Structures, Properties and Potential Applications of Corncob Residue Modified by Carboxymethylation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Composition Analysis
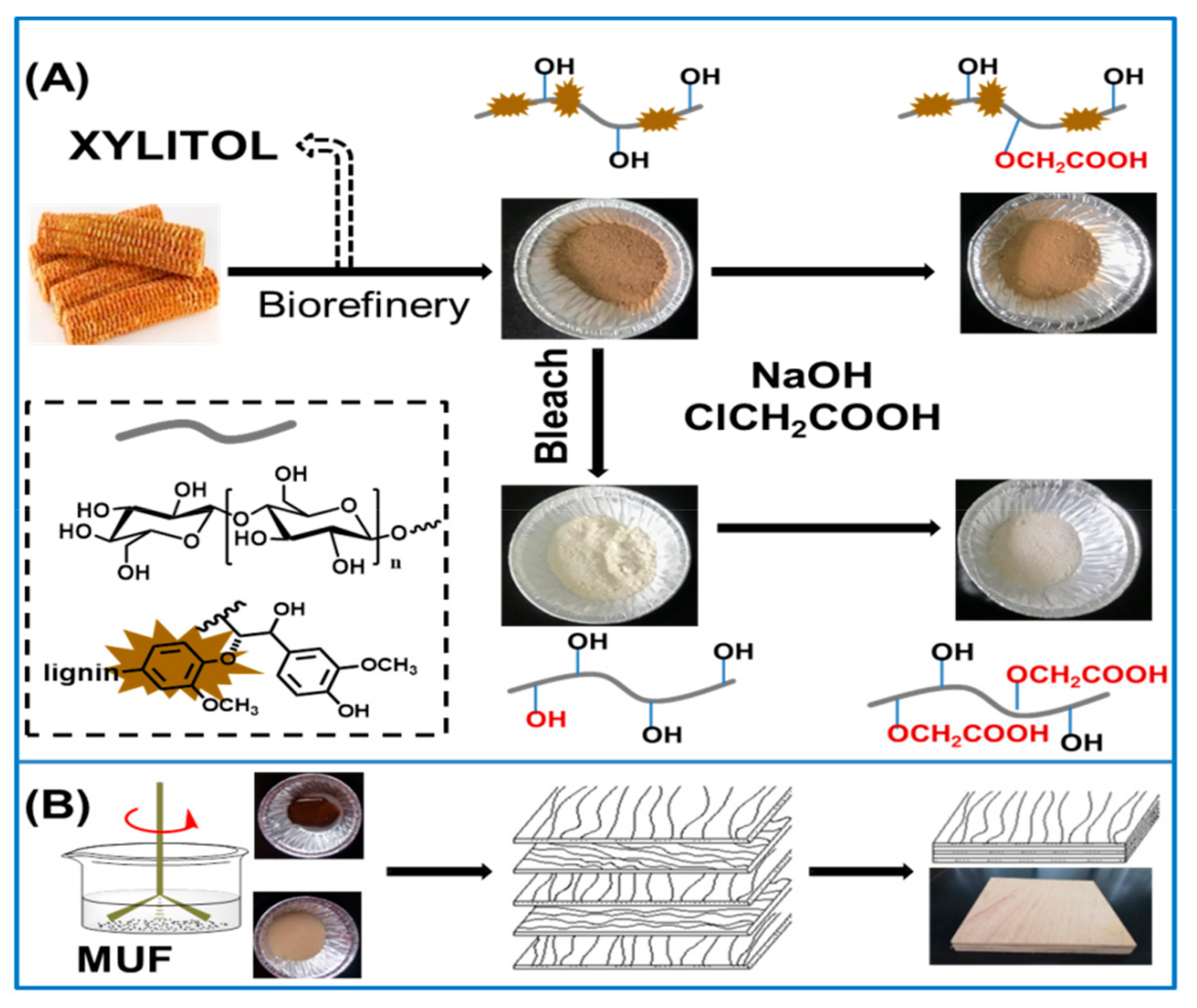
2.3. Cellulose Extraction and Carboxymethylation
2.4. Determination of Degree of Substitution
2.5. Characterization
2.6. Synthesis of Melamine-Urea-Formaldehyde Resin
2.7. Preparation of Three-ply Plywood and Adhesion Test
2.8. Formaldehyde Emission Test
3. Results and Discussion
3.1. Carboxymethylation of Cellulose and Corncob Residue
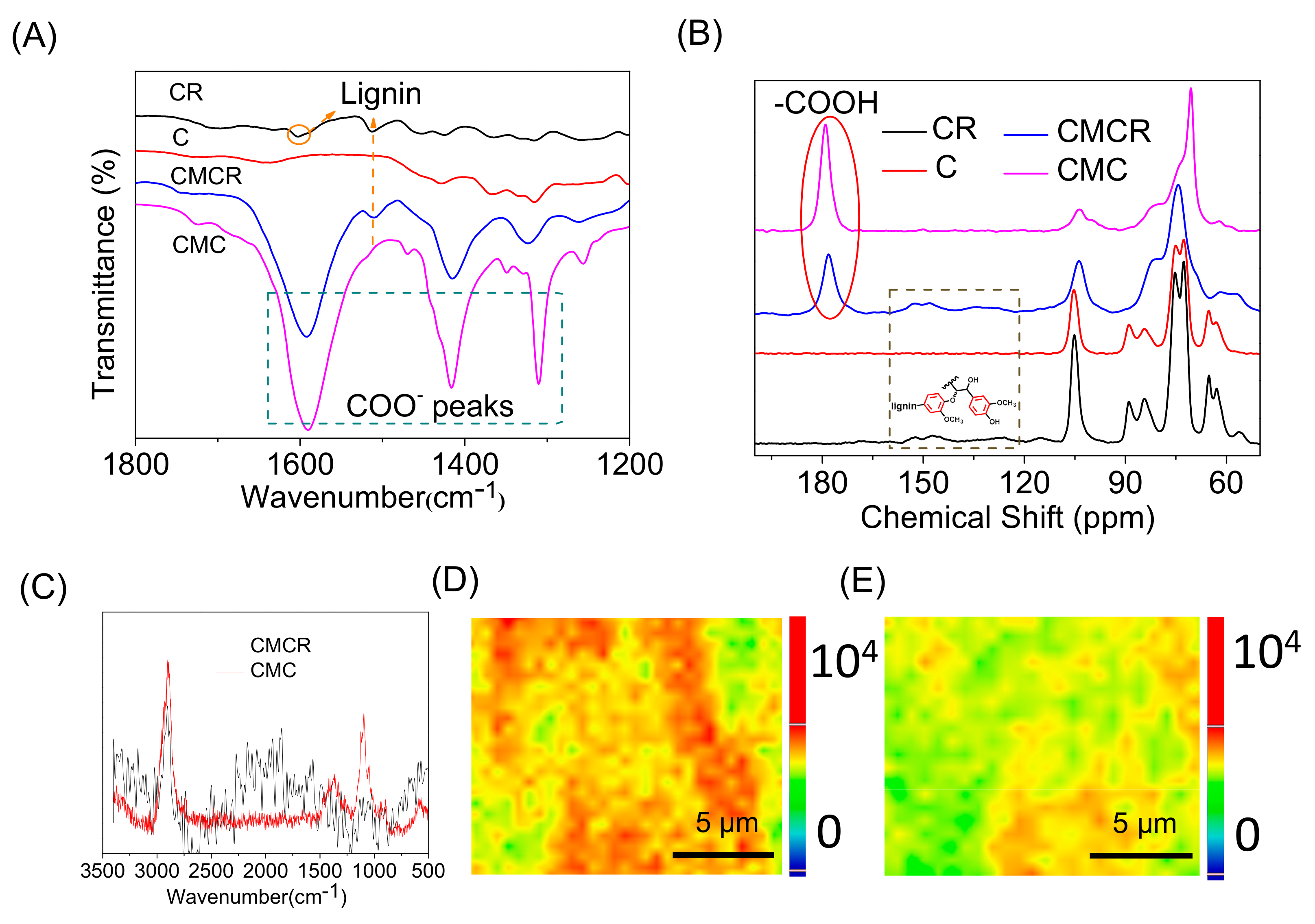
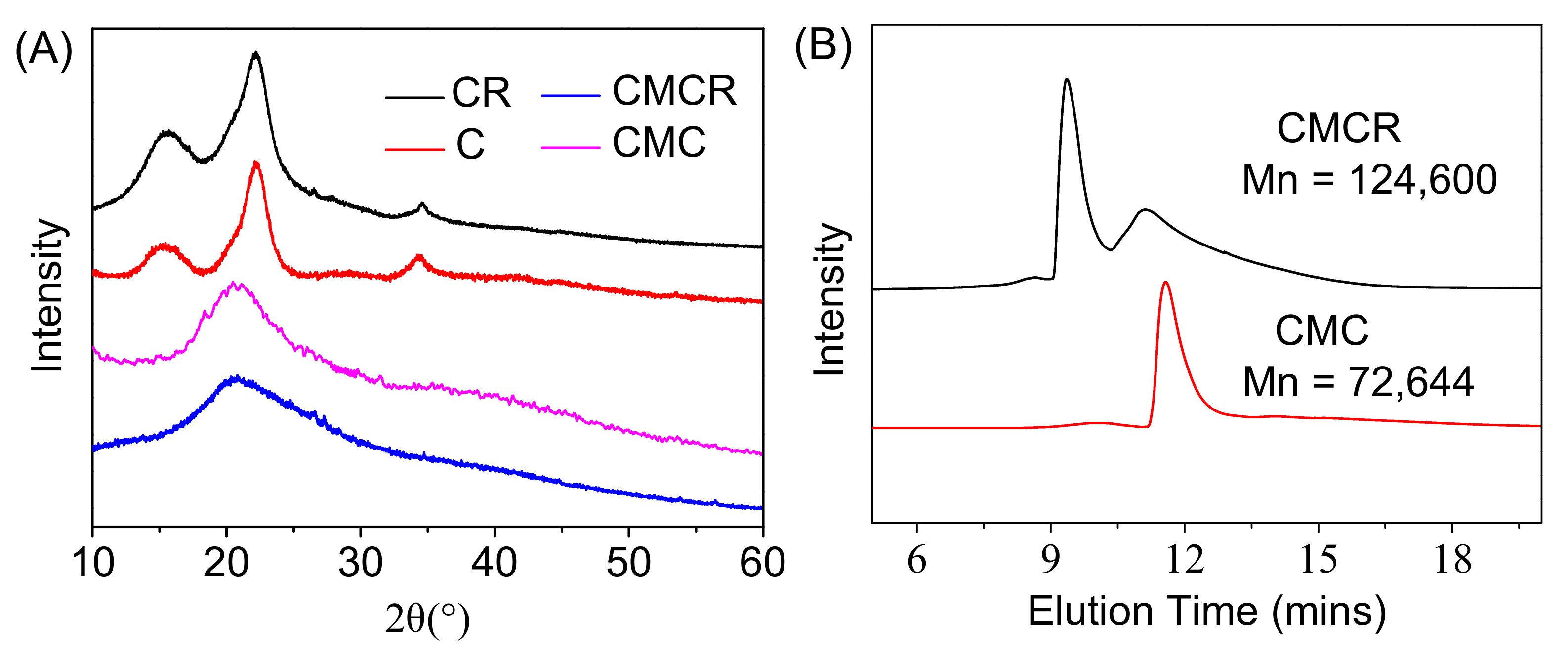
3.1.1. Structural Characterization of Modified Substrates
3.1.2. Thermal Stability of Modified Substrates
3.1.3. Morphology of Modified Substrates
3.2. Enhanced Performance of Modified Substrates in UF Resin Composite
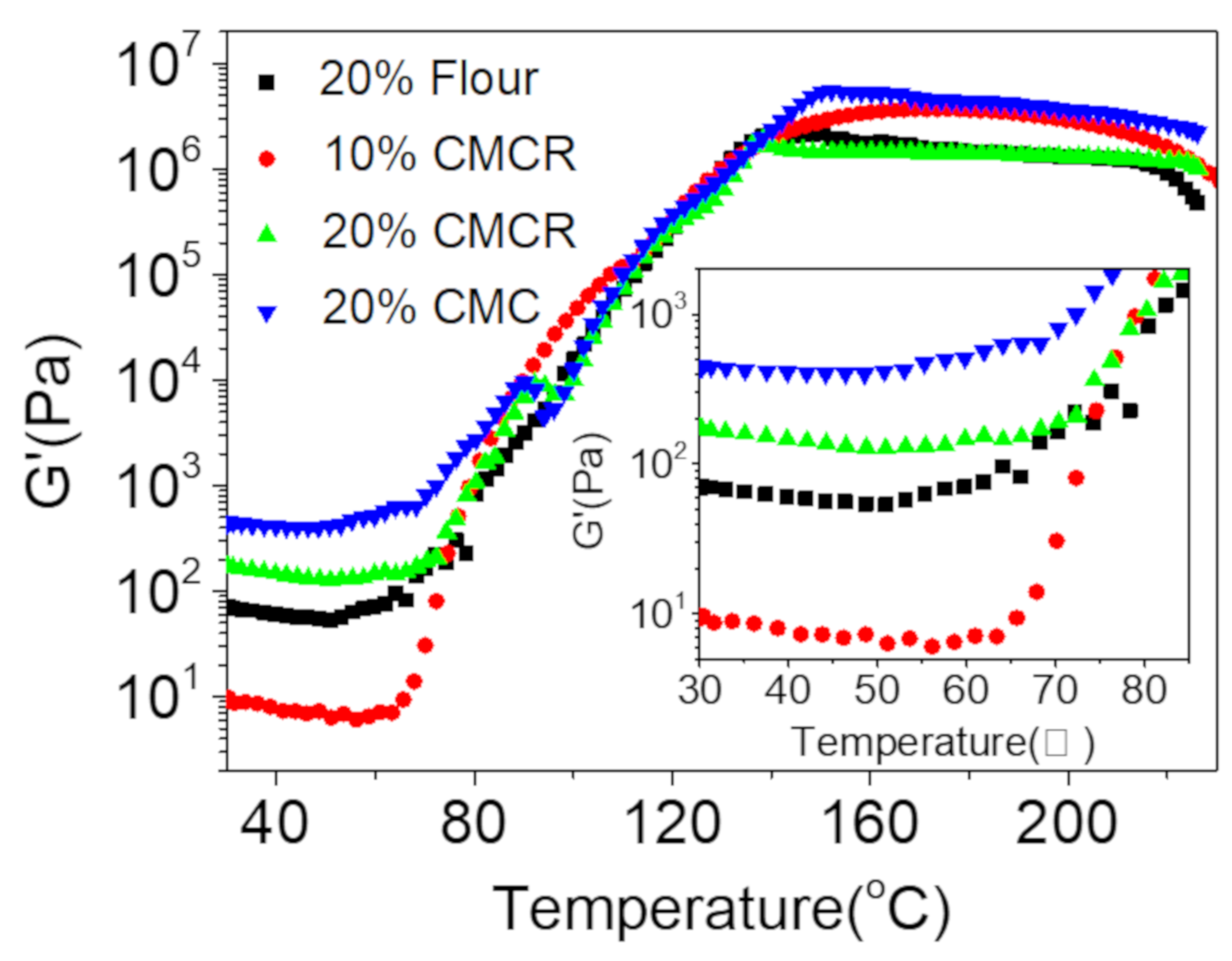
3.2.1. Effect on the Viscosity and Viscoelastic Properties
3.2.2. Effect on the Curing Process
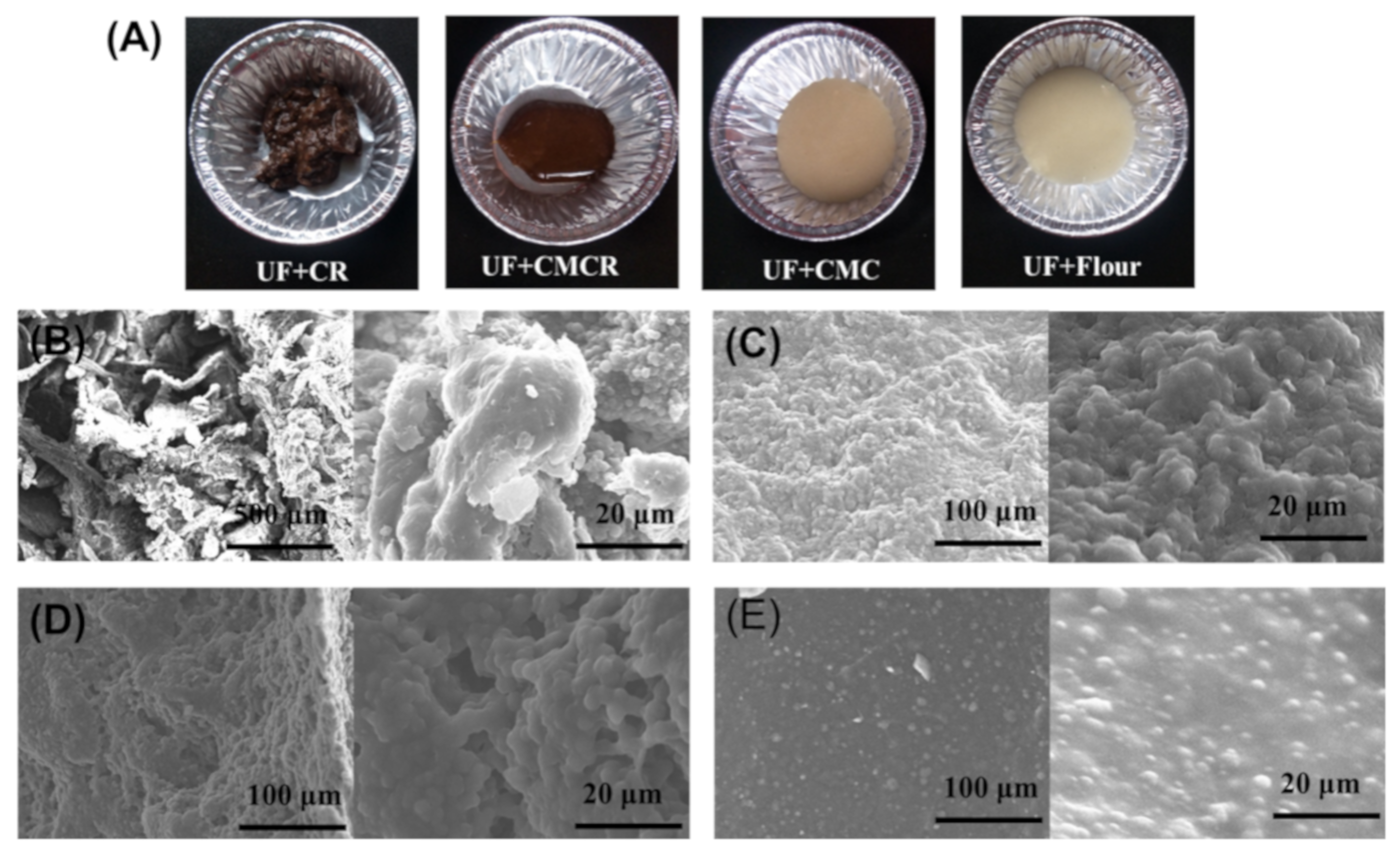
3.2.3. Effect on the Structural and Morphological Properties
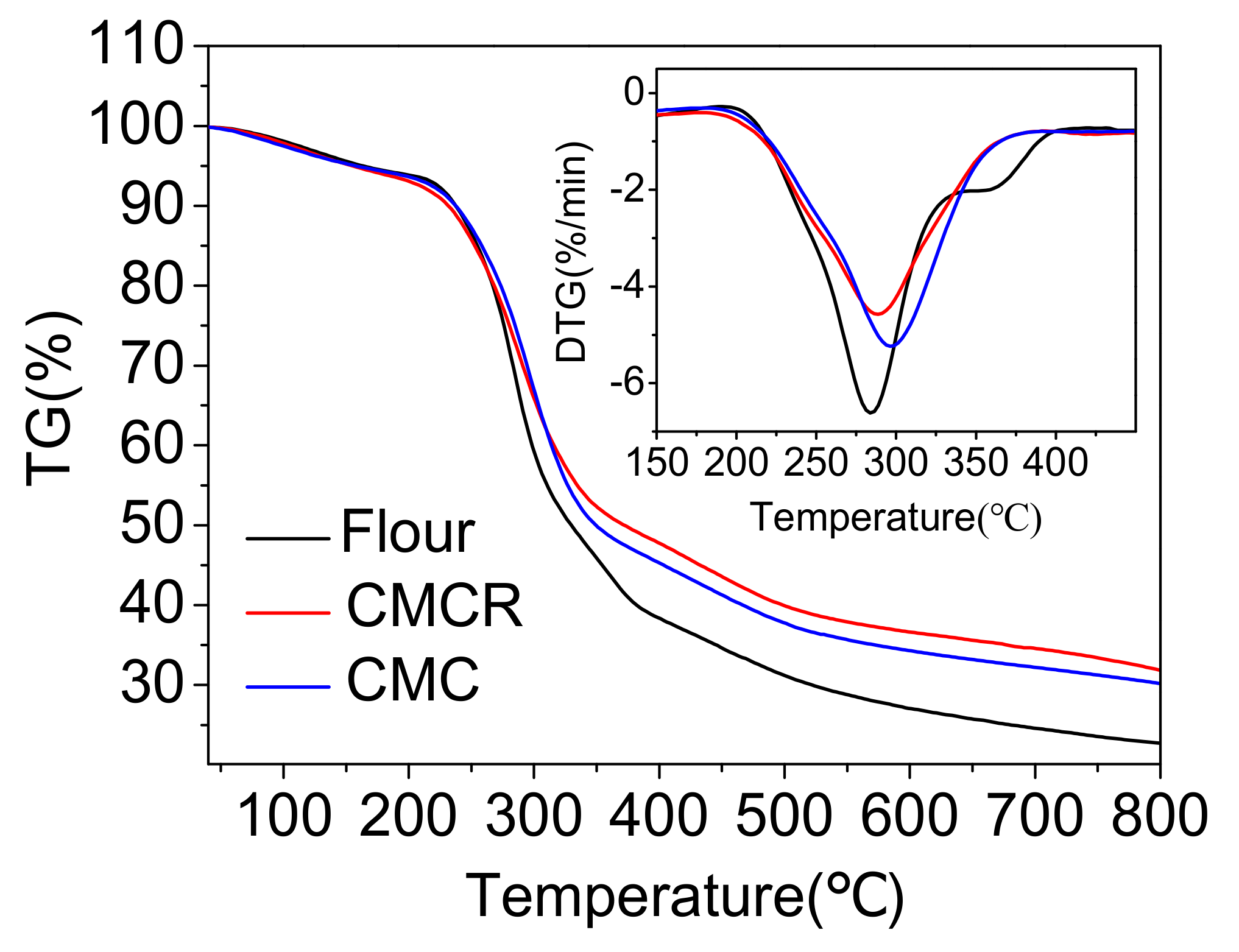
3.2.4. Effect on the Thermal Stability
