Organosoluble Starch-Cellulose Binary Polymer Blend as a Quasi-Solid Electrolyte in a Dye-Sensitized Solar Cell
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimentals
2.2.1. Phthaloylation of Starch
2.2.2. Preparation of Electrolytes
2.3. Characterizations of Electrolytes
2.4. Characterization of Dye-Sensitized Solar Cell
3. Results
3.1. Rheological Properties
3.1.1. Amplitude Sweep
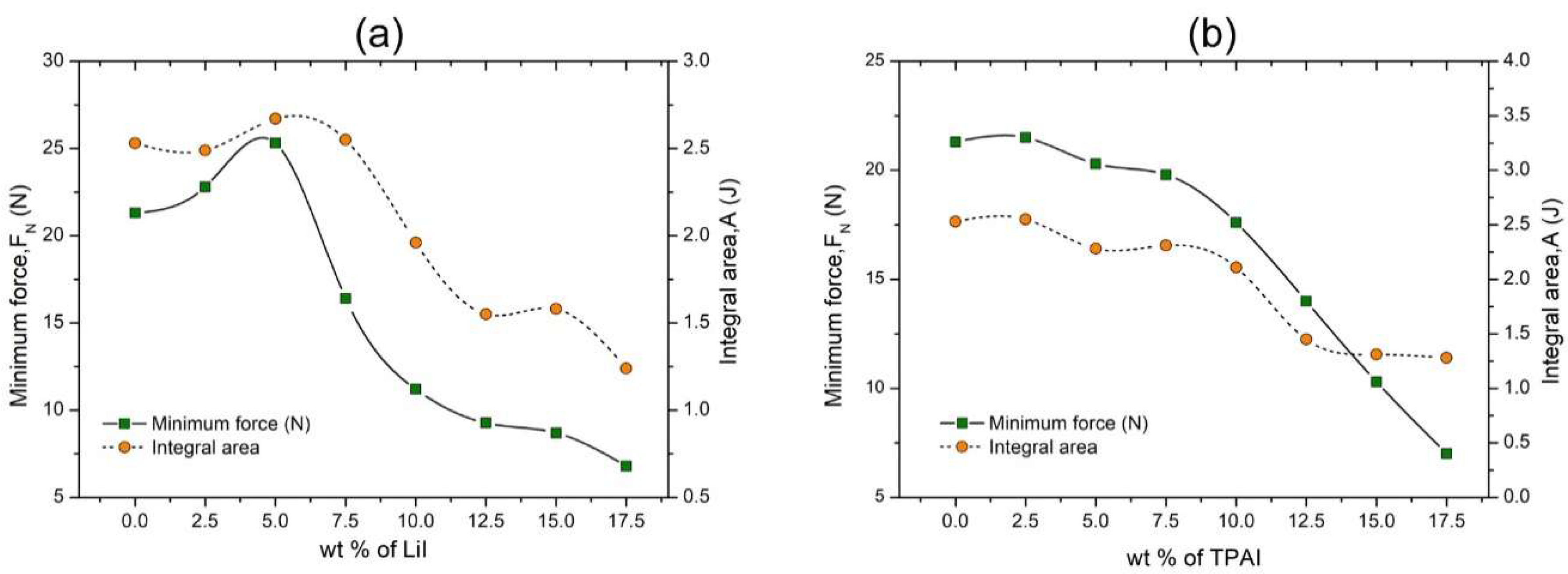
3.1.2. Tack Test
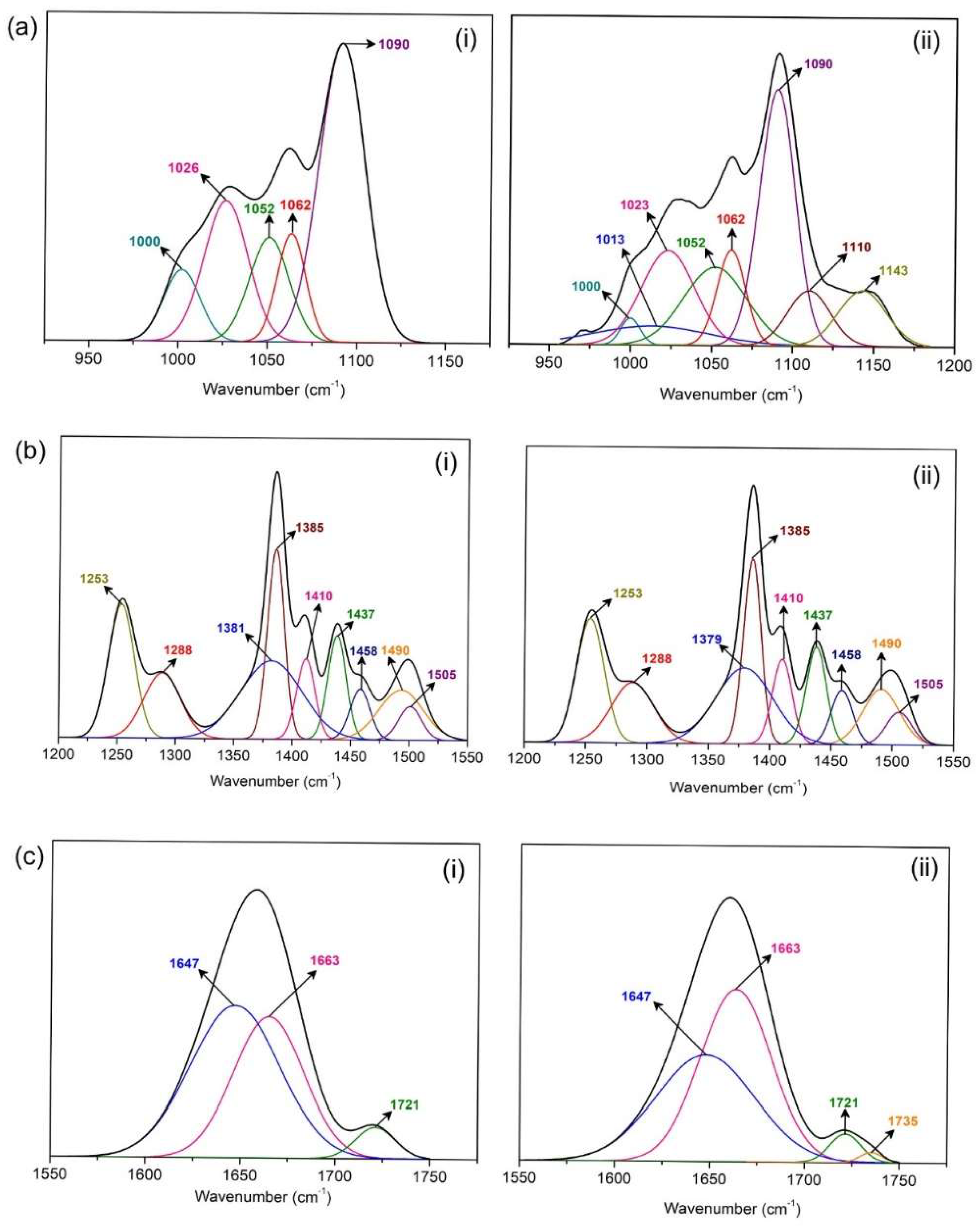
3.2. FTIR Analysis
