Effect of Aromatic Petroleum Resin on Damping Properties of Polybutyl Methacrylate
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of P(BMA-co-St).
2.3. Preparation of P(BMA-co-St)/Petroleum Resin Blends
2.4. Characterization
3. Results and Discussion
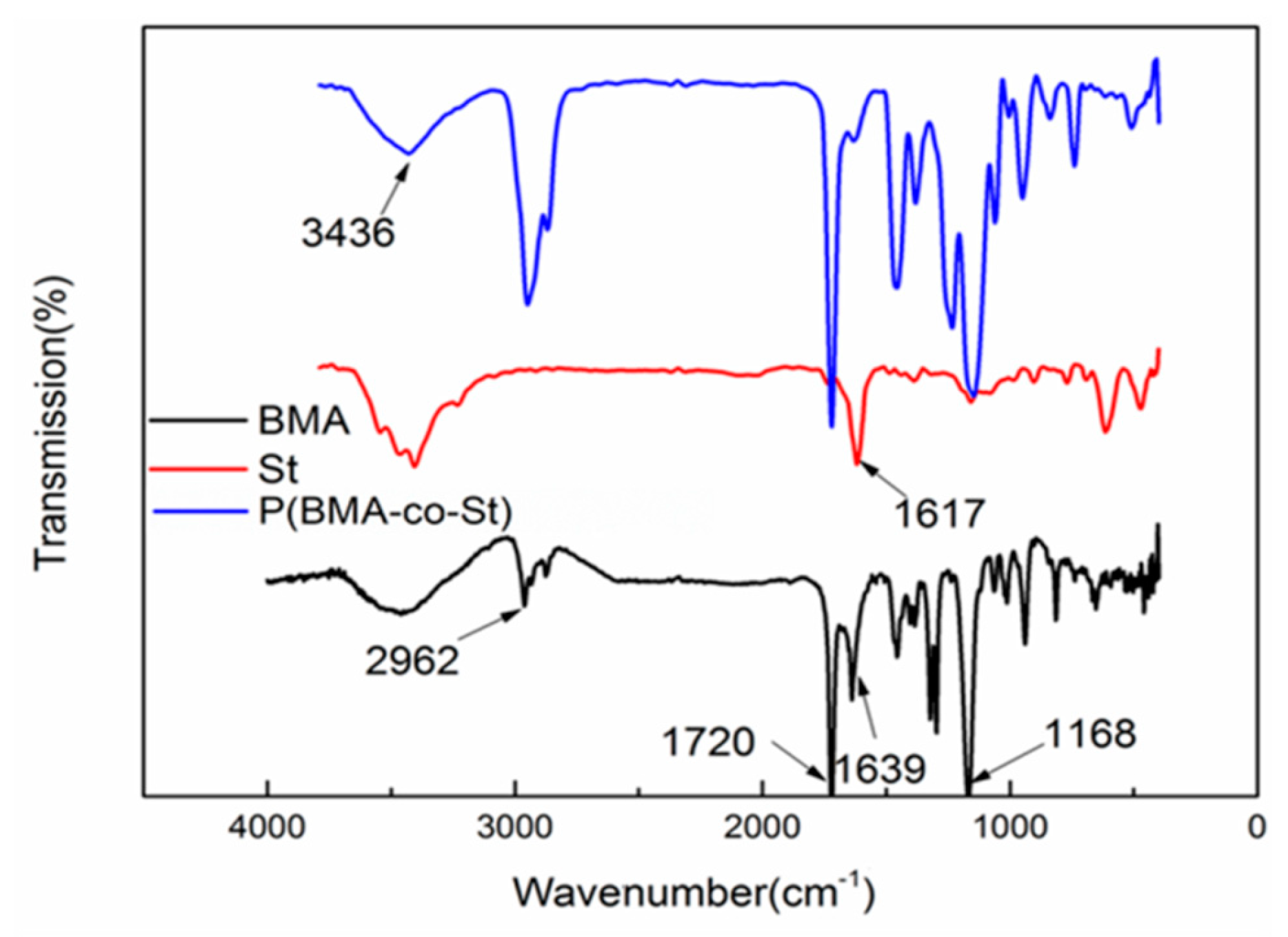
3.1. The Structure of P(BMA-co-St)
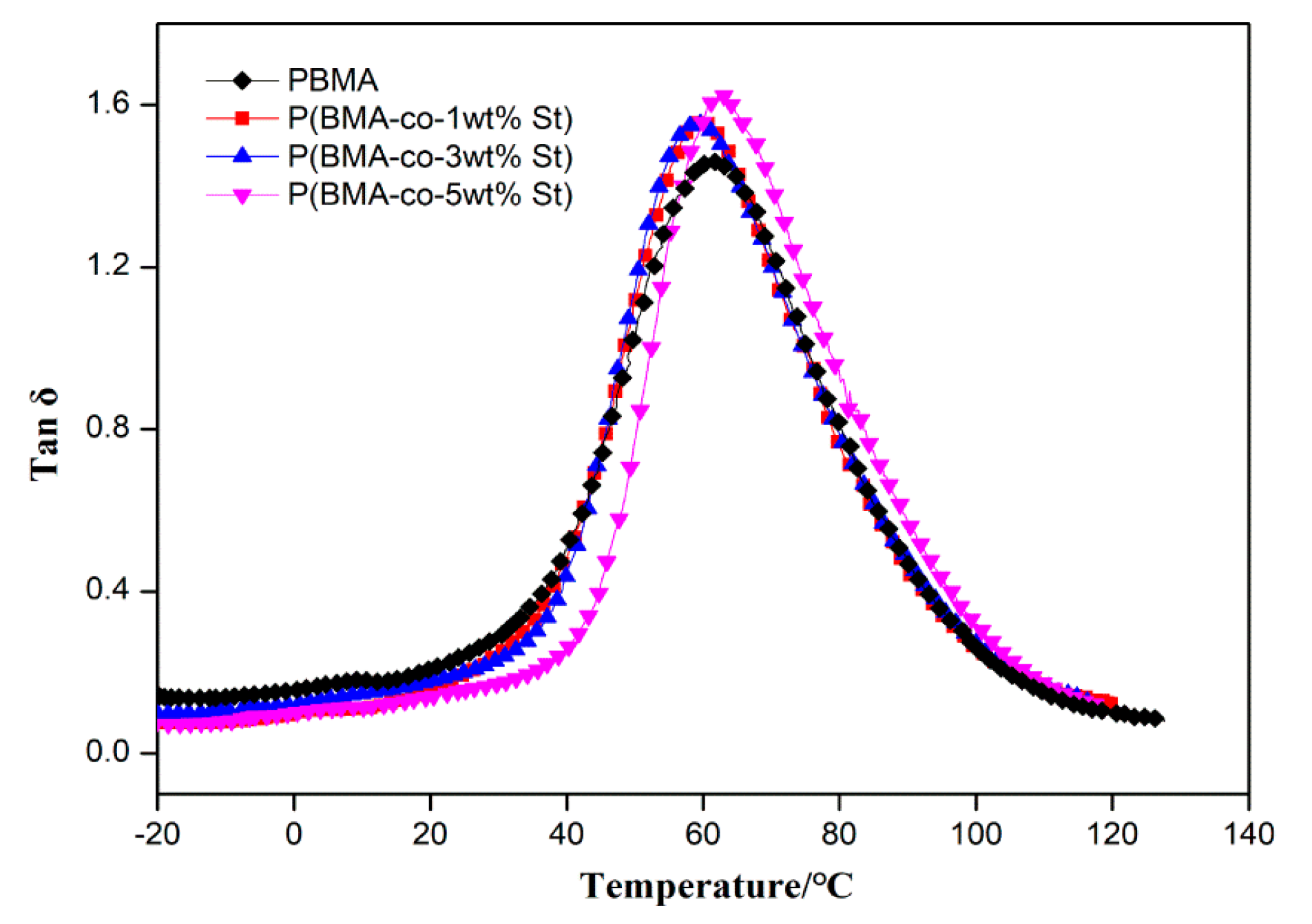
3.2. Selection of the P(BMA-co-St) with the Most Suitable Content of St
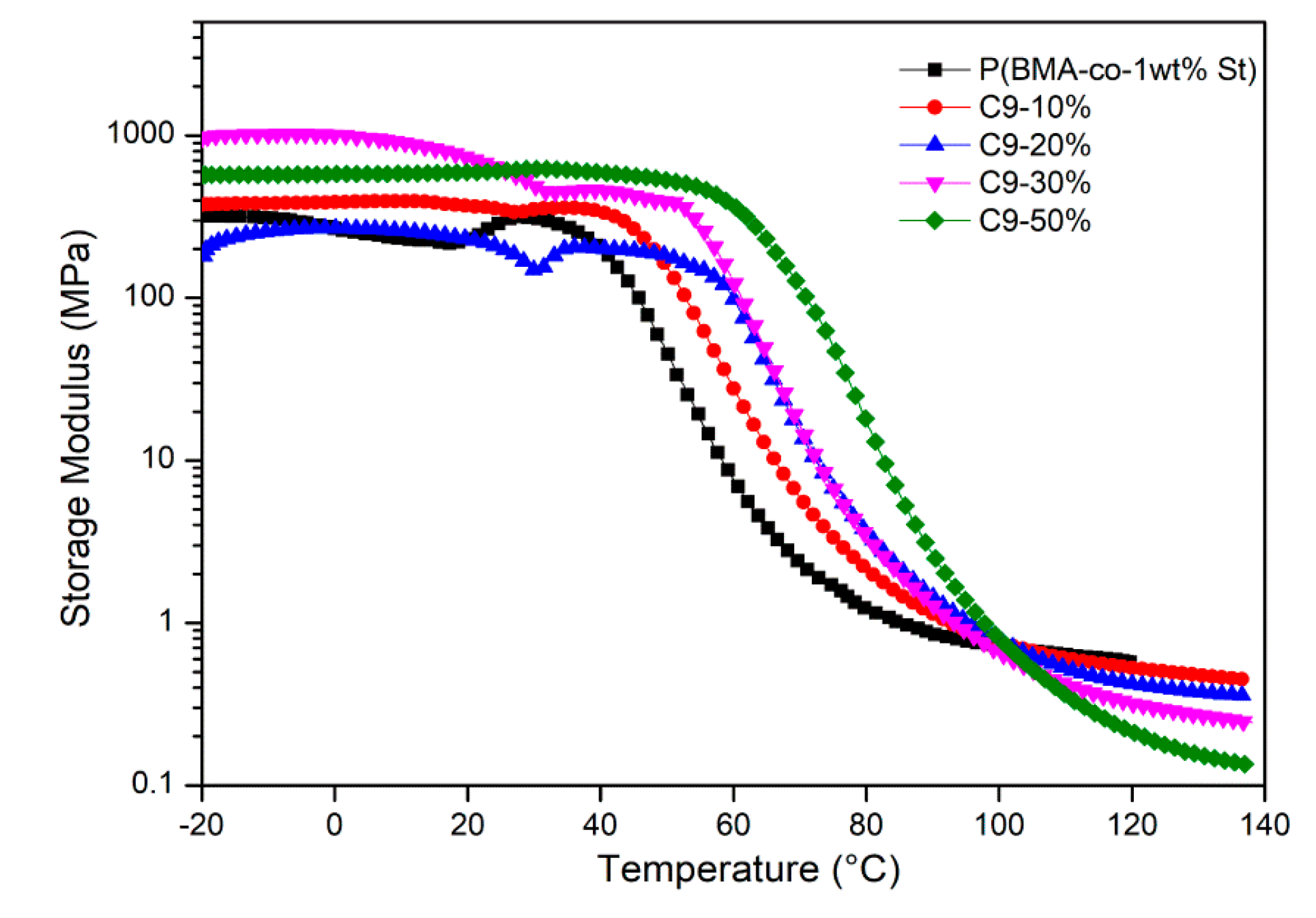
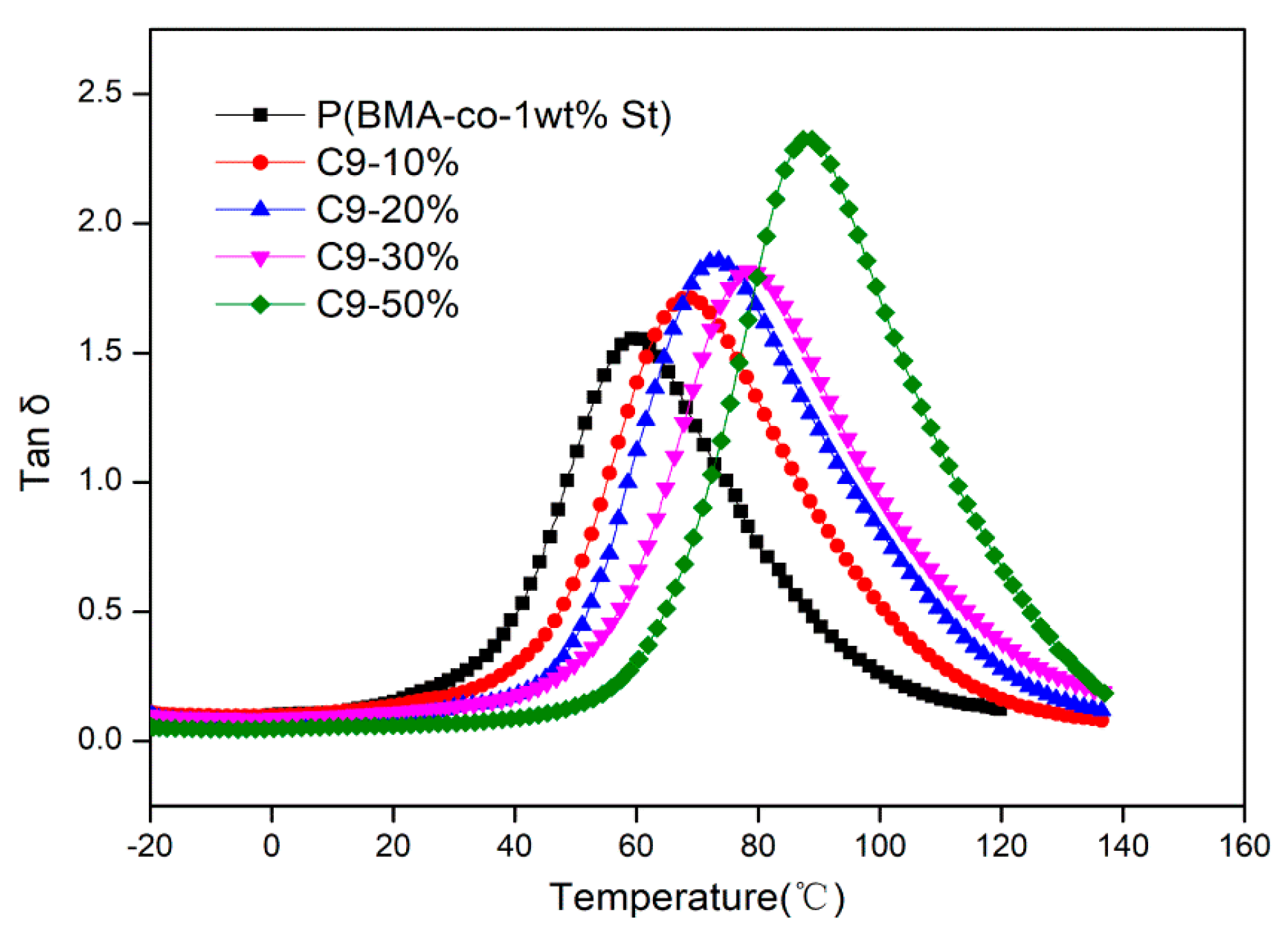
3.3. Study on Compatibility between C9 and P(BMA-co-St)
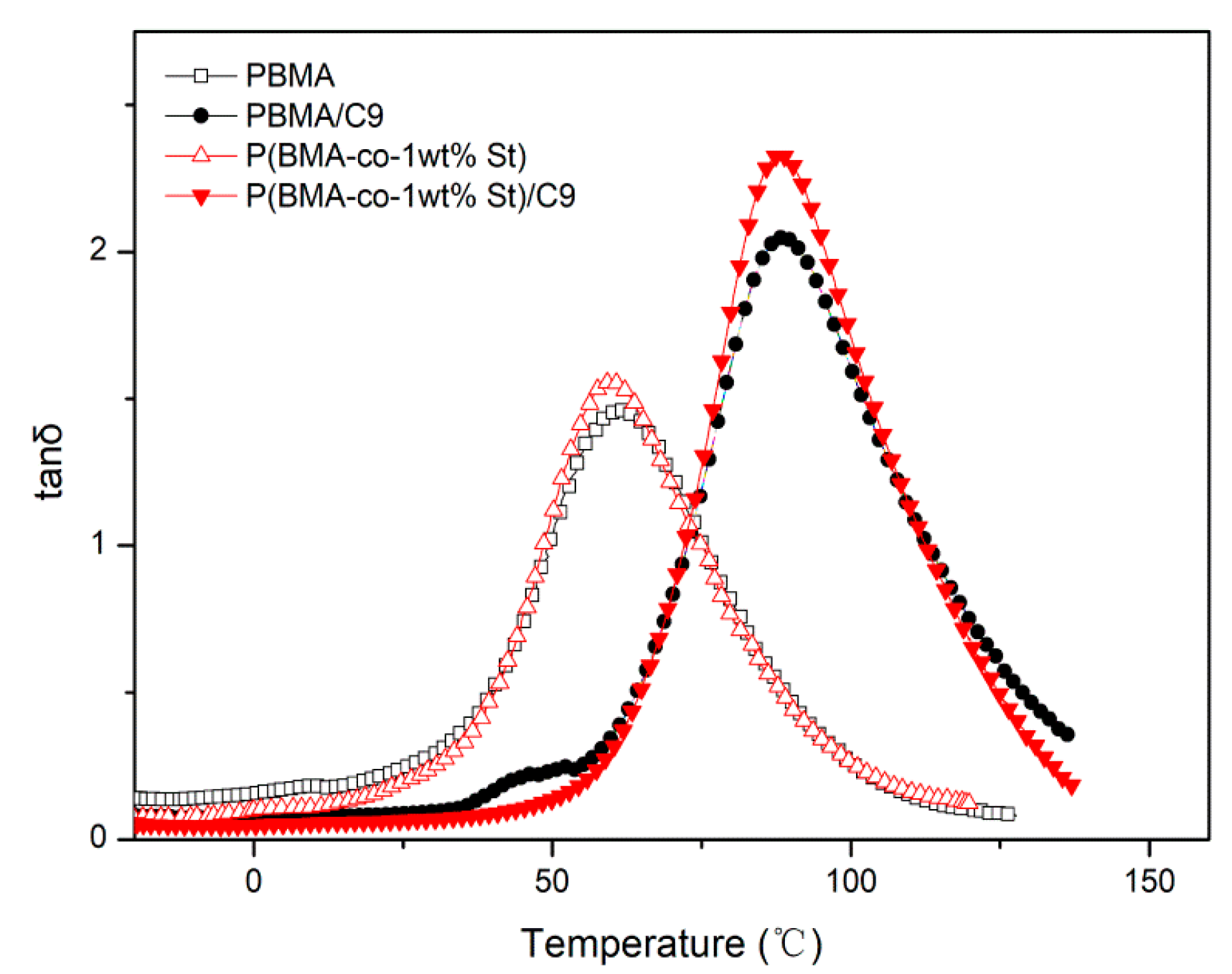
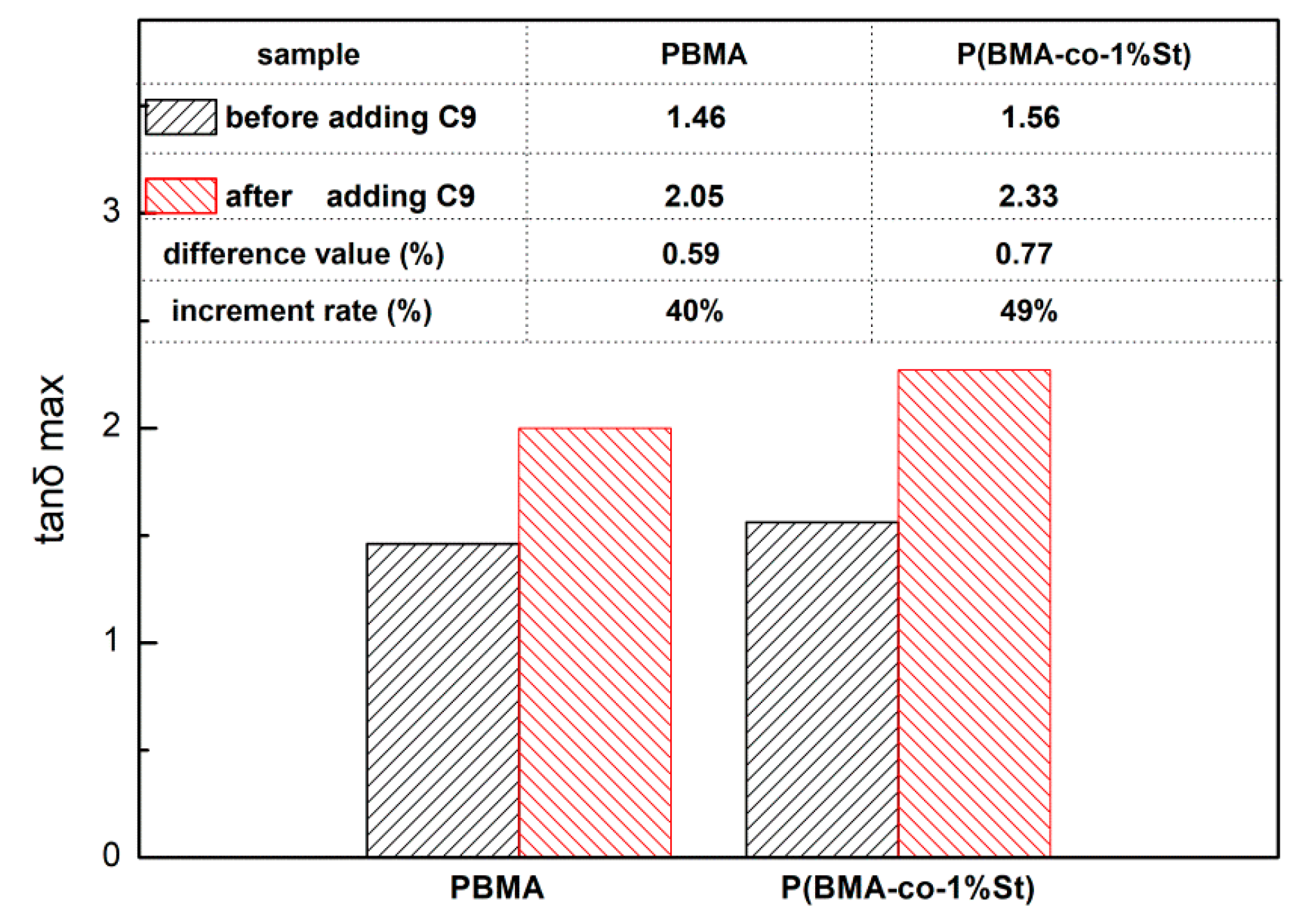
3.4. Damping Properties of P(BMA-co-1wt%St)/C9 Composites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chandra, R.; Singh, S.P.; Gupta, K. Damping studies in fiber-reinforced composites—A review. Compos. Struct. 1999, 46, 41–51. [Google Scholar] [CrossRef]
