Phosphonium-Based Porous Ionic Polymer with Hydroxyl Groups: A Bifunctional and Robust Catalyst for Cycloaddition of CO2 into Cyclic Carbonates
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Catalyst Preparation
2.3. Synthesis of Cyclic Carbonates
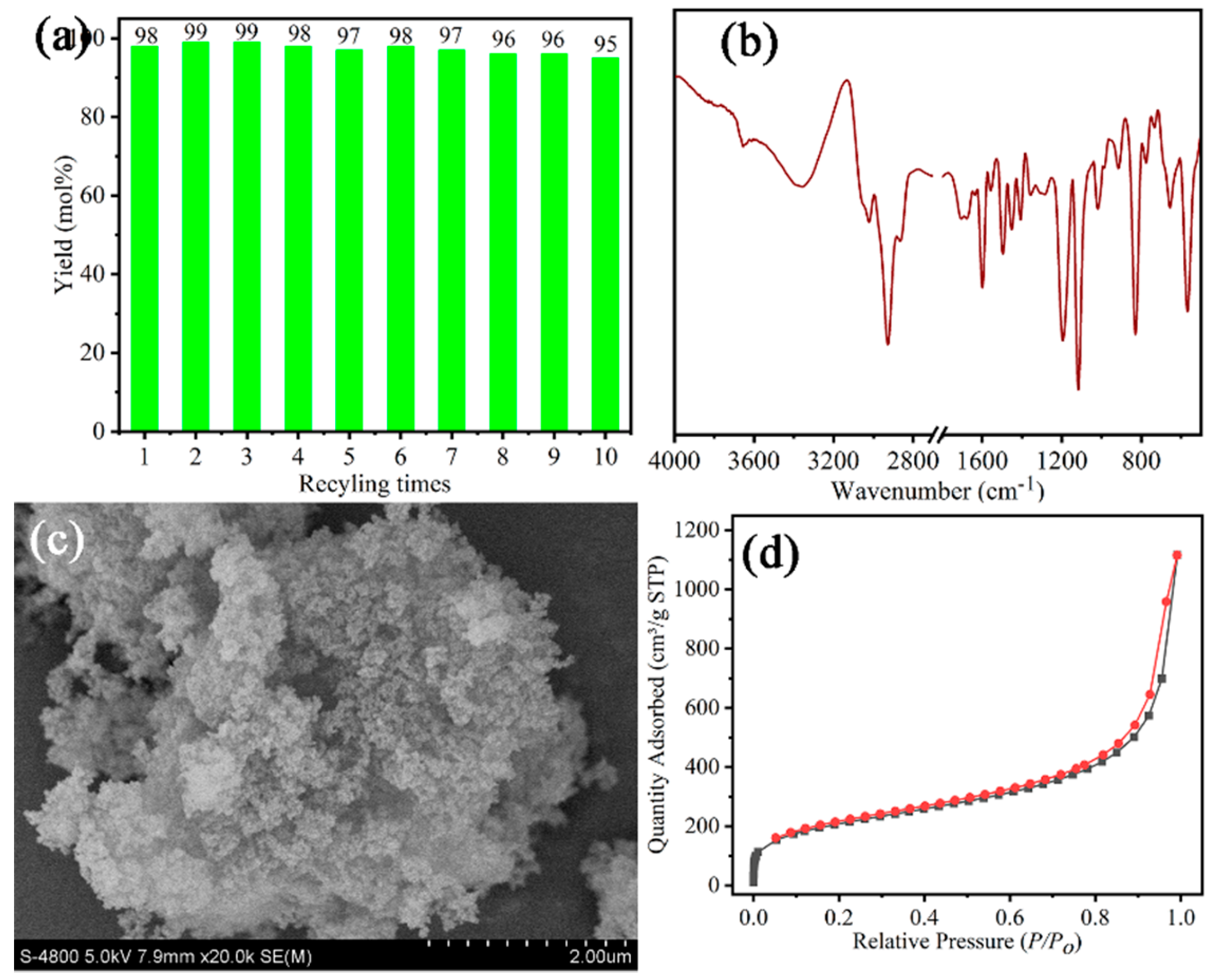
2.4. Catalyst Recycling
2.5. Characterizations
3. Results
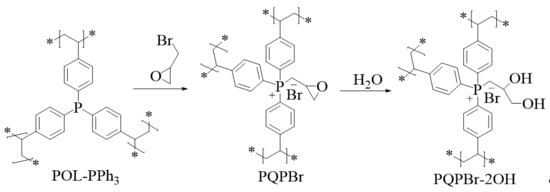
3.1. Characterizations of the Polymeric Catalysts
3.2. Catalytic Activity
3.3. Plausible Reaction Mechanism
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Liang, J.; Huang, Y.B.; Cao, R. Metal–organic frameworks and porous organic polymers for sustainable fixation of carbon dioxide into cyclic carbonates. Coord. Chem. Rev. 2019, 378, 32–65. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, Z.; Xu, X.; Wang, J. Review on porous nanomaterials for adsorption and photocatalytic conversion of CO2. Chin. J. Catal. 2017, 38, 1956–1969. [Google Scholar] [CrossRef]
- Li, X.; Yu, J.; Jaroniec, M.; Chen, X. Cocatalysts for selective photoreduction of CO2 into solar fuels. Chem. Rev. 2019, 119, 3962–4179. [Google Scholar] [CrossRef] [PubMed]
- Artz, J.; Müller, T.E.; Thenert, K.; Kleinekorte, J.; Meys, R.; Sternberg, A.; Bardow, A.; Leitner, W. Sustainable conversion of carbon dioxide: An integrated review of catalysis and life cycle assessment. Chem. Rev. 2018, 118, 434–504. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, R.R.; Pornpraprom, S.; D’Elia, V. Catalytic strategies for the cycloaddition of pure, diluted, and waste CO2 to epoxides under ambient conditions. ACS Catal. 2018, 8, 419–450. [Google Scholar] [CrossRef]
- Xu, W.; Chen, H.; Jie, K.; Yang, Z.; Li, T.; Dai, S. Entropy-driven mechanochemical synthesis of polymetallic zeolitic imidazolate frameworks for CO2 fixation. Angew. Chem. Int. Ed. 2019, 58, 5018–5022. [Google Scholar] [CrossRef]
- Yan, X.M.; Ding, X.; Pan, Y.; Xu, X.W.; Hao, C.; Zheng, W.J.; He, G.H. Quaternary-ammonium-immobilized polystyrenes as efficient and reusable heterogeneous catalysts for synthesis of cyclic carbonate: Effects of linking chains and pendent hydroxyl group. Chin. J. Catal. 2017, 38, 862–871. [Google Scholar] [CrossRef]