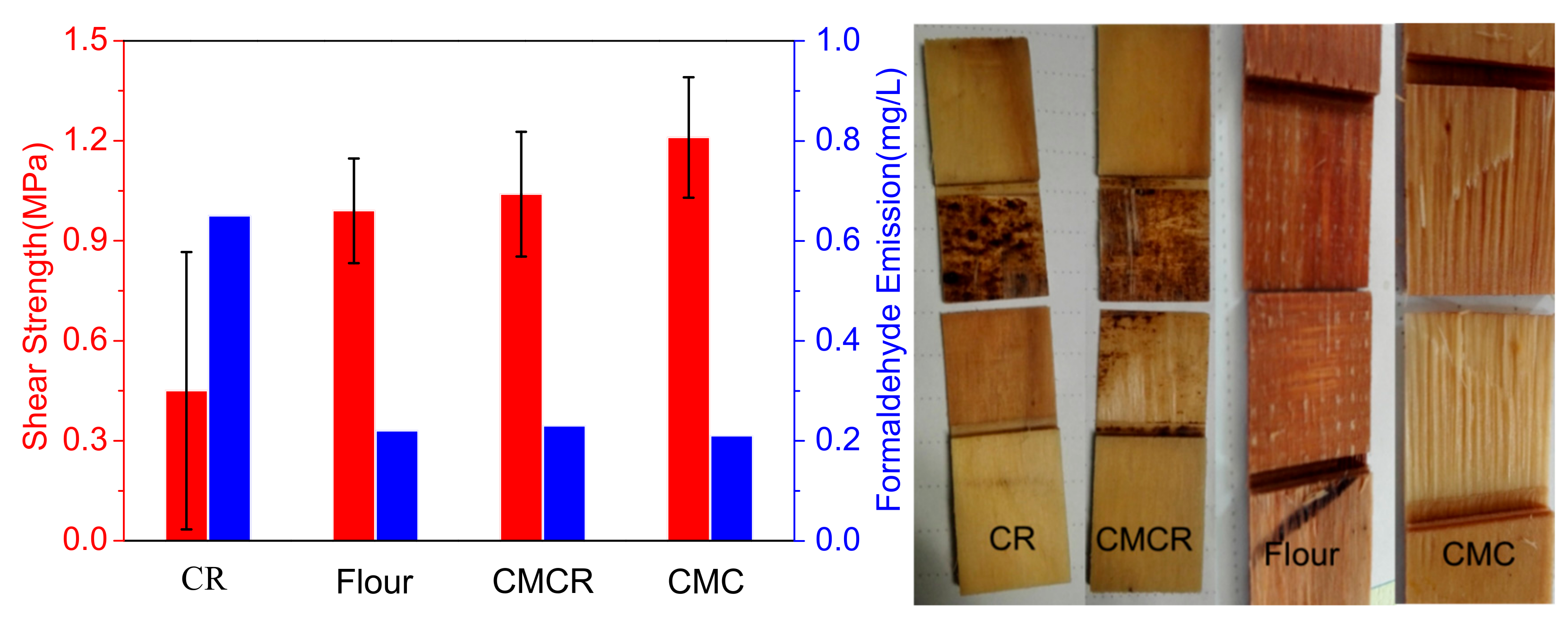
3.3. Adhesive Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pickering, K.L.; Efendy, M.A.; Le, T.M. A review of recent developments in natural fibre composites and their mechanical performance. Compos. Part A 2016, 83, 98–112. [Google Scholar] [CrossRef]
- De Prez, J.; Van Vuure, A.W.; Ivens, J.; Aerts, G.; Van de Voorde, I. Effect of enzymatic treatment of flax on fineness of fibers and mechanical performance of composites. Compos. Part A 2019, 123, 190–199. [Google Scholar] [CrossRef]
- El-Abbassi, F.E.; Assarar, M.; Ayad, R.; Bourmaud, A.; Baley, C. A review on alfa fibre (Stipa tenacissima L.): From the plant architecture to the reinforcement of polymer composites. Compos. Part A 2020, 128, 105677. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y.; Liu, W.; Ni, Y.; Hou, Q. Corncob residues as carbon quantum dots sources and their application in detection of metal ions. Ind. Crops Prod. 2019, 133, 18–25. [Google Scholar] [CrossRef]
- Cheng, K.-K.; Zhang, J.-A.; Chavez, E.; Li, J.-P. Integrated production of xylitol and ethanol using corncob. Appl. Microbiol. Biotechnol. 2010, 87, 411–417. [Google Scholar] [CrossRef]