- Maheo, L.; Rio, G.; Grolleau, V. On the use of some numerical damping methods of spurious oscillations in the case of elastic wave propagation. Mech. Res. Commun. 2011, 38, 81–88. [Google Scholar] [CrossRef]
- Moradi, A. Damping properties of plasmonic waves on graphene. Phys. Plasmas 2017, 24, 072114. [Google Scholar] [CrossRef]
- Rao, M.D. Recent applications of viscoelastic damping for noise control in automobiles and commercial airplanes. J. Sound Vib. 2003, 262, 457–474. [Google Scholar] [CrossRef]
- Lewis, C.; Buanpa, R.; Kiatkamjornwong, S. Effect of rubber ratio, carbon black level, and accelerator level on natural rubber/bromobutyl rubber blend properties. J. Appl. Polym. Sci. 2003, 90, 3059–3068. [Google Scholar] [CrossRef]
- Chang, M.C.; Thomas, D.A.; Sperling, L.H. Group Contribution Analysis of the Damping Behavior of Homopolymers, Statistical Copolymers, and Interpenetrating Polymer Networks Based on Acrylic, Vinyl, and Styrenic Mers. J. Polym. Sci. Part A Polym. Chem. 2010, 26. [Google Scholar] [CrossRef]
- Wang, T.; Chen, S.; Wang, Q.; Pei, X. Damping analysis of polyurethane/epoxy graft interpenetrating polymer network composites filled with short carbon fiber and micro hollow glass bead. Mater. Des. 2010, 31, 3810–3815. [Google Scholar] [CrossRef]
- Mok, M.M.; Kim, J.; Torkelson, J.M. Gradient copolymers with broad glass transition temperature regions: Design of purely interphase compositions for damping applications. J. Polym. Sci. Part B Polym. Phys. 2008, 46, 48–58. [Google Scholar] [CrossRef]
- Huang, G.S.; Jiang, L.-X.; Li, Q. Molecular design of damping rubber based on polyacrylate-containing silicone. J. Appl. Polym. Sci. 2002, 85, 746–751. [Google Scholar] [CrossRef]
- Su, C.; He, P.; Yan, R.; Zhao, C.; Zhang, C. Study of the orientation-controlled damping temperature based on selective distribution of oligo-phenol in acrylate rubber/chlorinated butyl rubber blends. Polym. Compos. 2012, 33, 860–865. [Google Scholar] [CrossRef]
