Coupling Effect of Molecular Chain Displacement and Carrier Trap Characteristics on DC Breakdown of HDPE/LDPE Blend Insulation
Abstract
1. Introduction
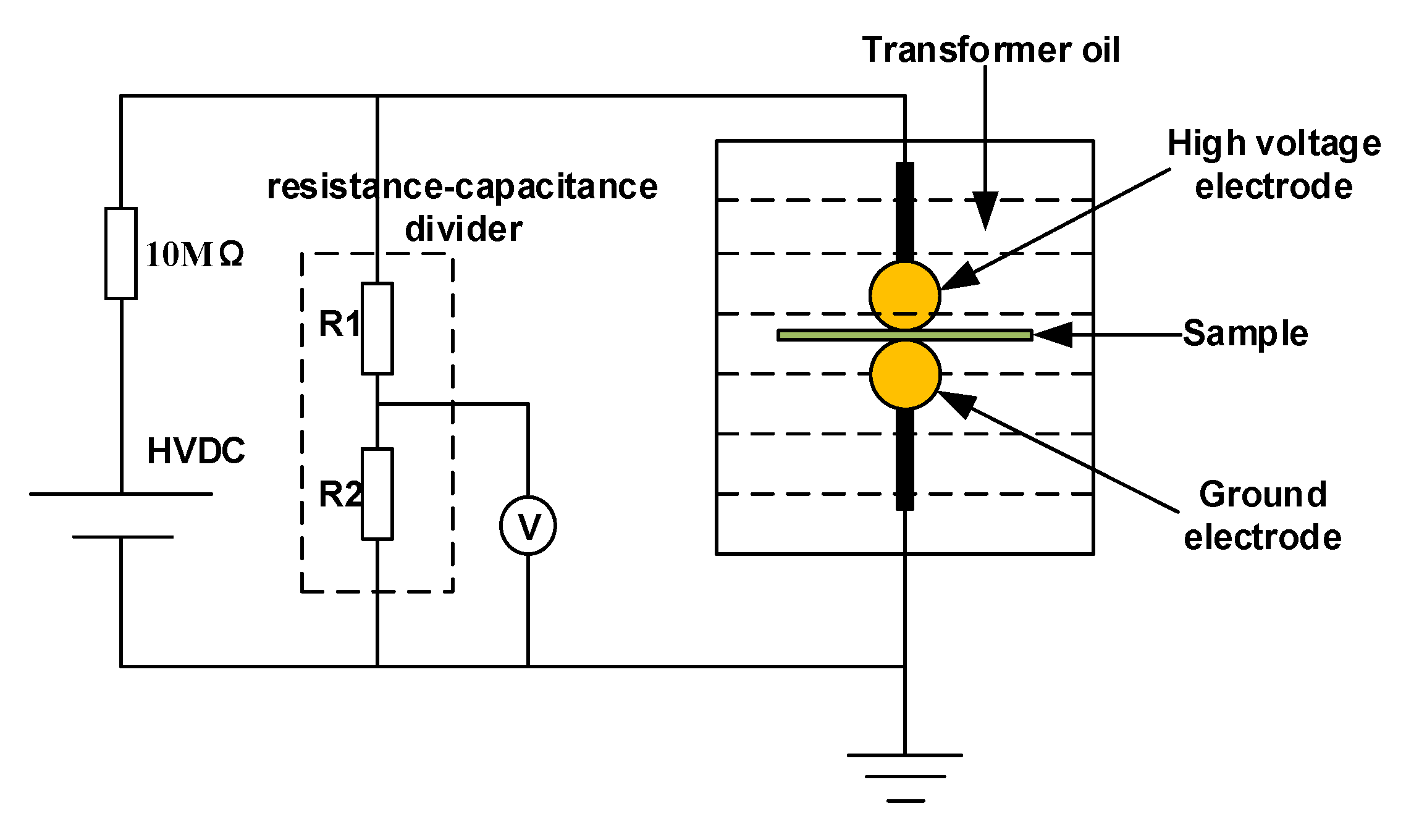
2. Experimental Arrangement
2.1. Process of Samples Preparation
2.2. Experimental Methods
2.3. Trap Distribution Characterization
3. Results and Analysis
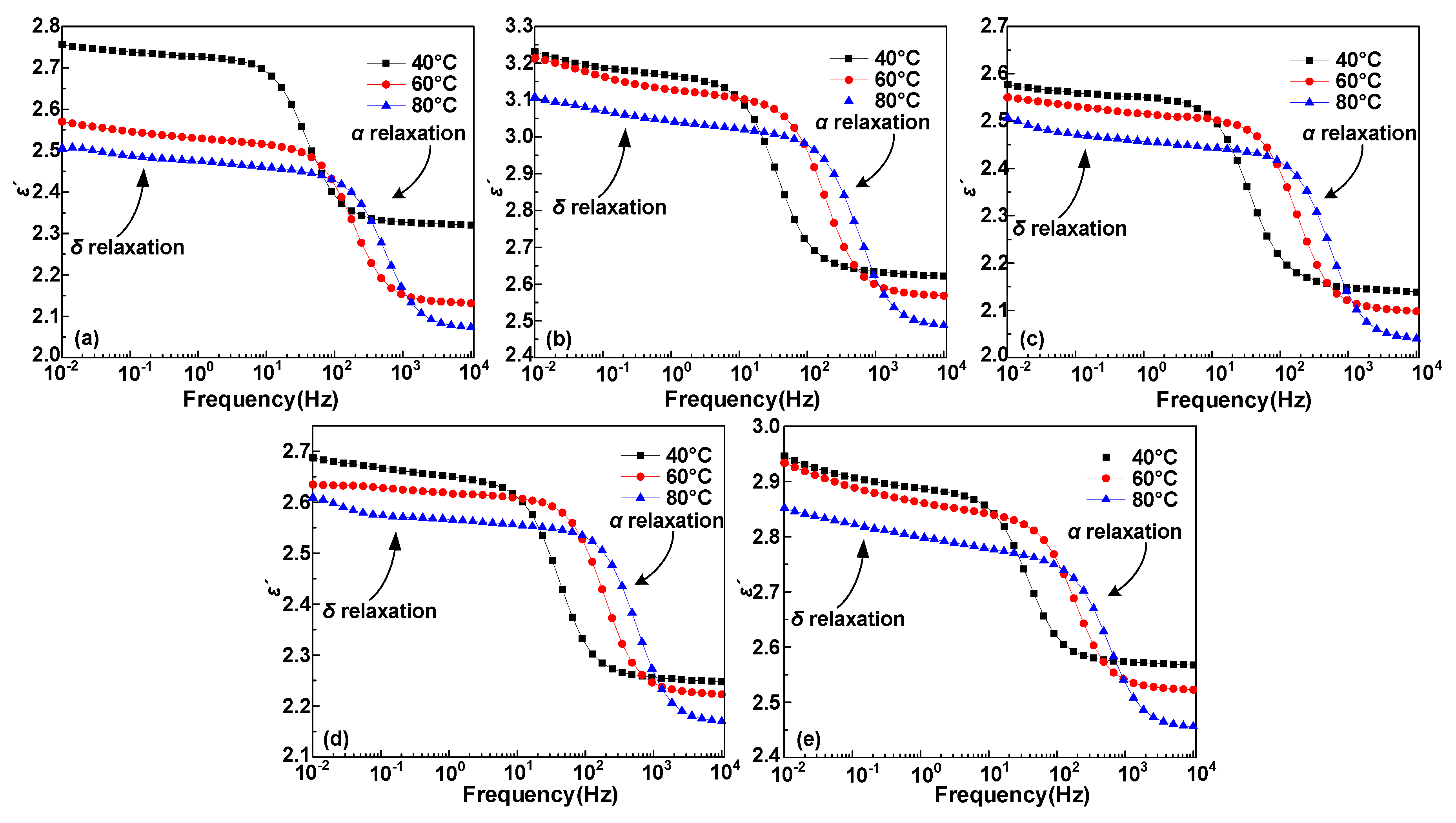