- Peng, J.; Wang, S.; Yang, H.J.; Ban, B.; Wei, Z.; Wang, L.; Lei, B. Highly efficient fixation of carbon dioxide to cyclic carbonates with new multi-hydroxyl bis-(quaternary ammonium) ionic liquids as metal-free catalysts under mild conditions. Fuel 2018, 224, 481–488. [Google Scholar] [CrossRef]
- Ziaee, M.A.; Tang, Y.; Zhong, H.; Tian, D.; Wang, R. Urea-functionalized imidazolium-based ionic polymer for chemical conversion of CO2 into organic carbonates. ACS Sustain. Chem. Eng. 2019, 7, 2380–2387. [Google Scholar] [CrossRef]
- Buaki-Sogó, M.; Vivian, A.; Bivona, L.A.; García, H.; Gruttadauria, M.; Aprile, C. Imidazolium functionalized carbon nanotubes for the synthesis of cyclic carbonates: Reducing the gap between homogeneous and heterogeneous catalysis. Catal. Sci. Technol. 2016, 6, 8418–8427. [Google Scholar] [CrossRef]
- Xie, Y.; Liang, J.; Fu, Y.; Lin, J.; Wang, H.; Tu, S.; Li, J. Poly (ionic liquid) s with high density of nucleophile/electrophile for CO2 fixation to cyclic carbonates at mild conditions. J. CO2 Utiliz. 2019, 32, 281–289. [Google Scholar] [CrossRef]
- Toda, Y.; Komiyama, Y.; Esaki, H.; Fukushima, K.; Suga, H. Methoxy groups increase reactivity of bifunctional tetraarylphosphonium salt catalysts for carbon dioxide fixation: A mechanistic study. J. Org. Chem. 2019, 84, 15578–15589. [Google Scholar] [CrossRef] [PubMed]
- Macarie, L.; Simulescu, V.; Ilia, G. Phosphonium-Based Ionic Liquids Used as Reagents or Catalysts. ChemistrySelect 2019, 4, 9285–9299. [Google Scholar] [CrossRef]
- Steinbauer, J.; Longwitz, L.; Frank, M.; Epping, J.; Kragl, U.; Werner, T. Immobilized bifunctional phosphonium salts as recyclable organocatalysts in the cycloaddition of CO2 and epoxides. Green Chem. 2017, 19, 4435–4445. [Google Scholar] [CrossRef]
- Tahir, N.; Krishnaraj, C.; Leus, K.; Van Der Voort, P. Development of covalent triazine frameworks as heterogeneous catalytic supports. Polymers 2019, 11, 1326. [Google Scholar] [CrossRef]