- Zhou, S.; Yang, C.; Hu, J.; He, X.; Zhang, R. Damping Analysis of Some Inorganic Particles on Poly(butyl-methacrylate). Materials 2018, 11, 992. [Google Scholar] [CrossRef]
- Chang, S.; Zong, D.; Xu, L.; Zhang, C. Dynamic mechanical properties of semi-interpenetrating polymer network-based on nitrile rubber and poly(methyl methacrylatbutyl acrylate). J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Suresh, K.I.; Vishwanatham, S.; Bartsch, E. Viscoelastic and damping characteristics of poly(n-butyl acrylate)-poly(n-butyl methacrylate) semi-IPN latex films. Polym. Adv. Technol. 2010, 18, 364–372. [Google Scholar] [CrossRef]
- Ngai, K.L.; Gopalakrishnan, T.R.; Beiner, M. Relaxation in poly(alkyl methacrylate)s: Change of intermolecular coupling with molecular structure, tacticity, molecular weight, copolymerization, crosslinking, and nanoconfinement. Polymer 2006, 47, 7222–7230. [Google Scholar] [CrossRef]
- Wang, J.; Liu, R.; Li, W.; Li, Y.; Tang, X. Studies on the Damping Performance of Polystyrene/Polyacrylate LatexIPN. Polym. Int. 1996, 39, 101–104. [Google Scholar] [CrossRef]