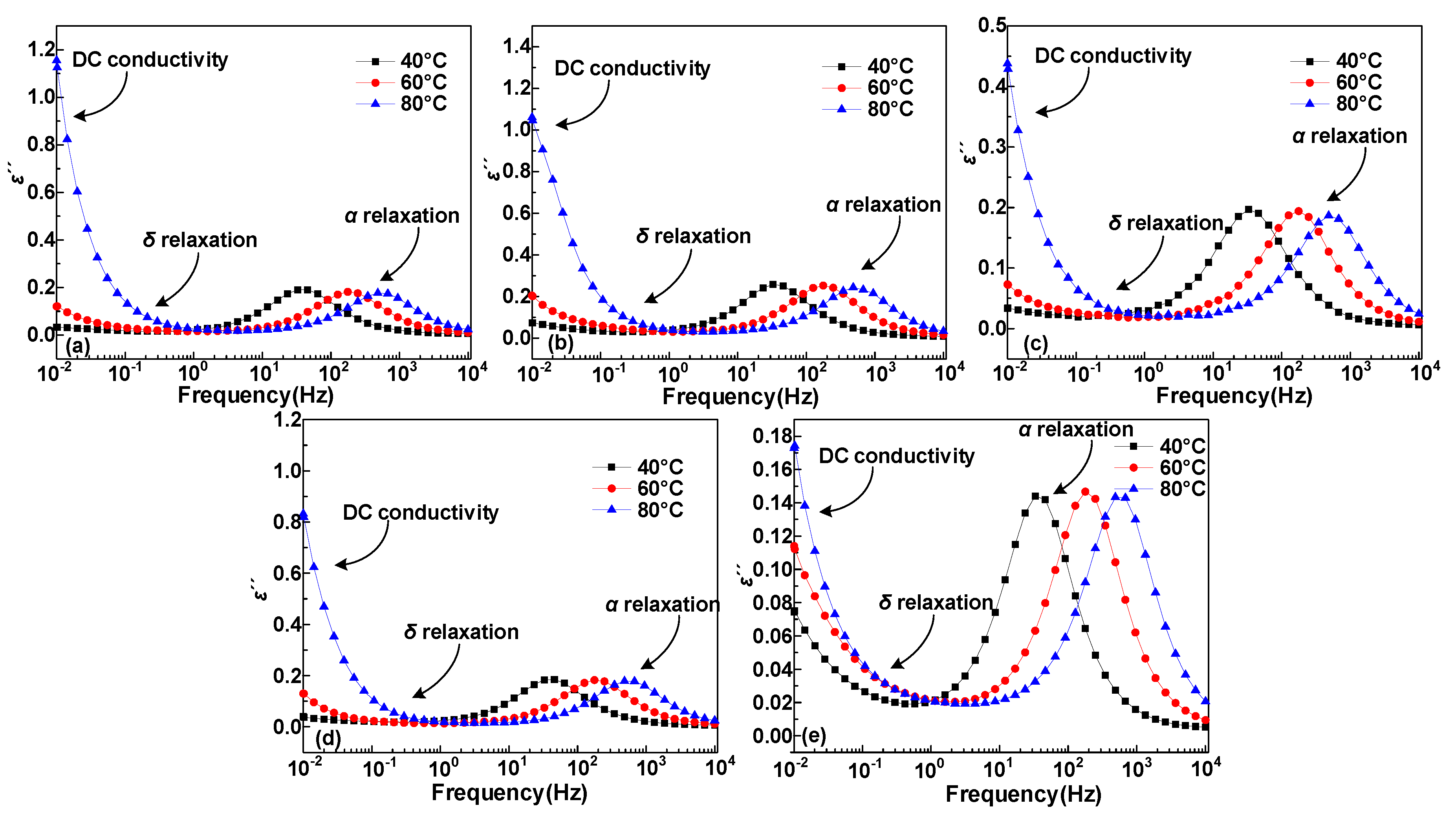
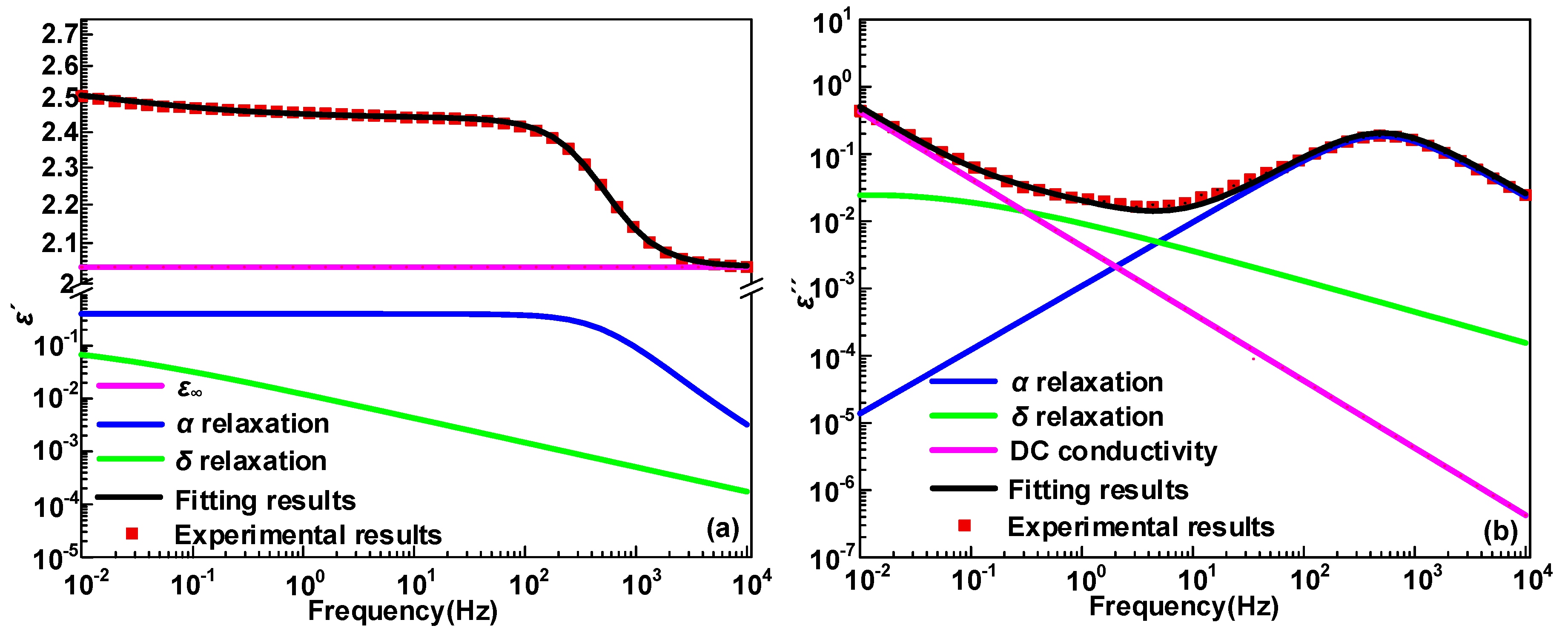
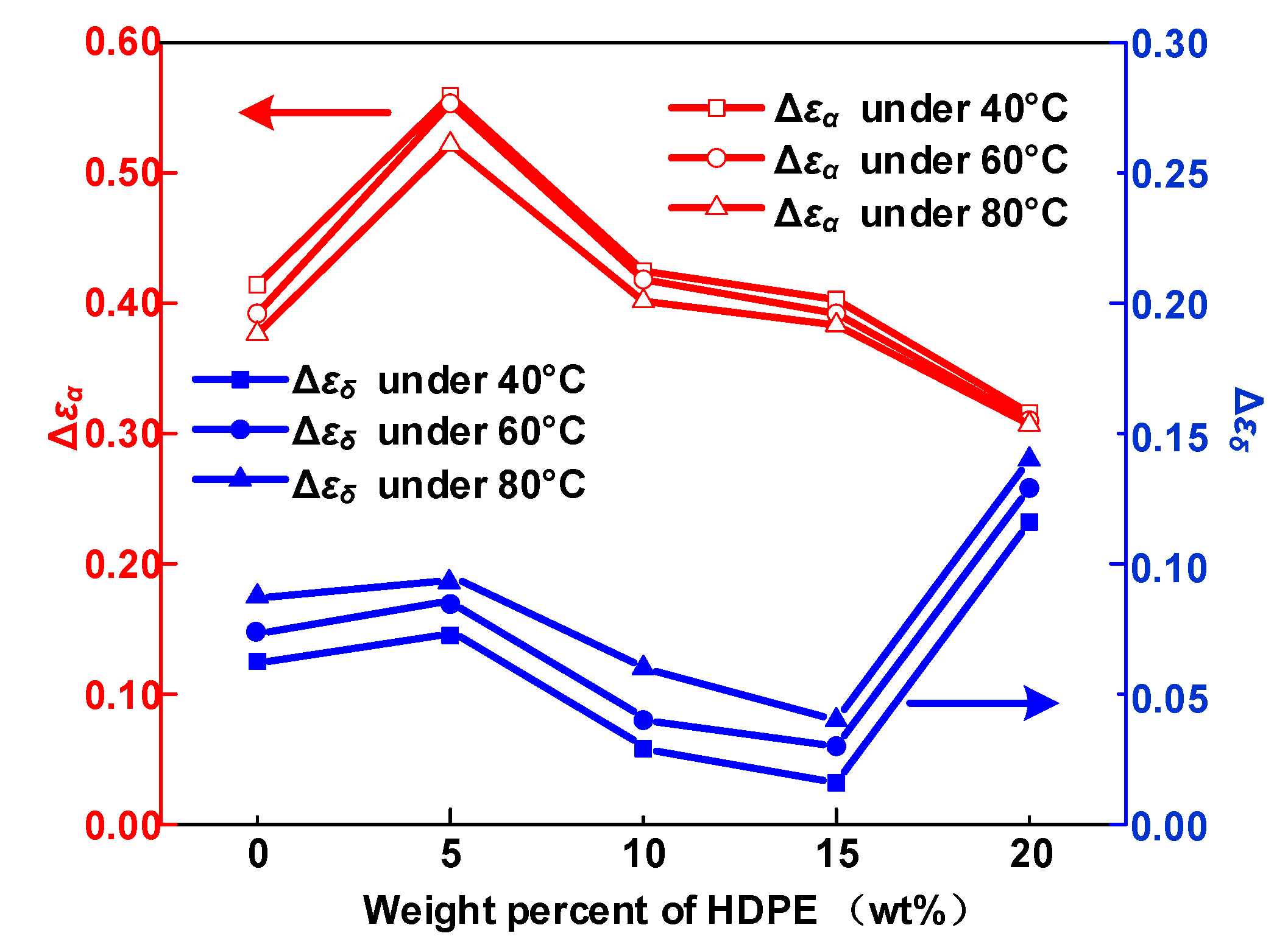
3.1. Dielectric Constant and Dielectric Loss
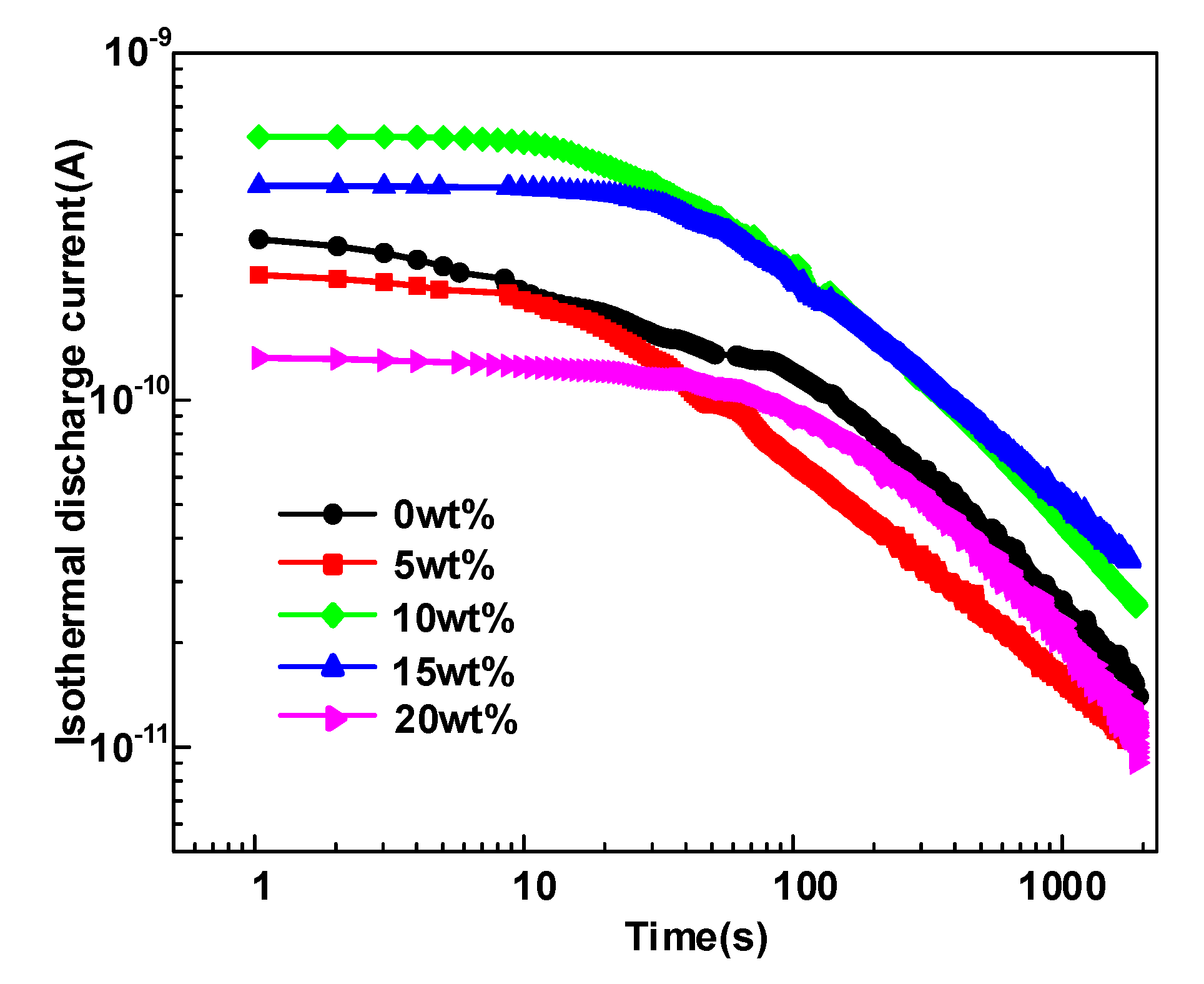
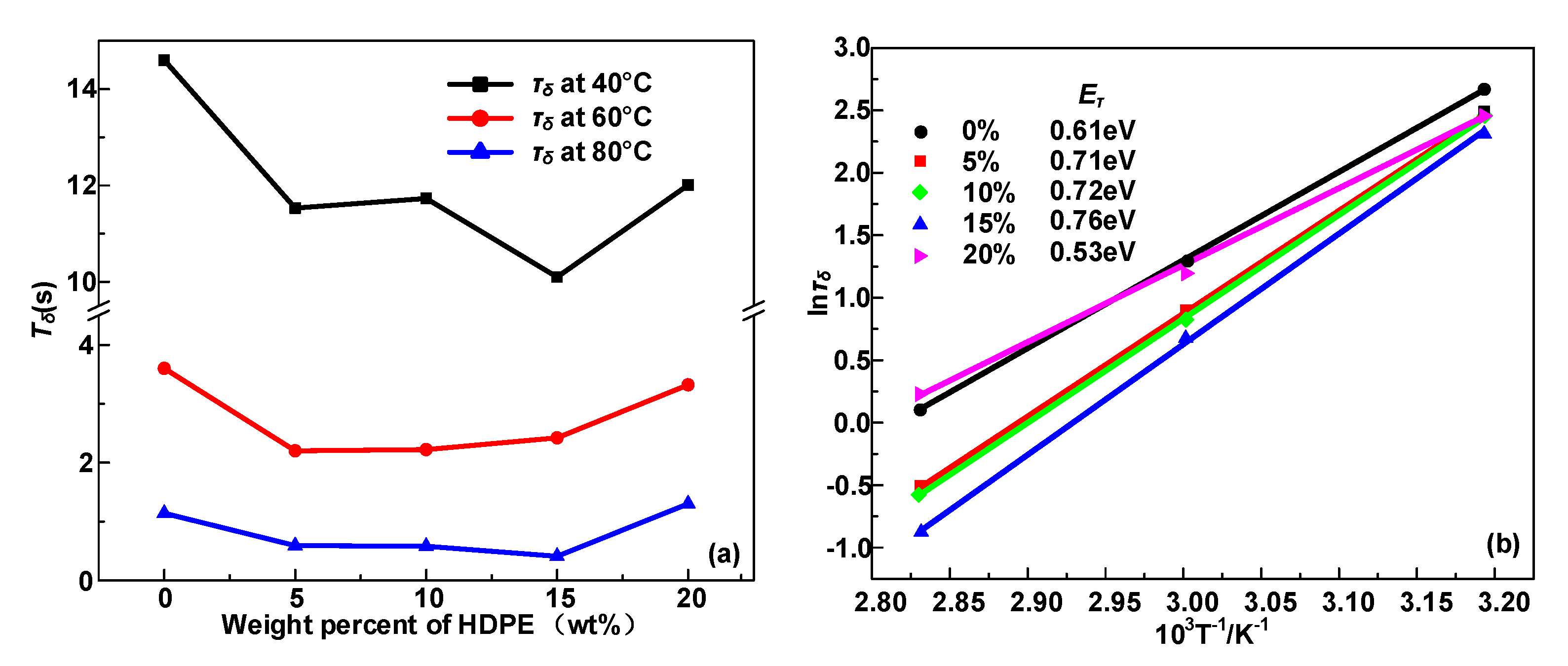
3.2. Trap Level Characteristics
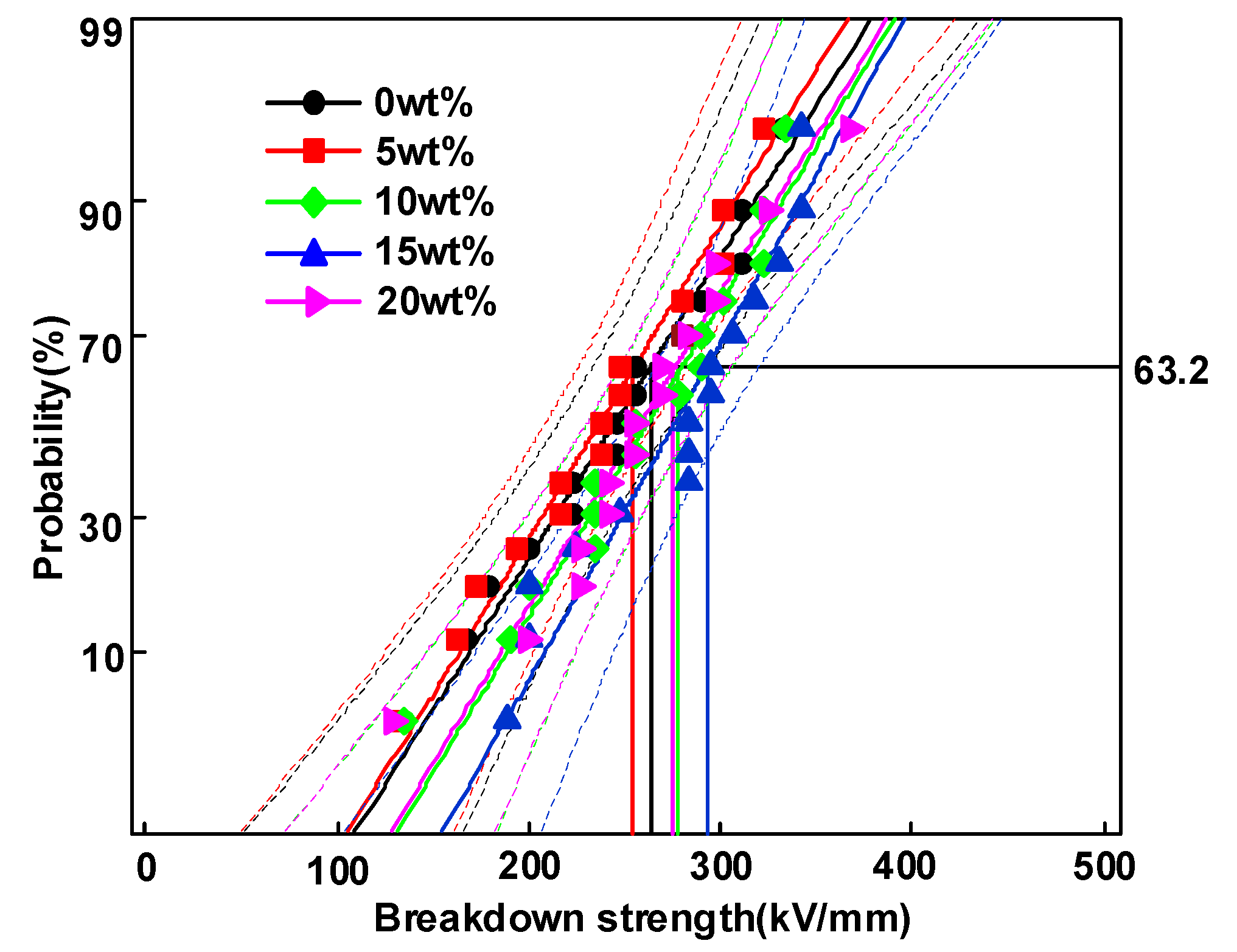
3.3. DC Breakdown Characteristics
4. Discussion
4.1. Dipole Motion Relaxation