- Kramer, S.; Bennedsen, N.R.; Kegnæs, S. Porous organic polymers containing active metal centers as catalysts for synthetic organic chemistry. ACS Catal. 2018, 8, 6961–6982. [Google Scholar] [CrossRef]
- Bhanja, P.; Modak, A.; Bhaumik, A. Porous organic polymers for CO2 storage and conversion reactions. ChemCatChem 2019, 11, 244–257. [Google Scholar] [CrossRef]
- Liu, Z.W.; Han, B.H. Ionic porous organic polymers for CO2 capture and conversion. Curr. Opin. Green Sustain. Chem. 2019, 16, 20–25. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, K.; Wu, L.; Zhong, H.; Luo, N.; Zhu, Y.; Tong, M.; Long, Z.; Chen, G. Silanol-enriched viologen-based ionic porous hybrid polymers for efficient catalytic CO2 fixation into cyclic carbonates under mild conditions. ACS Sustain. Chem. Eng. 2019, 7, 16907–16916. [Google Scholar] [CrossRef]
- Liu, M.; Wang, X.; Jiang, Y.; Sun, J.; Arai, M. Hydrogen bond activation strategy for cyclic carbonates synthesis from epoxides and CO2: Current state-of-the art of catalyst development and reaction analysis. Catal. Rev. 2019, 61, 214–269. [Google Scholar] [CrossRef]
- Dai, Z.; Sun, Q.; Chen, F.; Pan, S.; Wang, L.; Meng, X.; Li, J.; Xiao, F.S. Enhancement of catalytic activity in epoxide hydration by increasing the concentration of cobalt (III)/salen in porous polymer catalysts. ChemCatChem 2016, 8, 812–817. [Google Scholar] [CrossRef]
- Chen, Y.; Luo, R.; Xu, Q.; Jiang, J.; Zhou, X.; Ji, H. Charged metalloporphyrin polymers for cooperative synthesis of cyclic carbonates from CO2 under ambient conditions. ChemSusChem 2017, 10, 2534–2541. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhong, M.; Tao, L.; Liu, L.; Jayakumar, S.; Li, C.; Li, H.; Yang, Q. The cooperation of porphyrin-based porous polymer and thermal-responsive ionic liquid for efficient CO2 cycloaddition reaction. Green Chem. 2018, 20, 903–911. [Google Scholar] [CrossRef]
- Li, J.; Han, Y.; Lin, H.; Wu, N.; Li, Q.; Jiang, J.; Zhu, J. Cobalt-salen based porous ionic polymer: The role of valence for cooperative conversion of CO2 to cyclic carbonate. ACS Appl. Mater. Interfaces 2020, 12, 609–618. [Google Scholar] [CrossRef]