- Rivas, B.; Torre, P.; Domínguez, J.M.; Converti, A.; Parajó, J.C. Purification of Xylitol Obtained by Fermentation of Corncob Hydrolysates. J. Agric. Food. Chem. 2006, 54, 4430–4435. [Google Scholar] [CrossRef] [PubMed]
- Berber-Villamar, N.K.; Netzahuatl-Muñoz, A.R.; Morales-Barrera, L.; Chávez-Camarillo, G.M.; Flores-Ortiz, C.M.; Cristiani-Urbina, E. Corncob as an effective, eco-friendly, and economic biosorbent for removing the azo dye Direct Yellow 27 from aqueous solutions. PLoS ONE 2018, 13, e0196428. [Google Scholar] [CrossRef]
- Liu, K.; Lin, X.; Yue, J.; Li, X.; Fang, X.; Zhu, M.; Lin, J.; Qu, Y.; Xiao, L. High concentration ethanol production from corncob residues by fed-batch strategy. Bioresour. Technol. 2010, 101, 4952–4958. [Google Scholar] [CrossRef]
- Qu, W.-H.; Xu, Y.-Y.; Lu, A.-H.; Zhang, X.-Q.; Li, W.-C. Converting biowaste corncob residue into high value added porous carbon for supercapacitor electrodes. Bioresour. Technol. 2015, 189, 285–291. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Q.; Wang, B.; Yang, G.; Lucia, L.A.; Chen, J. Hydrothermal carbonization of corncob residues for hydrochar production. Energy Fuels 2015, 29, 872–876. [Google Scholar] [CrossRef]