- Wu, C.; Yamagishi, T.-A.; Nakamoto, Y.; Ishida, S.-I.; Kubota, S.; Nitta, K.-H. Organic hybrid of chlorinated polyethylene and hindered phenol. II. Influence of the chemical structure of small molecules on viscoelastic properties. J. Polym. Sci. Part B Polym. Phys. 2000, 38, 1496–1503. [Google Scholar] [CrossRef]
- Li, C.; Xu, S.-A.; Xiao, F.-Y.; Wu, C.-F. Dynamic mechanical properties of chlorinated butyl rubber blends. Eur. Polym. J. 2006, 42, 2507–2514. [Google Scholar] [CrossRef]
- Song, M.; Zhao, X.; Li, Y.; Hu, S.; Zhang, L.; Wu, S. Molecular dynamics simulations and microscopic analysis of the damping performance of hindered phenol AO-60/nitrile-butadiene rubber composites. RSC Adv. 2014, 4, 6719. [Google Scholar] [CrossRef]
- Yang, D.; Zhao, X.; Chan, T.; Zhang, L.; Wu, S. Investigation of the damping properties of hindered phenol AO-80/polyacrylate hybrids using molecular dynamics simulations in combination with experimental methods. J. Mater. Sci. 2016, 51, 5760–5774. [Google Scholar] [CrossRef]