- Li, J.; Han, Y.; Ji, T.; Wu, N.; Lin, H.; Jiang, J.; Zhu, J. Porous metallosalen hypercrosslinked ionic polymers for cooperative CO2 cycloaddition conversion. Ind. Eng. Chem. Res. 2020, 59, 676–684. [Google Scholar] [CrossRef]
- Xu, D.; Guo, J.; Yan, F. Porous ionic polymers: Design, synthesis, and applications. Prog. Polym. Sci. 2018, 79, 121–143. [Google Scholar] [CrossRef]
- Sun, J.-K.; Antonietti, M.; Yuan, J. Nanoporous ionic organic networks: From synthesis to materials applications. Chem. Soc. Rev. 2016, 45, 6627–6656. [Google Scholar] [CrossRef]
- Chen, Y.; Luo, R.; Bao, J.; Xu, Q.; Jiang, J.; Zhou, X.; Ji, H. Function-oriented ionic polymers having high-density active sites for sustainable carbon dioxide conversion. J. Mater. Chem. 2018, 6, 9172–9182. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, G.; Wu, L.; Liu, K.; Zhong, H.; Long, Z.; Tong, M.; Yang, Z.; Dai, S. Two-in-one: Construction of hydroxyl and imidazolium-bifunctionalized ionic networks in one-pot toward synergistic catalytic CO2 fixation. Chem. Commun. 2020. [Google Scholar] [CrossRef]
- Li, J.; Jia, D.; Guo, Z.; Liu, Y.; Lyu, Y.; Zhou, Y.; Wang, J. Imidazolinium based porous hypercrosslinked ionic polymers for efficient CO2 capture and fixation with epoxides. Green Chem. 2017, 19, 2675–2686. [Google Scholar] [CrossRef]
- Zhang, W.; Ma, F.; Ma, L.; Zhou, Y.; Wang, J. Imidazolium-functionalized ionic hypercrosslinked porous polymers for efficient synthesis of cyclic carbonates from simulated flue gas. ChemSusChem 2020, 13, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, C.; Yan, L.; Wang, Y.; Jiang, M.; Ding, Y. Ionic liquid/Zn-PPh3 integrated porous organic polymers featuring multifunctional sites: Highly active heterogeneous catalyst for cooperative conversion of CO2 to cyclic carbonates. ACS Catal. 2016, 6, 6091–6100. [Google Scholar] [CrossRef]
- Chen, G.; Zhang, Y.; Xu, J.; Liu, X.; Liu, K.; Tong, M.; Long, Z. Imidazolium-based ionic porous hybrid polymers with POSS-derived silanols for efficient heterogeneous catalytic CO2 conversion under mild conditions. Chem. Eng. J. 2020, 381, 122765. [Google Scholar] [CrossRef]