- El-Korashy, S.A.; Elwakeel, K.Z.; El-Hafeiz, A.A. Fabrication of bentonite/thiourea-formaldehyde composite material for Pb (II), Mn (VII) and Cr (VI) sorption: A combined basic study and industrial application. J. Clean. Prod. 2016, 137, 40–50. [Google Scholar] [CrossRef]
- Guo, M.; Liu, M.; Liang, R.; Niu, A. Granular urea-formaldehyde slow-release fertilizer with superabsorbent and moisture preservation. J. Appl. Polym. Sci. 2006, 99, 3230–3235. [Google Scholar] [CrossRef]
- Tiwari, D.; Bhunia, H.; Bajpai, P.K. Development of chemically activated N-enriched carbon adsorbents from urea-formaldehyde resin for CO2 adsorption: Kinetics, isotherm, and thermodynamics. J. Environ. Manage. 2018, 218, 579–592. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Ru, X.; Shi, J.; Song, J.; Zhao, H.; Liu, Y.; Zhao, G. Granular, Slow-Release Fertilizer from Urea-formaldehyde, Ammonium Polyphosphate, and Amorphous Silica Gel: A New Strategy Using Cold Extrusion. J. Agric. Food. Chem. 2018, 66, 7606–7615. [Google Scholar] [CrossRef]