4.2. Thermal Ion Polarization Behaviours
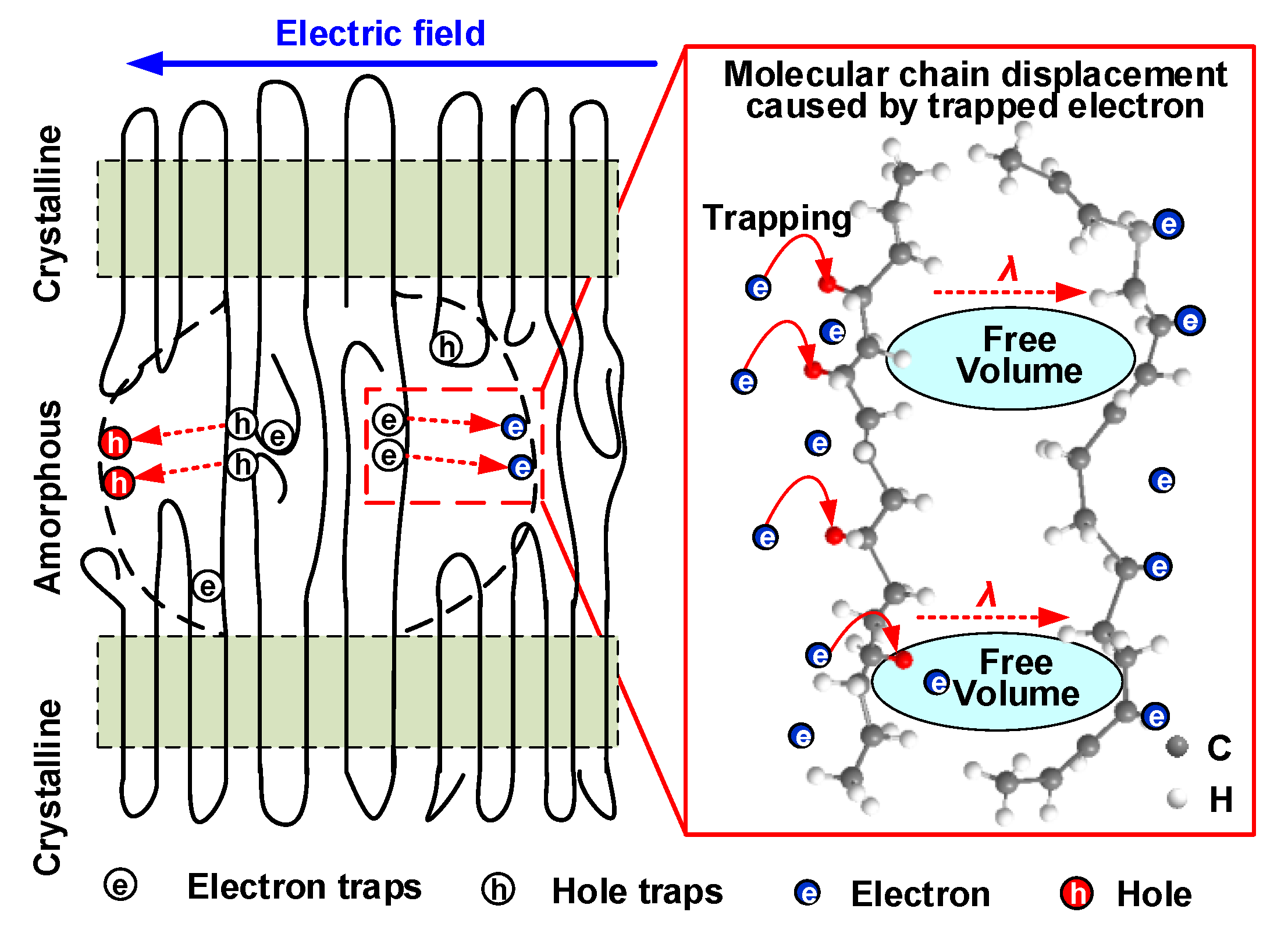
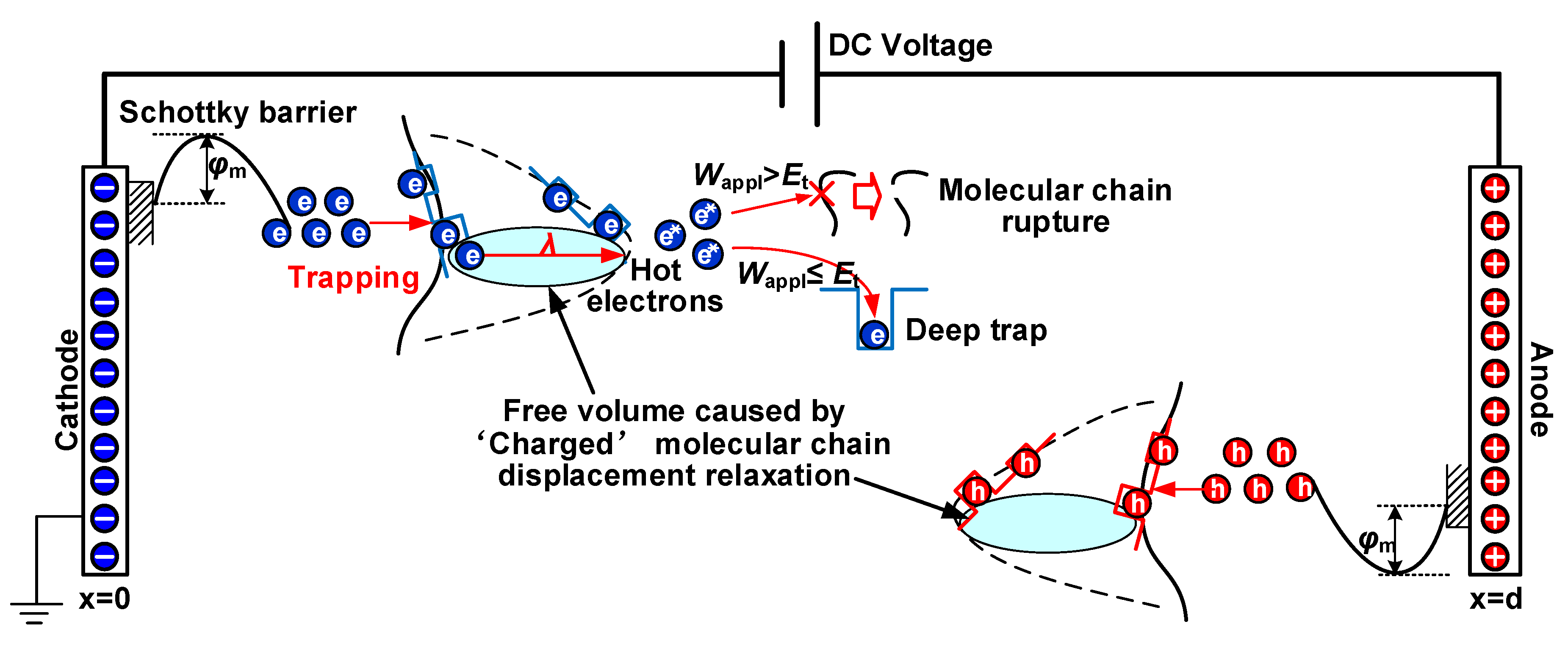
4.3. Electrical Breakdown Model of Coupling Molecular Chain Displacement and Carrier Trap Characteristics
5. Conclusions
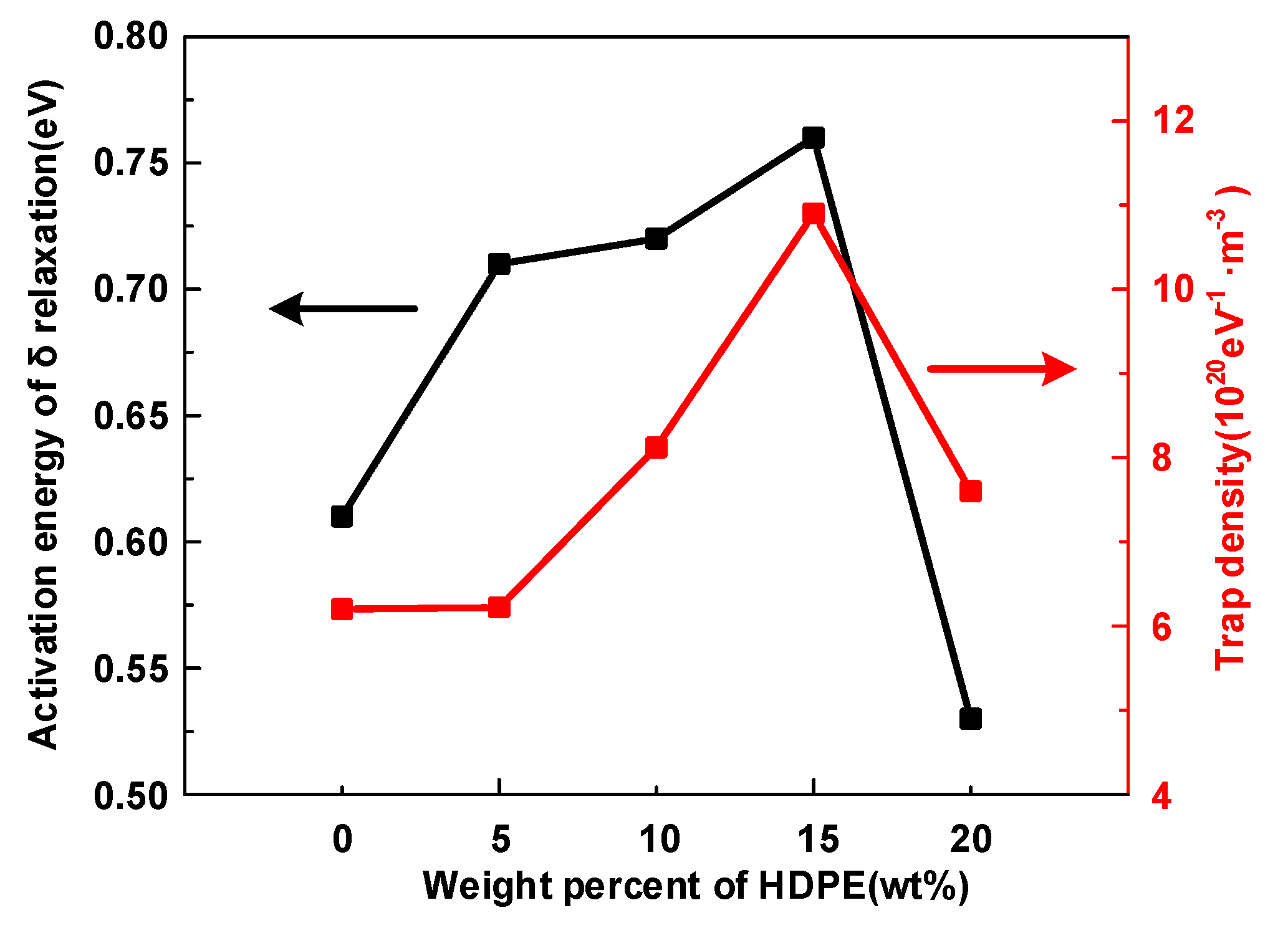
- The α and δ relaxation in HDPE/LDPE blends turn out to contain the molecular segments displacement and carrier’s