- Guo, Z.; Jiang, Q.; Shi, Y.; Li, J.; Yang, X.; Hou, W.; Zhou, Y.; Wang, J. Tethering dual hydroxyls into mesoporous poly (ionic liquid) s for chemical fixation of CO2 at ambient conditions: A combined experimental and theoretical study. ACS Catal. 2017, 7, 6770–6780. [Google Scholar] [CrossRef]
- Anthofer, M.H.; Wilhelm, M.E.; Cokoja, M.; Drees, M.; Herrmann, W.A.; Kühn, F.E. Hydroxy-functionalized imidazolium bromides as catalysts for the cycloaddition of CO2 and epoxides to cyclic carbonates. ChemCatChem 2015, 7, 94–98. [Google Scholar] [CrossRef]
- Wang, X.; Li, J.; Chen, G.; Guo, Z.; Zhou, Y.; Wang, J. Hydrophobic mesoporous poly(ionic liquid)s towards highly efficient and contamination-resistant solid-base catalysts. ChemCatChem 2015, 7, 993–1003. [Google Scholar] [CrossRef]
- Bernard, F.L.; Duczinski, R.B.; Rojas, M.F.; Fialho, M.C.C.; Carreno, L.A.; Chaban, V.; Vecchia, F.D.; Einloft, S. Cellulose based poly (ionic liquids): Tuning cation-anion interaction to improve carbon dioxide sorption. Fuel 2018, 211, 76–86. [Google Scholar] [CrossRef]
- Dai, W.L.; Chen, L.; Yin, S.F.; Luo, S.L.; Au, C.T. 3-(2-Hydroxyl-ethyl)-1-propylimidazolium bromide immobilized on SBA-15 as efficient catalyst for the synthesis of cyclic carbonates via the coupling of carbon dioxide with epoxides. Catal. Lett. 2010, 135, 295–304. [Google Scholar] [CrossRef]
- Dai, W.-L.; Jin, B.; Luo, S.-L.; Luo, X.-B.; Tu, X.-M.; Au, C.-T. Functionalized phosphonium-based ionic liquids as efficient catalysts for the synthesis of cyclic carbonate from epoxides and carbon dioxide. Appl. Catal. Gen. 2014, 470, 183–188. [Google Scholar]
- Dai, W.; Zhang, Y.; Tan, Y.; Luo, X.; Tu, X. Reusable and efficient polymer nanoparticles grafted with hydroxyl-functionalized phosphonium-based ionic liquid catalyst for cycloaddition of CO2 with epoxides. Appl. Catal. Gen. 2016, 514, 43–50. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, S.; Li, S. Novel functional organic network containing quaternary phosphonium and tertiary phosphorus. Macromolecules 2012, 45, 2981–2988. [Google Scholar] [CrossRef]