- Liang, J.; Chang, S.; Na, F. Effect of C5 petroleum resin content on damping behavior, morphology, and mechanical properties of BIIR/BR vulcanizates. J. Appl. Polym. Sci. 2013, 130, 510–515. [Google Scholar] [CrossRef]
- Cong, L.; Wu, G.; Xiao, F.; Wu, C. Damping behavior of sandwich beam laminated with CIIR/petroleum resins blends by DMA measurement. J. Appl. Polym. Sci. 2007, 106, 2472–2478. [Google Scholar]
- Zhang, F.; He, G.; Xu, K.; Wu, H.; Guo, S.; Zhang, C. Damping mechanism and different modes of molecular motion through the glass transition of chlorinated butyl rubber and petroleum resin blends. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Wu, C.; Wu, G.; Wu, C. Dynamic mechanical properties in blends of poly(styrene-b-isoprene-b-styrene) with aromatic hydrocarbon resin. J. Appl. Polym. Sci. 2006, 102, 4157–4164. [Google Scholar] [CrossRef]
- Semsarzadeh, M.A.; Abdollahi, M. Atom transfer radical polymerization of styrene and methyl (meth)acrylates initiated with poly(dimethylsiloxane) macroinitiator: Synthesis and characterization of triblock copolymers. J. Appl. Polym. Sci. 2012, 123, 2423–2430. [Google Scholar] [CrossRef]
- Kumar, R.; Sekhon, S.S. Conductivity, FTIR studies, and thermal behavior of PMMA-based proton conducting polymer gel electrolytes containing triflic acid. Ionics 2013, 19, 1627–1635. [Google Scholar] [CrossRef]
- Hualin, C.; Deng, X.; Hou, X.; Luo, R.; Liu, B. Preparation and Characterization of PDMS-PMMA Interpenetrating Polymer Networks with Indistinct Phase Separation. J. Macromol. Sci. Part A 2008, 46, 83–89. [Google Scholar]
- Paluch, M.; Pawlus, S.; Sokolov, A.P.; Ngai, K.L. Sub-Rouse Modes in Polymers Observed by Dielectric Spectroscopy. Macromolecules 2010, 43, 3103–3106. [Google Scholar] [CrossRef]






| Chemical Displacements/ppm | Proton Type |
|---|---|
| 1.00~0.50 | –CH3 (h) |
| 2.00~1.75 | –CH2– (c,e) |
| 4.10~3.80 | –OCH2– (a) |
| 6.80~7.20 | =CH– (b,f,g) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wan, S.; Zhou, S.; Huang, X.; Chen, S.; Cai, S.; He, X.; Zhang, R. Effect of Aromatic Petroleum Resin on Damping Properties of Polybutyl Methacrylate. Polymers 2020, 12, 543. https://doi.org/10.3390/polym12030543
Wan S, Zhou S, Huang X, Chen S, Cai S, He X, Zhang R. Effect of Aromatic Petroleum Resin on Damping Properties of Polybutyl Methacrylate. Polymers. 2020; 12(3):543. https://doi.org/10.3390/polym12030543
Chicago/Turabian StyleWan, Songhan, Saisai Zhou, Xing Huang, Songbo Chen, Shuwei Cai, Xianru He, and Rui Zhang. 2020. "Effect of Aromatic Petroleum Resin on Damping Properties of Polybutyl Methacrylate" Polymers 12, no. 3: 543. https://doi.org/10.3390/polym12030543
APA StyleWan, S., Zhou, S., Huang, X., Chen, S., Cai, S., He, X., & Zhang, R. (2020). Effect of Aromatic Petroleum Resin on Damping Properties of Polybutyl Methacrylate. Polymers, 12(3), 543. https://doi.org/10.3390/polym12030543