hopping, respectively. Δεα declines with the increase of temperature, because of random thermal motion of dipoles caused by Brownian motion. Δεδ increases with the temperature, since higher temperatures provide more energy for the carriers to hop polarization. The activation energy of the dielectric relaxation process δ is positive related to the trap density obtained by IDC test.
- Δεα increases as the amount of HDPE increases from 0 to 5 wt%, then declines with a further increase of HDPE content to 20 wt%. The increase of Δεα will increase the displacement of the molecular chain inside the sample, resulting in a larger free volume, which will provide electrons with larger mean free path to obtain energy, and eventually continue to hit the molecular chain to cause breakdown.
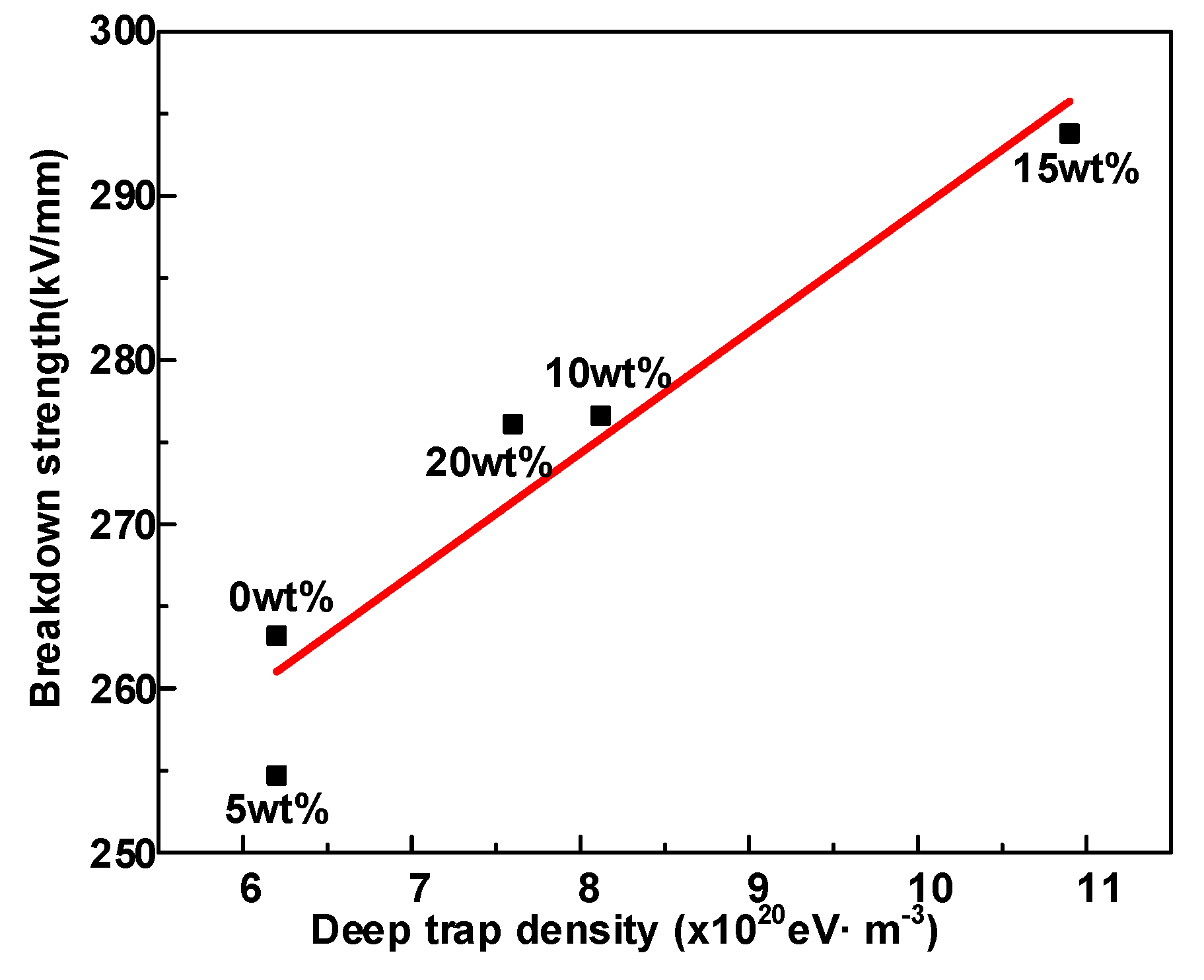
- Δεα and deep trap density Nt synergistically influence the average free path of the carrier and ultimately affect the breakdown performance of the dielectric. Moreover, the growth of deep traps density decreases the mean free path of electrons and prevents hot electrons from hitting the molecular chain directly, leading to an improvement in the DC breakdown field.