- Gao, S.; Liu, Y.; Wang, C.; Chu, F.; Xu, F.; Zhang, D. Synthesis of Lignin-Based Polyacid Catalyst and Its Utilization to Improve Water Resistance of Urea–formaldehyde Resins. Polymers 2020, 12, 175. [Google Scholar] [CrossRef]
- Aydin, I.; Demirkir, C.; Colak, S.; Colakoglu, G. Utilization of bark flours as additive in plywood manufacturing. Eur. J. Wood Wood Prod. 2017, 75, 63–69. [Google Scholar] [CrossRef]
- Ding, R.; Su, C.; Yang, Y.; Li, C.; Liu, J. Effect of Wheat Flour on the Viscosity of Urea-formaldehyde Adhesive. Int. J. Adhes. Adhes. 2013, 41, 1–5. [Google Scholar] [CrossRef]
- Lei, H.; Du, G.; Pizzi, A.; Celzard, A. Influence of nanoclay on urea-formaldehyde resins for wood adhesives and its model. J. Appl. Polym. Sci. 2008, 109, 2442–2451. [Google Scholar] [CrossRef]
- Bekhta, P.; Sedliačik, J.; Kačík, F.; Noshchenko, G.; Kleinová, A. Lignocellulosic waste fibers and their application as a component of urea-formaldehyde adhesive composition in the manufacture of plywood. Eur. J. Wood Wood Prod. 2019, 77, 495–508. [Google Scholar] [CrossRef]
- Ong, H.R.; Khan, M.M.R.; Prasad, D.R.; Yousuf, A.; Chowdhury, M. Palm kernel meal as a melamine urea formaldehyde adhesive filler for plywood applications. Int. J. Adhes. Adhes. 2018, 85, 8–14. [Google Scholar] [CrossRef]