- Wang, J.; Yang, J.G.W.; Yi, G.; Zhang, Y. Phosphonium salt incorporated hypercrosslinked porous polymers for CO 2 capture and conversion. Chem. Commun. 2015, 51, 15708–15711. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Jin, Y.; Aguila, B.; Meng, X.; Ma, S.; Xiao, F.S. Porous ionic polymers as a robust and efficient platform for capture and chemical fixation of atmospheric CO2. ChemSusChem 2017, 10, 1160–1165. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Tang, Y.; Cui, J.; Gong, Q.; Hu, C.; Wang, S.; Dong, K.; Meng, X.; Sun, Q.; Xiao, F.S. Location matters: Cooperativity of catalytic partners in porous organic polymers for enhanced CO2 transformation. Chem. Commun. 2019, 55, 9180–9183. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Song, F.; Ye, T.; Li, G.; Liu, D.; Lei, Y. Carbonylative Suzuki coupling and alkoxycarbonylation of aryl halides using palladium supported on phosphorus-doped porous organic polymer as an active and robust catalyst. Appl. Organometal. Chem. 2019, 33, e4714. [Google Scholar] [CrossRef]
- Chen, G.J.; Zhou, Y.; Zhao, P.P.; Long, Z.Y.; Wang, J. Mesostructured dihydroxy-functionalized guanidinium-based polyoxometalate with enhanced heterogeneous catalytic activity in epoxidation. ChemPlusChem 2013, 78, 561–569. [Google Scholar] [CrossRef]
- Sun, Q.; Dai, Z.; Liu, X.; Sheng, N.; Deng, F.; Meng, X.; Xiao, F.S. Highly efficient heterogeneous hydroformylation over Rh-metalated porous organic polymers: Synergistic effect of high ligand concentration and flexible framework. J. Am. Chem. Soc. 2015, 137, 5204–5209. [Google Scholar] [CrossRef]
- Sun, Q.; Jiang, M.; Shen, Z.; Jin, Y.; Pan, S.; Wang, L.; Meng, X.; Chen, W.; Ding, Y.; Li, J.; et al. Porous organic ligands (POLs) for synthesizing highly efficient heterogeneous catalysts. Chem. Commun. 2014, 50, 11844–11847. [Google Scholar] [CrossRef]
- Wang, D.H.; Sihn, S.; Roy, A.K.; Baek, J.B.; Tan, L.S. Nanocomposites based on vapor-grown carbon nanofibers and an epoxy: Functionalization, preparation and characterization. Eur. Polym. J. 2010, 46, 1404–1416. [Google Scholar] [CrossRef]