Author Contributions
Funding
Conflicts of Interest
References
- Montanari, G.C.; Laurent, C.; Teyssedre, G.; Campus, A.; Nilssone, U.H. From LDPE to XLPE: Investigating the change of electrical properties. Part, I. space charge, conduction and lifetime. IEEE Trans. Dielectr. Electr. Insul. 2005, 12, 438–446. [Google Scholar] [CrossRef]
- Teyssedre, G.; Laurent, C.; Montanari, G.C.; Campus, A.; Nilssone, U.H. From LDPE to XLPE: Investigating the change of electrical properties. Part II. Luminescence. IEEE Trans. Dielectr. Electr. Insul. 2005, 12, 447–454. [Google Scholar] [CrossRef]
- Li, Z.L.; Du, B.X. Polymeric insulation for high-voltage DC extruded cables: Challenges and development directions. IEEE Electr. Insul. Mag. 2018, 34, 30–43. [Google Scholar] [CrossRef]
- Du, B.X.; Li, Z.L.; Yang, Z.R. Progress in application and research of HVDC XLPE cable. High Volt. Eng. 2017, 43, 344–354. [Google Scholar]
- Tian, F.Q.; Lei, Q.Q.; Wang, X.; Wang, Y. Investigation of electrical properties of LDPE/ZnO nanocomposite dielectrics. IEEE Trans. Dielectr. Electr. Insul. 2012, 19, 763–769. [Google Scholar] [CrossRef]
- Zhou, C.R.; Chen, G. Space charge and AC electrical breakdown strength in polyethylene. IEEE Trans. Dielectr. Electr. Insul. 2017, 24, 559–566. [Google Scholar] [CrossRef]
- Huang, X.Y.; Zhang, J.; Jiang, P.K.; Tanaka, T. Material progress toward recyclable insulation of power cables. Part 1: Polyethylene-based thermoplastic materials: Dedicated to the 80th birthday of professor Toshikatsu Tanaka. IEEE Electr. Insul. Mag. 2019, 35, 7–19. [Google Scholar] [CrossRef]
- Chen, G.; Xu, Z. Charge trapping and detrapping in polymeric materials. J. Appl. Phys. 2009, 106, 123707. [Google Scholar] [CrossRef]
- Lin, Y.J.; Du, W.C.; Tu, D.M.; Zhong, W.; Du, Q.G. Space charge distribution and crystalline structure in low density polyethylene (LDPE) blended with high density polyethylene (HDPE). Polym. Int. 2005, 54, 465–470. [Google Scholar] [CrossRef]
- Andersson, M. Polyethylene Blends a Material Concept for Future HVDC-cable Insulation. Ph.D. Thesis, Chalmers University of Technology, Gothenburg, Sweden, 2017. [Google Scholar]
- Smith, R.C.; Liang, C.; Landry, M.; Nelson, J.K.; Schadler, L.S. The mechanisms leading to the useful electrical properties of polymer nanodielectrics. IEEE Trans. Dielectr. Electr. Insul. 2008, 15, 187–196. [Google Scholar] [CrossRef]
- Murakami, Y.; Nemoto, M.; Okuzumi, S.; Masuda, S.; Nagao, M.; Hozumi, N.; Murata, Y. DC conduction and electrical breakdown of MgO/LDPE nanocomposite. IEEE Trans. Dielectr. Electr. Insul. 2008, 15, 33–39. [Google Scholar] [CrossRef]
- Dang, B.; He, J.; Hu, J.; Zhou, Y. Large improvement in trap level and space charge distribution of polypropylene by enhancing the crystalline-amorphous interface effect in blends. Polym. Int. 2016, 65, 371–379. [Google Scholar] [CrossRef]
- Wang, W.; Min, D.; Li, S. Understanding the conduction and breakdown properties of polyethylene nanodielectrics: Effect of deep traps. IEEE Trans. Dielectr. Electr. Insul. 2016, 23, 564–572. [Google Scholar] [CrossRef]
- Jiang, X.W.; Sima, W.X.; Peng, Q.J.; Sun, P.T. Electrical Breakdown of Sandwiched Polymers- The Effect of Dielectric Properties and Trapped Electrons. IEEE Trans. Dielectr. Electr. Insul. 2019, 26, 1627–1635. [Google Scholar] [CrossRef]
- Liu, N.; Zhou, C.R.; Chen, G.X.Y.; Cao, J.Z.; Wang, H.T. Model to estimate the trapping parameters of cross-linked polyethylene cable peelings of different service years and their relationships with dc breakdown strengths. High 2016, 1, 95–105. [Google Scholar] [CrossRef]
- Schonhals, A.; Goering, H.; Costa, F.R.; Wagenknecht, U.; Heinrich, G. Dielectric properties of nanocomposites based on polyethylene and layered double hydroxide. Macromolecules 2009, 42, 4165–4174. [Google Scholar] [CrossRef]
- Carretero–González, J.; Ezquerra, T.A.; Amnuaypornsri, S.; Toki, S.; Verdejo, R.; Sanz, A.; Sakdapipanich, J.; Hsiao, B.S.; López–Manchado, M.A. Molecular dynamics of natural rubber as revealed by dielectric spectroscopy: The role of natural cross–linking. Soft Matter 2010, 6, 3636–3642. [Google Scholar] [CrossRef]
- Min, D.M.; Li, S.T.; Ohki, Y. Numerical simulation on molecular displacement and DC breakdown of LDPE. IEEE Trans. Dielectr. Electr. Insul. 2016, 23, 507–516. [Google Scholar] [CrossRef]
- Xie, D.R.; Min, D.M.; Yang, L.Q.; Li, S.T. Temperature- and thickness-dependent electrical breakdown modulated by a coupling model of charge transport and molecular chain dynamics. J. Phys. D Appl. Phys. 2019, 52, 365305. [Google Scholar] [CrossRef]
- Simmons, J.G.; Tam, M.C. Theory of Isothermal Currents and the Direct Determination of Trap Parameters in Semiconductors and Insulators Containing Arbitrary Trap Distributions. Phys. Rev. B 1973, 7, 3706–3713. [Google Scholar] [CrossRef]
- Min, D.M.; Cui, H.Z.; Wang, W.W.; Wu, Q.Z.; Xing, Z.L.; Li, S.T. The coupling effect of interfacial traps and molecular motion on the electrical breakdown in polyethylene nanocomposites. Compos. Sci. Technol. 2019, 184, 107873. [Google Scholar] [CrossRef]
- Huang, Y.; Min, D.; Li, S.; Wang, X.; Lin, S. Dielectric relaxation and carrier transport in epoxy resin and its microcomposite. IEEE Trans. Dielectr. Electr. Insul. 2017, 24, 3083–3091. [Google Scholar] [CrossRef]
- Kao, K.C. Dielectric Phenomena in Solids; Elsevier: San Diego, CA, USA, 2004. [Google Scholar]