- Chiang, T.C.; Hamdan, S.; Osman, M.S. Urea formaldehyde composites reinforced with Sago fibres analysis by FTIR, TGA, and DSC. Adv. Mater. Sci. Eng. 2016. [Google Scholar] [CrossRef]
- De Almeida Mesquita, R.G.; Mendes, L.M.; Sanadi, A.R.; de Sena Neto, A.R.; Claro, P.I.C.; Corrêa, A.C.; Marconcini, J.M. Urea formaldehyde and cellulose nanocrystals adhesive: Studies applied to sugarcane bagasse particleboards. J. Polym. Environ. 2018, 26, 3040–3050. [Google Scholar] [CrossRef]
- Qiao, Z.; Gu, J.; Zuo, Y.; Tan, H.; Zhang, Y. The effect of carboxymethyl cellulose addition on the properties of starch-based wood adhesive. BioResources 2014, 9, 6117–6129. [Google Scholar] [CrossRef]
- Liu, J.; Yue, K.; Xu, L.; Wu, J.; Chen, Z.; Wang, L.; Liu, W.; Lu, W. Bonding performance of melamine-urea–formaldehyde and phenol-resorcinol–formaldehyde adhesive glulams at elevated temperatures. Int. J. Adhes. Adhes. 2019, 98, 102500. [Google Scholar] [CrossRef]
- Gulati, I.; Park, J.; Maken, S.; Lee, M.-G. Production of carboxymethylcellulose fibers from waste lignocellulosic sawdust using NaOH/NaClO 2 pretreatment. Fibers Polym. 2014, 15, 680–686. [Google Scholar] [CrossRef]