- Cai, J.; Ma, H.; Zhang, J.; Du, Z.; Huang, Y.; Gao, J.; Xu, J. Catalytic oxidation of glycerol to tartronic acid over Au/HY catalyst under mild conditions. Chin. J. Catal. 2014, 35, 1653–1660. [Google Scholar] [CrossRef]
- Lei, Y.; Chen, Z.; Lan, G.; Wang, R.; Zhou, X.Y. Pd nanoparticles stabilized by phosphine-functionalized porous ionic polymer for efficient catalytic hydrogenation of nitroarenes in water. New J. Chem. 2020. [Google Scholar] [CrossRef]
- Liu, M.; Li, X.; Liang, L.; Sun, J. Protonated triethanolamine as multi-hydrogen bond donors catalyst for efficient cycloaddition of CO2 to epoxides under mild and cocatalyst-free conditions. J. CO2 Util. 2016, 16, 384–390. [Google Scholar] [CrossRef]
- Foltran, S.; Mereau, R.; Tassaing, T. Theoretical study on the chemical fixation of carbon dioxide with propylene oxide catalyzed by ammonium and guanidinium salts. Catal. Sci. Technol. 2014, 4, 1585–1597. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, D.J.J. Density functional theory study on the cycloaddition of carbon dioxide with propylene oxide catalyzed by alkylmethylimidazolium chlorine ionic liquids. Phys. Chem. 2007, 111, 8036–8043. [Google Scholar] [CrossRef] [PubMed]
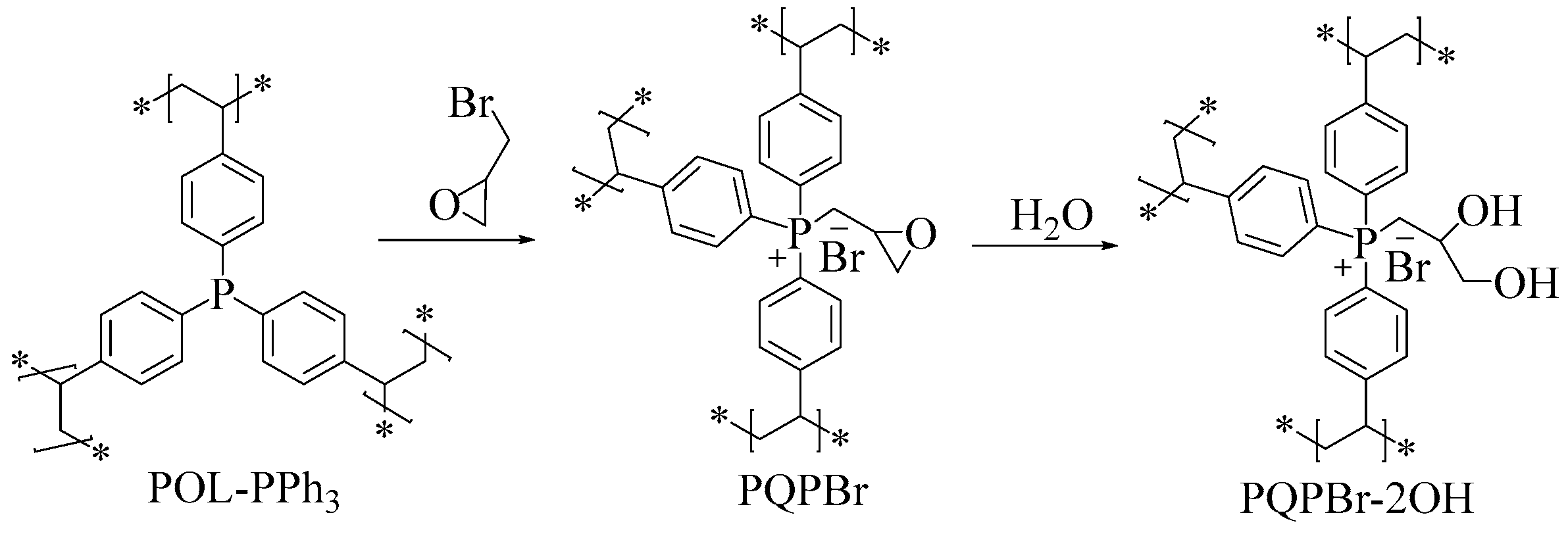
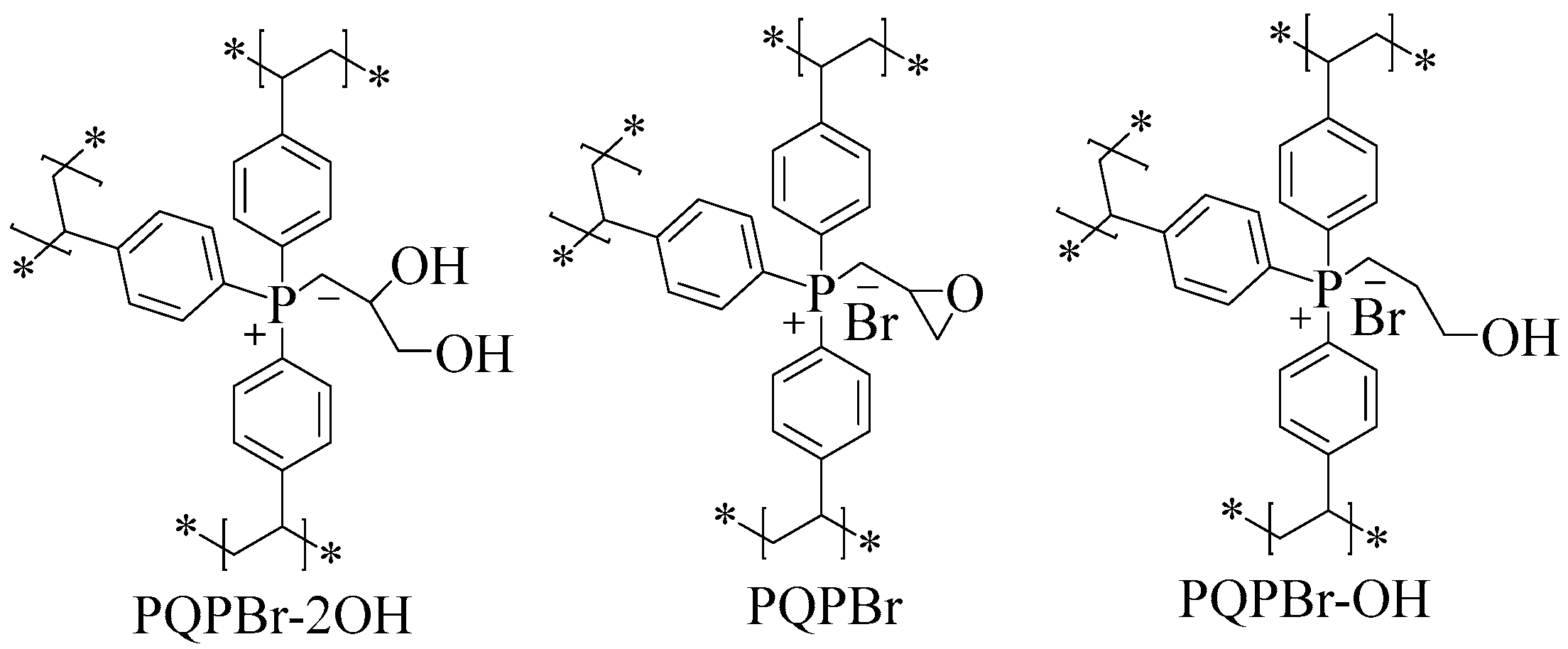
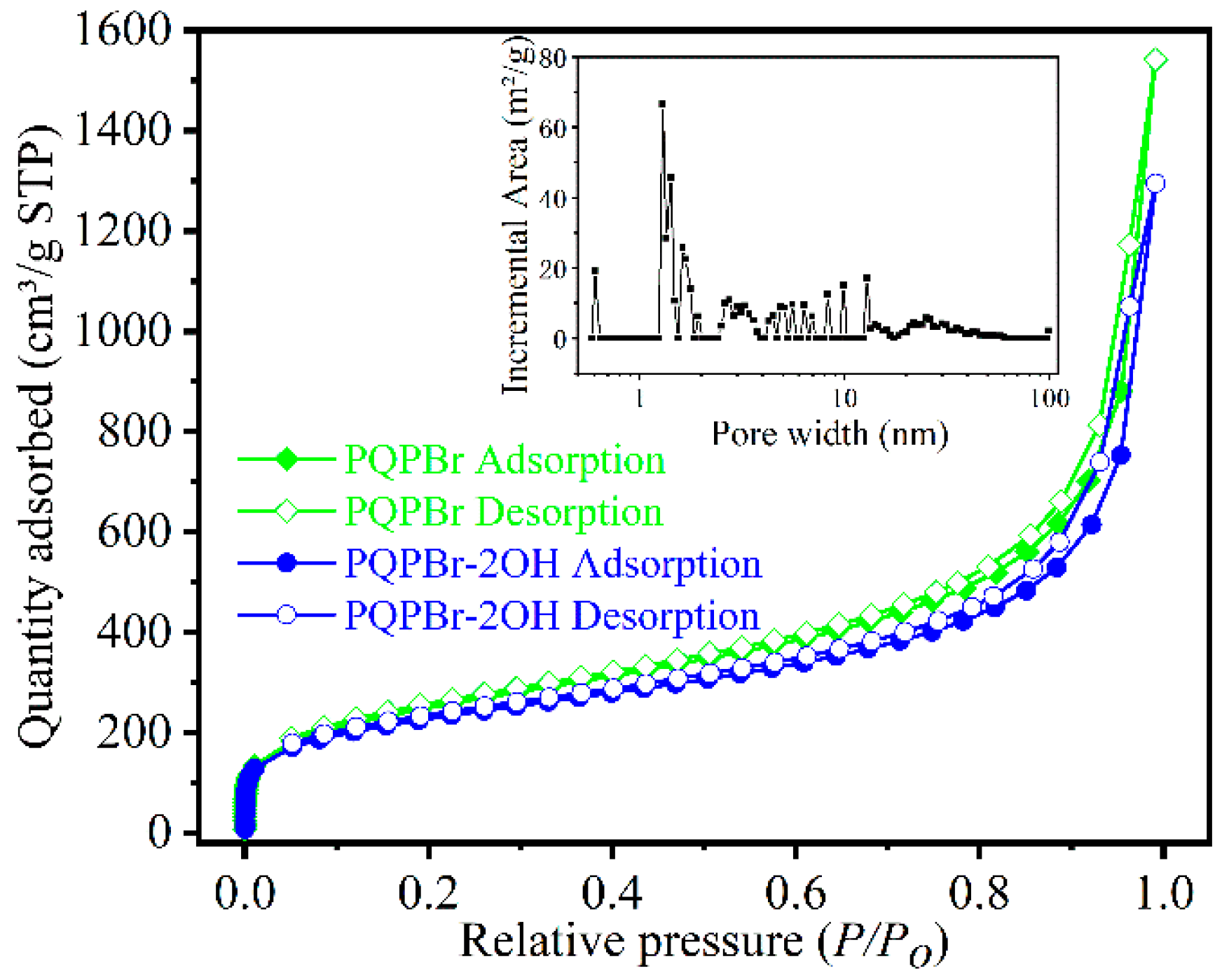
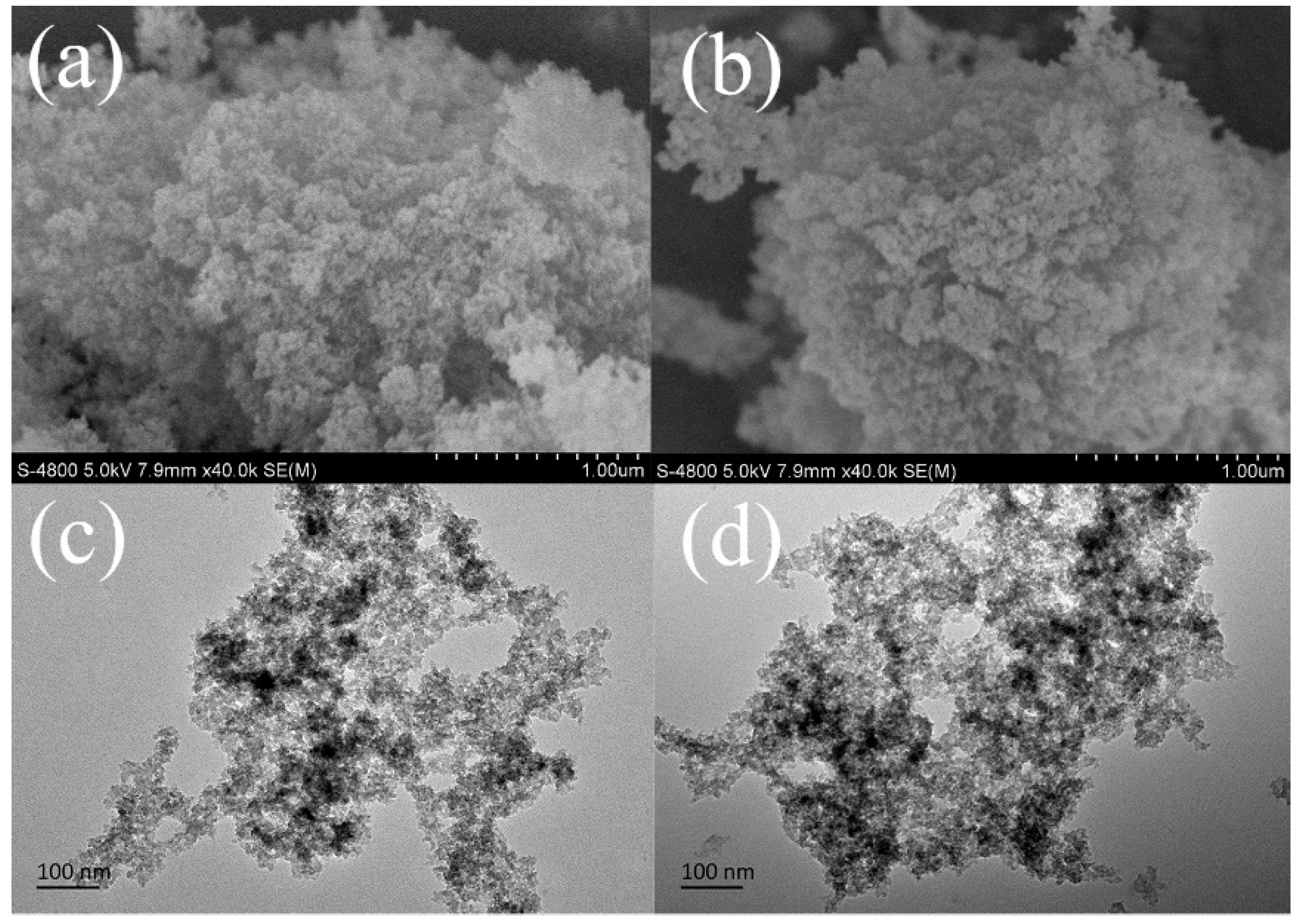

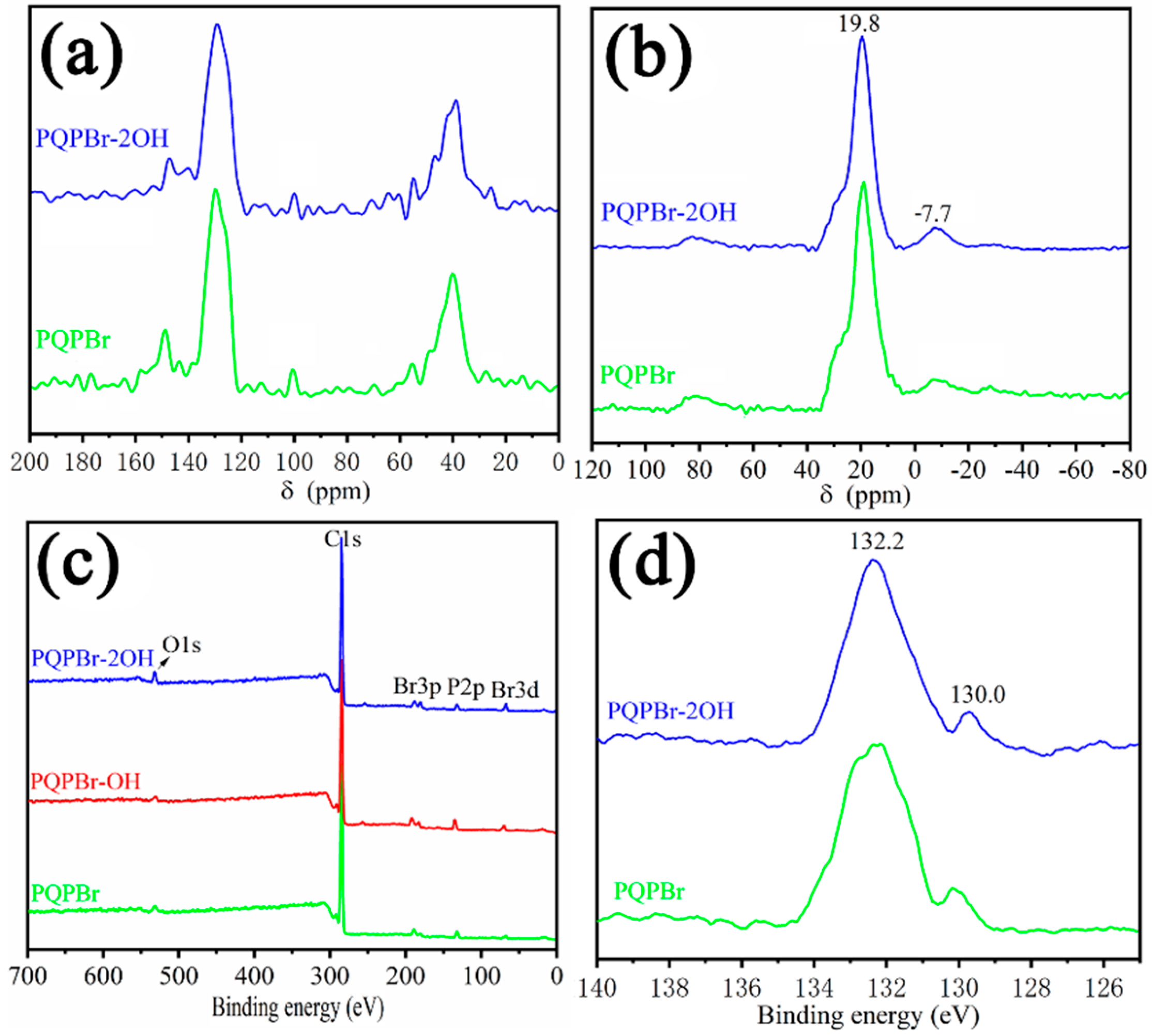
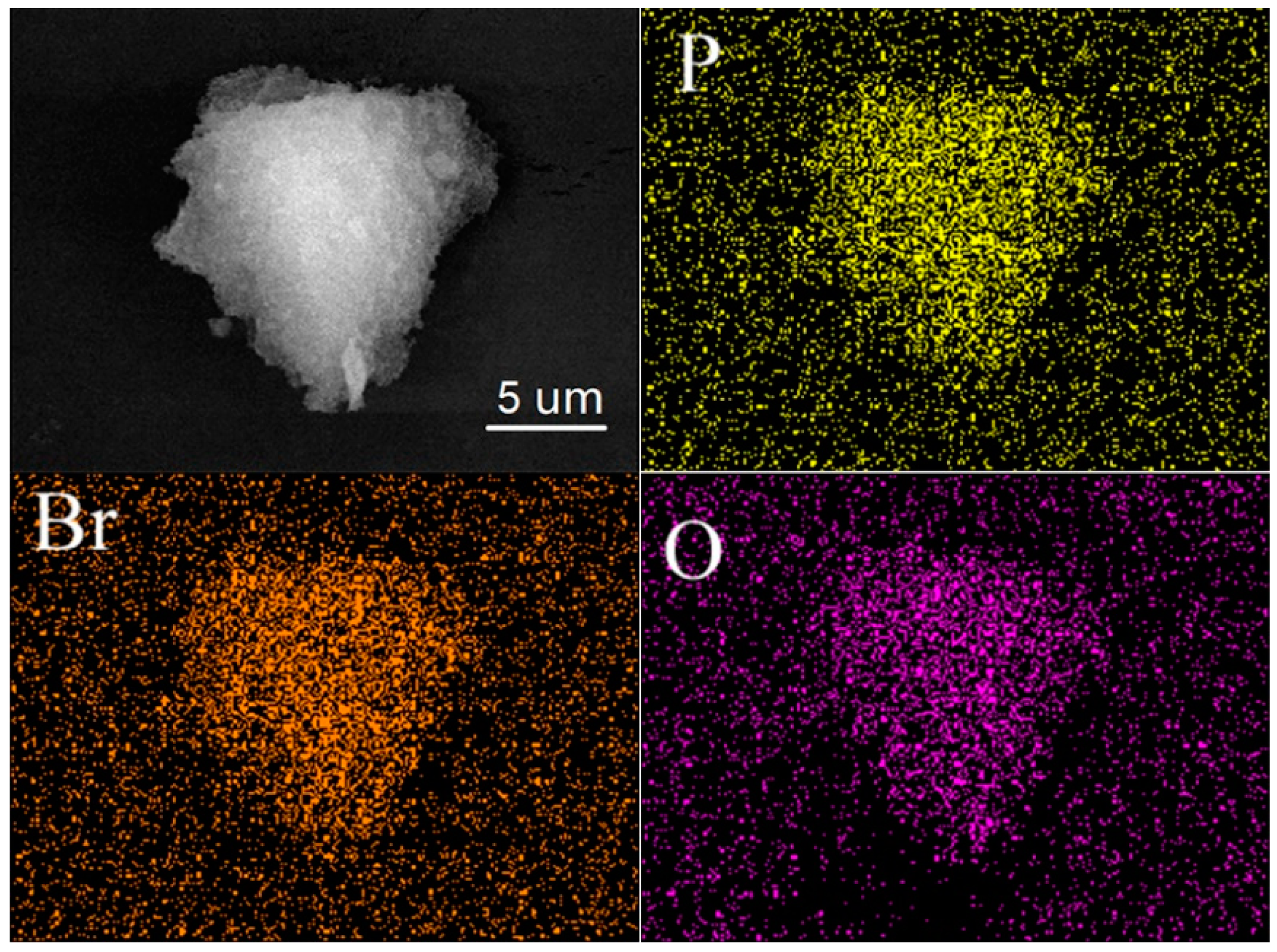
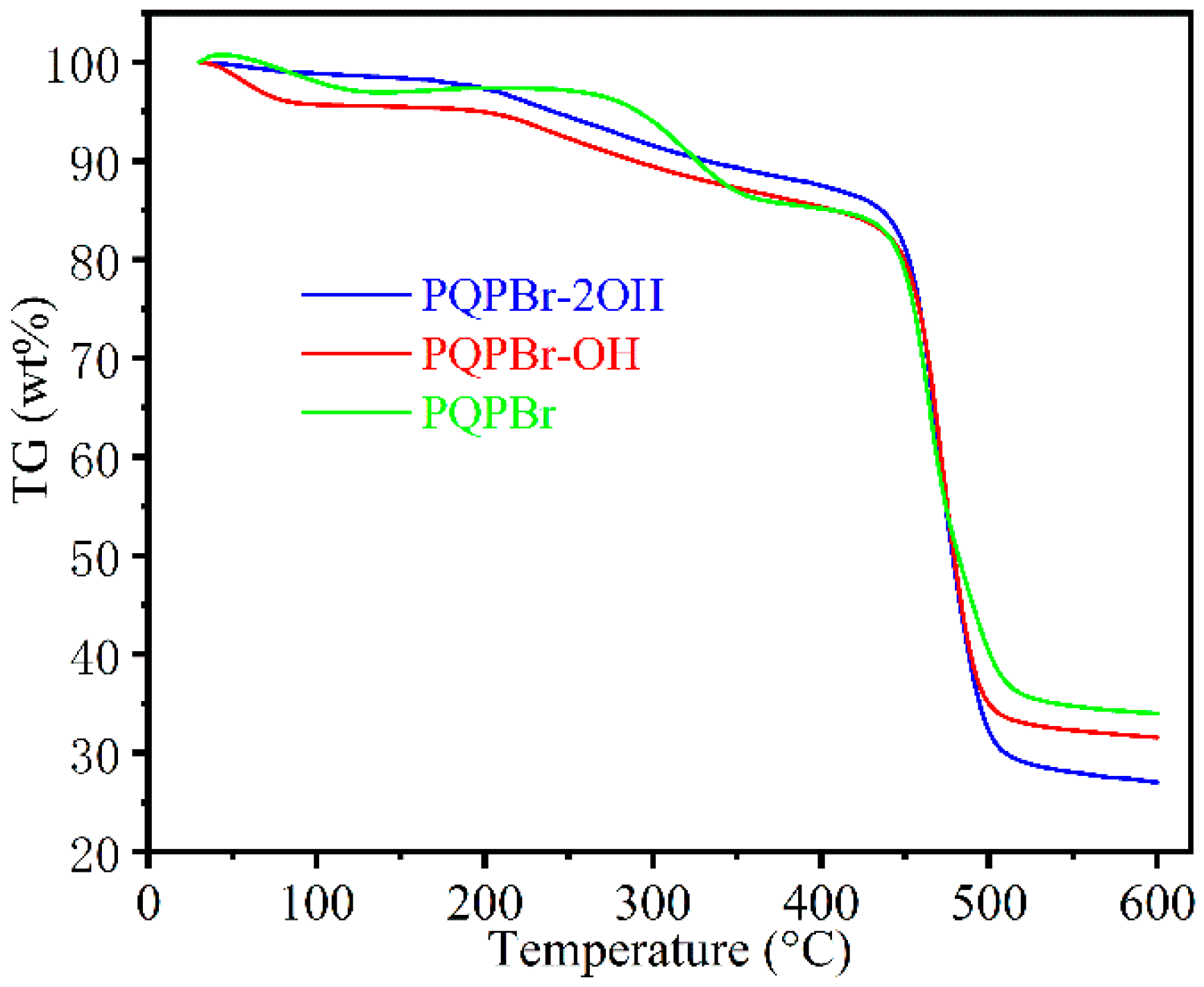

| Entry | Sample | SBET (m2 g−1) a | Vp (cm3 g−1) b | Dave (nm) c |
|---|---|---|---|---|
| 1 | POP-PPh3 | 1146 | 2.41 | 8.42 |
| 2 | PQPBr | 879 | 2.20 | 6.25 |
| 3 | PQPBr-OH | 848 | 1.57 | 3.39 |
| 4 | PQPBr-2OH | 794 | 1.90 | 6.26 |
| Sample | P (mmol/g) | Br (mmol/g) | O (mmol/g) |
|---|---|---|---|
| PQPBr | 2.06 | 1.78 | 2.2 |
| PQPBr-OH | 2.10 | 1.80 | 2.3 |
| PQPBr-2OH | 2.03 | 1.77 | 4.3 |
| Entry | Sample | T (°C) | Yield (mol %) | Selectivity (mol %) | TOF (h−1) |
|---|---|---|---|---|---|