- Sy, J.W.; Mijovic, J. Reorientational dynamics of poly (vinylidene fluoride)/poly (methyl methacrylate) blends by broad-band dielectric relaxation spectroscopy. Macromolecules 2000, 33, 933–946. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, W.; Jiang, X.; Liu, K.; Peng, G.; Zhan, Z. Exploring the relationship of dielectric relaxation behavior and discharge efficiency of P(VDF-HFP)/PMMA blends by dielectric spectroscopy. Mater. Res. Express 2016, 3, 075304. [Google Scholar] [CrossRef]
- Migahed, M.D.; Fahmy, T. Structural relaxation around the glass transition temperature in amorphous polymer blends: Temperature and composition dependence. Polymers 1994, 35, 1688–1693. [Google Scholar] [CrossRef]
- Ma, C.; Min, D.M.; Li, S.T.; Zheng, X.; Li, X.; Min, C.; Zhan, H. Trap distribution and direct current breakdown characteristics in polypropylene/Al2O3 nanodielectrics. Acta Phys. Sin. 2017, 66, 067701. [Google Scholar]
- Han, Y.S.; Li, S.T.; Min, D.M. Trap energy distribution in polymeric insulating materials through surface potential decay method. IEEE Trans. Dielectr. Electr. Insul. 2018, 25, 639–648. [Google Scholar] [CrossRef]
- Li, S. Linking traps to insulation failure. IEEE. Int. Conf. Appl. Dielectr. Mater. 2015, 1–14. [Google Scholar]
- Li, S.; Min, D.; Wang, W.; Chen, G.; Xia, R. Modelling of dielectric breakdown through charge dynamics for polymer nanocomposites. IEEE Trans. Dielectr. Electr. Insul. 2016, 23, 3476–3485. [Google Scholar] [CrossRef]
- Meunier, M.; Quirke, N.; Aslanides, A. Molecular modeling of electron traps in polymer insulators: Chemical defects and impurities. J. Chem. Phys. 2001, 115, 2876. [Google Scholar] [CrossRef]
- Teyssedre, G.; Laurent, C.; Aslanides, A.; Quirke, N.; Dissado, L.A.; Montanari, G.C.; Campus, A.; Martinotto, L. Deep trapping centers in crosslinked polyethylene investigated by molecular modeling and luminescence techniques IEEE. Trans. Dielectr. Electr. Insul. 2001, 8, 744–752. [Google Scholar] [CrossRef]
- Artbauer, J. Electric strength of polymers. J. Phys. D Appl. Phys. 1996, 29, 446. [Google Scholar] [CrossRef]
- Li, X.; Du, Q.; Kang, J.; Tu, D. Influence of microstructure on space charges of polypropylene. J. Polym. Sci. Part B 2002, 40, 365–374. [Google Scholar] [CrossRef]
- Khan, M.R.; Varade, V.; Koteswara Rao, K.S.R.; Menon, R. Injection barrier induced deviations in space charge limited conduction in doped poly(3-methylthiophene) based devices. J. Appl. Phys. 2015, 118, 164503. [Google Scholar] [CrossRef]
- Min, D.; Li, Y.; Yan, C.; Xie, D.; Li, S.; Wu, Q.; Xing, Z. Thickness-Dependent DC Electrical Breakdown of Polyimide Modulated by Charge Transport and Molecular Displacement. Polymers 2018, 10, 1012. [Google Scholar] [CrossRef] [PubMed]
- Kuik, M.; Koster, L.J.A.; Wetzelaer, G.A.H.; Blom, P.W.M. Trap-assisted recombination in disordered organic semiconductors. Phys. Rev. Lett. 2011, 25, 256805. [Google Scholar] [CrossRef] [PubMed]
- Simmons, J.G.; Taylor, G.W. Nonequilibrium steady-state statistics and associated effects for insulators and semiconductors containing an arbitrary distribution of traps. Phys. Rev. B Condens. Matter. 1971, 4, 502. [Google Scholar] [CrossRef]
- Teyssedre, G.; Laurent, C. Charge transport modeling in insulating polymers: From molecular to macroscopic scale. IEEE Trans. Dielectr. Electr. Insul. 2005, 12, 857–875. [Google Scholar] [CrossRef]
- Skinner, D.E.; Colombo, D.P.; Cavaleri, J.J.; Bowman, R.M. Femtosecond investigation of electron trapping in semiconductor nanoclusters. J. Phys. Chem. 1995, 99, 7853–7856. [Google Scholar] [CrossRef]
- Lowell, J. Absorption and conduction currents in polymers: A unified model. J. Phys. D Appl. Phys. 1990, 23, 205–210. [Google Scholar] [CrossRef]
- Toomer, R.; Lewis, T.J. Charge trapping in corona-charge polyethylene films. J. Phys. D Appl. Phys. 1980, 13, 1343–1356. [Google Scholar] [CrossRef]
- Kao, K.C. Electrical Conduction and breakdown in insulating polymers. Proc. IEEE. Int. Conf. Prop. Appl. Dielectr. Mater. 2000, 1, 1–17. [Google Scholar]
- Li, S.; Yin, G.; Bai, S.; Li, J. A new potential barrier model in epoxy resin nanodielectrics. IEEE Trans. Dielectr. Electr. Insul. 2011, 18, 1535–1543. [Google Scholar] [CrossRef]
- Li, Z.Z.; Zhong, Z.Y.; Du, B.X. Dielectric relaxation and trap-modulated DC breakdown of polypropylene blend insulation. Polymers 2019, 185, 121935. [Google Scholar] [CrossRef]


| Weight Percent of HDPE (wt%) | τα at 40 °C |
|---|---|
| 0 | 4.32 × 10−3 |
| 5 | 5.05 × 10−3 |
| 10 | 4.81 × 10−3 |
| 15 | 4.42 × 10−3 |
| 20 | 4.35 × 10−3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Fan, M.; Zhong, Z.; Du, B. Coupling Effect of Molecular Chain Displacement and Carrier Trap Characteristics on DC Breakdown of HDPE/LDPE Blend Insulation. Polymers 2020, 12, 589. https://doi.org/10.3390/polym12030589
Li Z, Fan M, Zhong Z, Du B. Coupling Effect of Molecular Chain Displacement and Carrier Trap Characteristics on DC Breakdown of HDPE/LDPE Blend Insulation. Polymers. 2020; 12(3):589. https://doi.org/10.3390/polym12030589
Chicago/Turabian StyleLi, Zhonglei, Mingsheng Fan, Zhuoyan Zhong, and Boxue Du. 2020. "Coupling Effect of Molecular Chain Displacement and Carrier Trap Characteristics on DC Breakdown of HDPE/LDPE Blend Insulation" Polymers 12, no. 3: 589. https://doi.org/10.3390/polym12030589
APA StyleLi, Z., Fan, M., Zhong, Z., & Du, B. (2020). Coupling Effect of Molecular Chain Displacement and Carrier Trap Characteristics on DC Breakdown of HDPE/LDPE Blend Insulation. Polymers, 12(3), 589. https://doi.org/10.3390/polym12030589