- Adinugraha, M.P.; Marseno, D.W. Synthesis and characterization of sodium carboxymethylcellulose from cavendish banana pseudo stem (Musa cavendishii LAMBERT). Carbohydr. Polym. 2005, 62, 164–169. [Google Scholar] [CrossRef]
- Lee, Y.-J.; Chung, C.-H.; Day, D.F. Sugarcane bagasse oxidation using a combination of hypochlorite and peroxide. Bioresour. Technol. 2009, 100, 935–941. [Google Scholar] [CrossRef]
- Casaburi, A.; Rojo, Ú.M.; Cerrutti, P.; Vázquez, A.; Foresti, M.L. Carboxymethyl cellulose with tailored degree of substitution obtained from bacterial cellulose. Food Hydrocoll. 2018, 75, 147–156. [Google Scholar] [CrossRef]
- Haleem, N.; Arshad, M.; Shahid, M.; Tahir, M.A. Synthesis of carboxymethyl cellulose from waste of cotton ginning industry. Carbohydr. Polym. 2014, 113, 249–255. [Google Scholar] [CrossRef]
- Oun, A.A.; Rhim, J.-W. Preparation and characterization of sodium carboxymethyl cellulose/cotton linter cellulose nanofibril composite films. Carbohydr. Polym. 2015, 127, 101–109. [Google Scholar] [CrossRef]
