name | |
|---|---|---|---|---|---|---|
| Base | Aliphatic polyurethane acrylate | Aliphatic-PUA | Oligomer | 2 | DBC | DM 553 |
| Aromatic polyurethane Dimethacrylate | Aromatic-PUA | Oligomer | 2 | DBC | DM 7201M | |
| Polyester acrylate | PEA | Oligomer | 4 | DSM-AGI Co. | AgiSyn 003 | |
| Isobornyl Acrylate | IBOA | Monomer | 1 | DBC | DM IBOA | |
| Tripropylene glycol diacrylate | TPGDA | Crosslinker | 2 | DBC | DM TPGDA | |
| Hexafunctional Polyester acrylate | 6F-PEA | Crosslinker | 6 | Sartomer Americas | CN2303 | |
| Fifteen-functional Polyester acrylate | 15F-PEA | Crosslinker | 15 | DBC | DM 2015 | |
| Diphenyl-(2,4,6-trimethylbenzoyl)-phosphine oxide | TPO | Free radical photoinitiator | Chembridge | Cgemcure-TPO | ||
| Additive | Acrylic Block Copolymer | PX-4701 | Dispersant | BASF | PX-4701 | |
| Di-functional Silicone Acrylate | DM-9137 | Leveling agent | DBC | DM 9137 | ||
| CMYK primary color UV ink | Pigment | ChengFeng Group | ||||
| W primary color UV ink | Pigment | ITRI | ||||
| Formulated Resins | Viscosity @ 70 °C, cP | Surface Tension, mN/m |
|---|---|---|
| Resin 65/5/10 | 15.8 | 29.9 |
| Resin 65/5/10-C_2% | 12.3 | 28.1 |
| Resin 65/5/10-M_3% | 12.0 | 28.1 |
| Resin 65/5/10-Y_3% | 12.1 | 27.7 |
| Resin 65/5/10-K_2% | 12.6 | 28.3 |
| Resin 65/5/10-W_3% | 11.8 | 27.3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, Y.-L.; Huang, K.-C. Preparation and Characterization of Color Photocurable Resins for Full-Color Material Jetting Additive Manufacturing. Polymers 2020, 12, 650. https://doi.org/10.3390/polym12030650
Cheng Y-L, Huang K-C. Preparation and Characterization of Color Photocurable Resins for Full-Color Material Jetting Additive Manufacturing. Polymers. 2020; 12(3):650. https://doi.org/10.3390/polym12030650
Chicago/Turabian StyleCheng, Yih-Lin, and Kuan-Chi Huang. 2020. "Preparation and Characterization of Color Photocurable Resins for Full-Color Material Jetting Additive Manufacturing" Polymers 12, no. 3: 650. https://doi.org/10.3390/polym12030650
APA StyleCheng, Y.-L., & Huang, K.-C. (2020). Preparation and Characterization of Color Photocurable Resins for Full-Color Material Jetting Additive Manufacturing. Polymers, 12(3), 650. https://doi.org/10.3390/polym12030650