| 1 | PQPBr | 100 | 41 | >99 | 20.5 |
| 2 | PQPBr-OH | 100 | 75 | >99 | 37.5 |
| 3 | PQPBr-2OH | 100 | 89 | >99 | 44.5 |
| 4 | POP-PPh3 | 100 | <3 | - | - |
| 5 | PQPBr-2OH | 60 | 7 | >99 | 3.5 |
| 6 | PQPBr-2OH | 80 | 36 | >99 | 18 |
| 7 | PQPBr-2OH | 120 | 99 | >99 | 49.5 |
| 8 | PQPBr-2OH | 140 | 98 | 98 | 49 |
| 9 b | PQPBr-2OH | 120 | 98 | >99 | 123 |
| Entry | Substrate | Product | Catalyst Loading (mol %) | Yield (mol %) | Selectivity (mol %) |
|---|---|---|---|---|---|
| 1 | 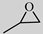 | 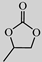 | 0.2 | 99 | >99 |
| 2 | 0.1 | 99 | >99 | ||
| 3 | 0.05 | 98 | >99 | ||
| 4 |  | 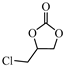 | 0.1 | 99 | >99 |
| 5 | 0.05 | 91 | >99 | ||
| 6 | 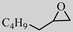 | 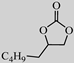 | 0.1 | 99 | >99 |
| 7 | 0.05 | 95 | >99 | ||
| 8 |  | 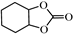 | 0.1 | 99 | >99 |
| 9 | 0.05 | 74 | >99 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lei, Y.; Wan, Y.; Zhong, W.; Liu, D.; Yang, Z. Phosphonium-Based Porous Ionic Polymer with Hydroxyl Groups: A Bifunctional and Robust Catalyst for Cycloaddition of CO2 into Cyclic Carbonates. Polymers 2020, 12, 596. https://doi.org/10.3390/polym12030596
Lei Y, Wan Y, Zhong W, Liu D, Yang Z. Phosphonium-Based Porous Ionic Polymer with Hydroxyl Groups: A Bifunctional and Robust Catalyst for Cycloaddition of CO2 into Cyclic Carbonates. Polymers. 2020; 12(3):596. https://doi.org/10.3390/polym12030596
Chicago/Turabian StyleLei, Yizhu, Yali Wan, Wei Zhong, Dingfu Liu, and Zhou Yang. 2020. "Phosphonium-Based Porous Ionic Polymer with Hydroxyl Groups: A Bifunctional and Robust Catalyst for Cycloaddition of CO2 into Cyclic Carbonates" Polymers 12, no. 3: 596. https://doi.org/10.3390/polym12030596
APA StyleLei, Y., Wan, Y., Zhong, W., Liu, D., & Yang, Z. (2020). Phosphonium-Based Porous Ionic Polymer with Hydroxyl Groups: A Bifunctional and Robust Catalyst for Cycloaddition of CO2 into Cyclic Carbonates. Polymers, 12(3), 596. https://doi.org/10.3390/polym12030596