- Onsree, T.; Tippayawong, N.; Zheng, A.; Li, H. Pyrolysis behavior and kinetics of corn residue pellets and eucalyptus wood chips in a macro thermogravimetric analyzer. Case Stud. Therm. Eng. 2018, 12, 546–556. [Google Scholar] [CrossRef]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, S.; Liu, Y.; Wang, C.; Chu, F.; Xu, F.; Zhang, D. Structures, Properties and Potential Applications of Corncob Residue Modified by Carboxymethylation. Polymers 2020, 12, 638. https://doi.org/10.3390/polym12030638
Gao S, Liu Y, Wang C, Chu F, Xu F, Zhang D. Structures, Properties and Potential Applications of Corncob Residue Modified by Carboxymethylation. Polymers. 2020; 12(3):638. https://doi.org/10.3390/polym12030638
Chicago/Turabian StyleGao, Shishuai, Yupeng Liu, Chunpeng Wang, Fuxiang Chu, Feng Xu, and Daihui Zhang. 2020. "Structures, Properties and Potential Applications of Corncob Residue Modified by Carboxymethylation" Polymers 12, no. 3: 638. https://doi.org/10.3390/polym12030638
APA StyleGao, S., Liu, Y., Wang, C., Chu, F., Xu, F., & Zhang, D. (2020). Structures, Properties and Potential Applications of Corncob Residue Modified by Carboxymethylation. Polymers, 12(3), 638. https://doi.org/10.3390/polym12030638