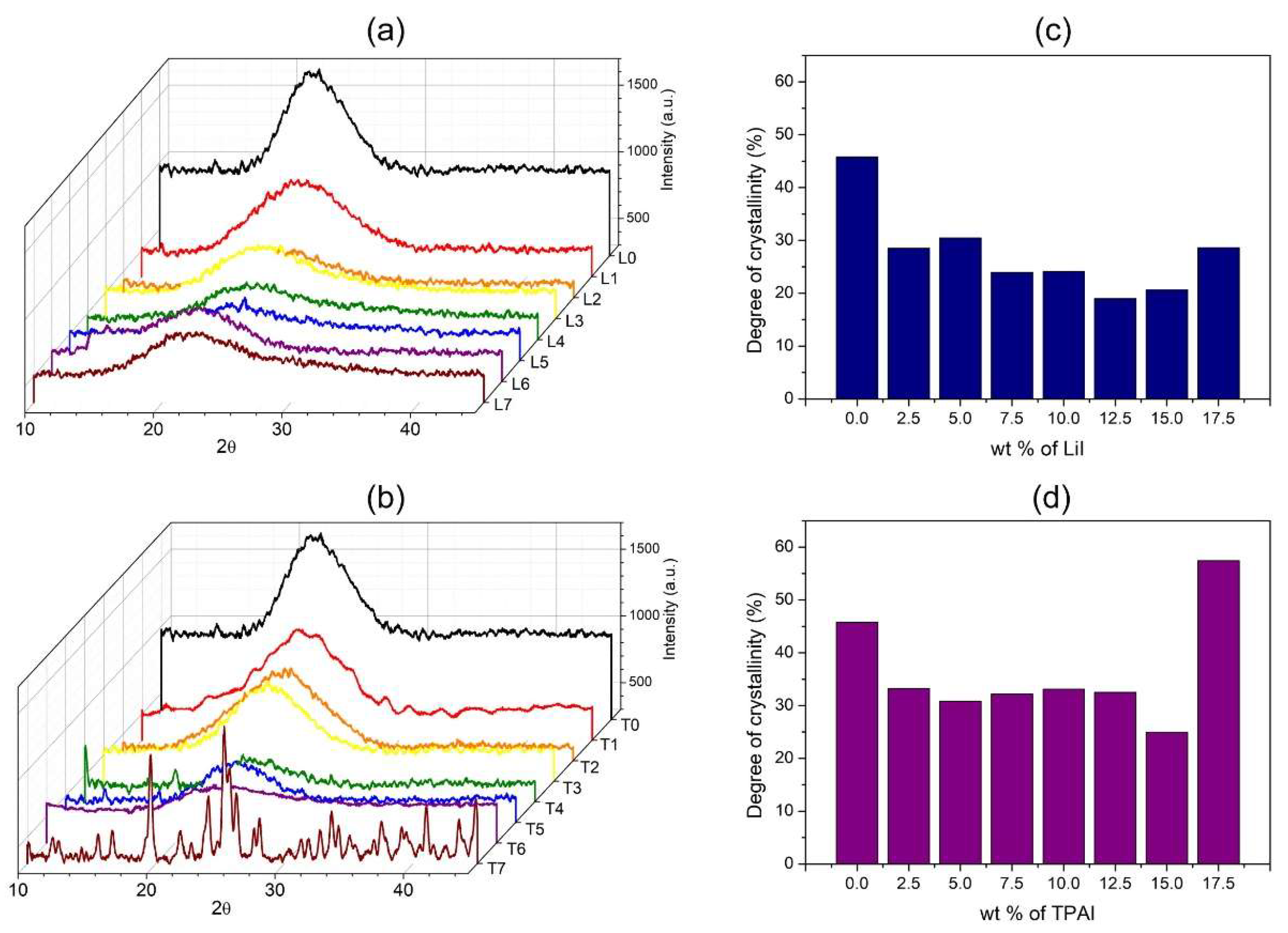
3.3. Crystallinity
3.4. Electrochemical Properties
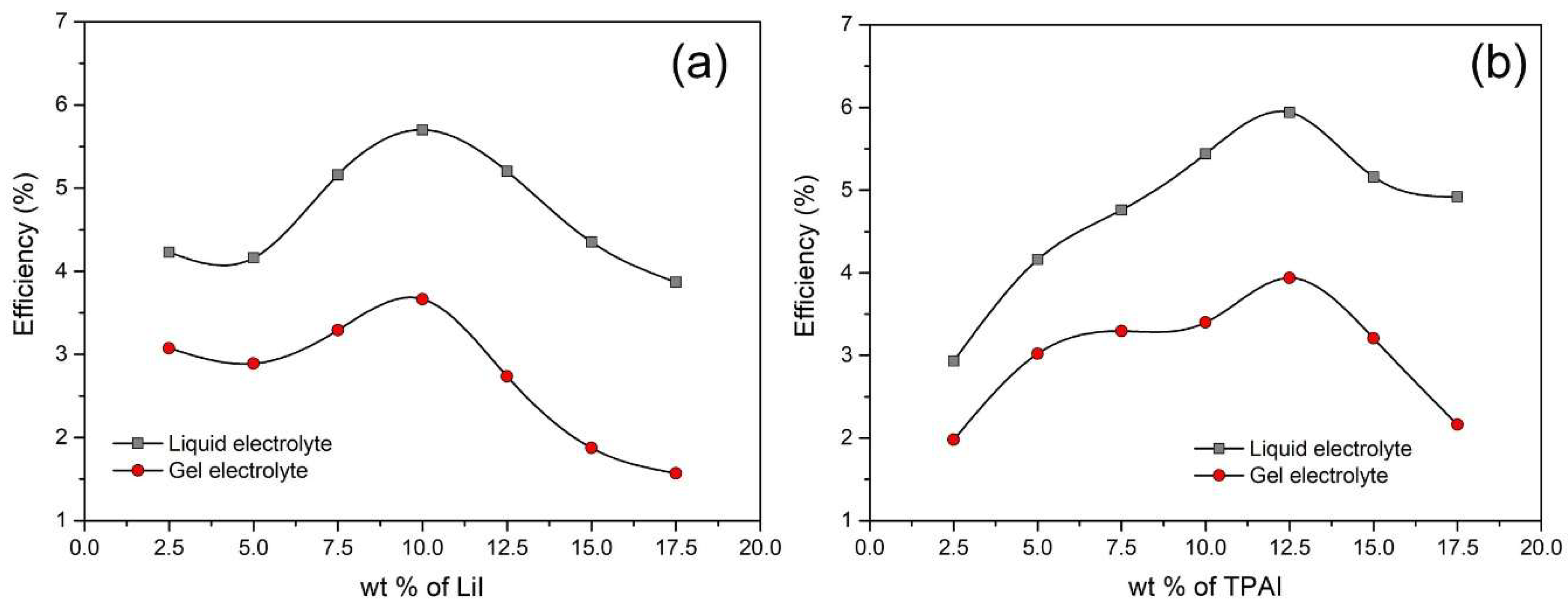
3.5. Photovoltaic Performances
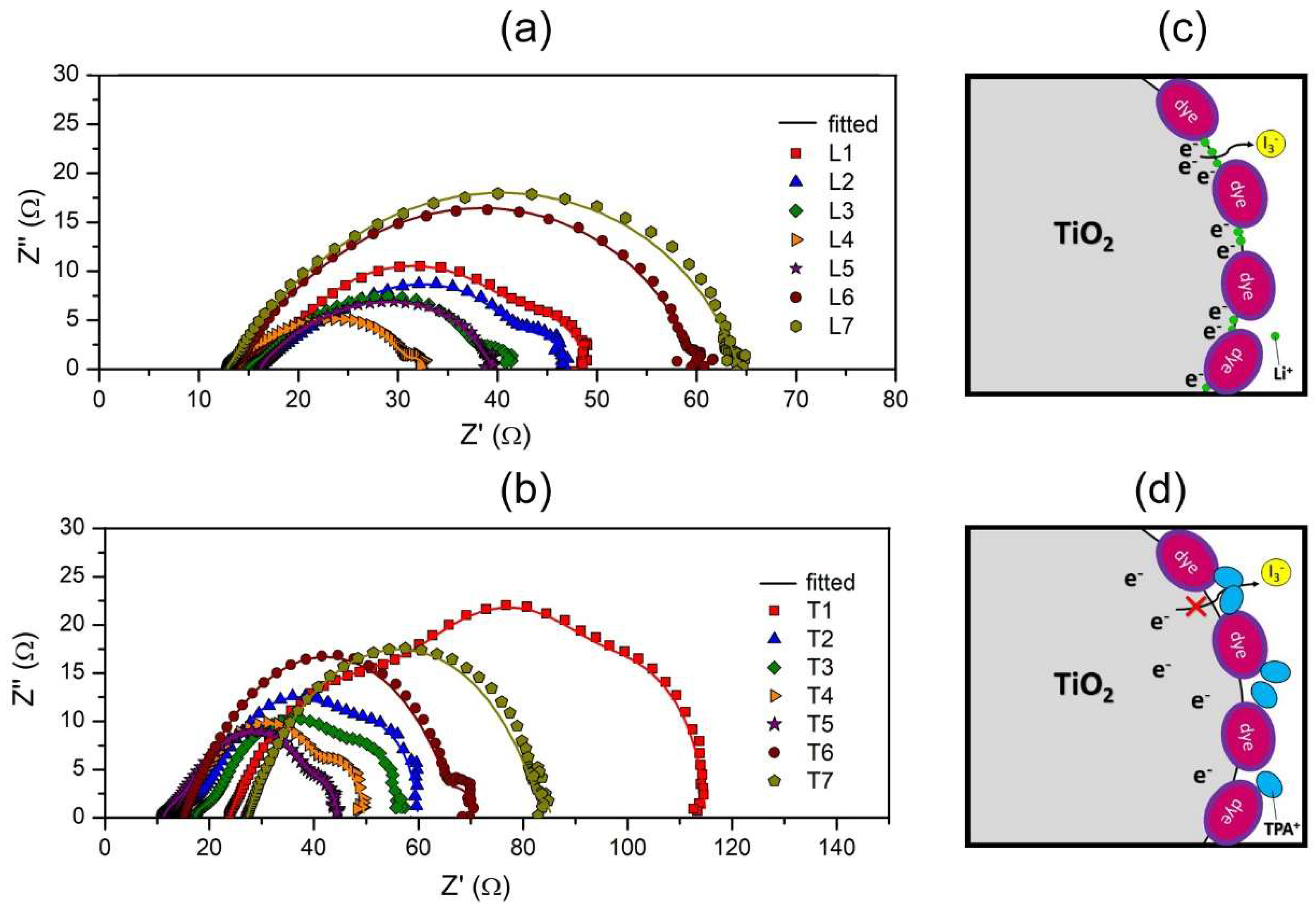
3.6. Impedance Study of DSSC
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- O’Regan, B.; Grätzel, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 1991, 353, 737. [Google Scholar] [CrossRef]
- Nazeeruddin, M.K.; De Angelis, F.; Fantacci, S.; Selloni, A.; Viscardi, G.; Liska, P.; Ito, S.; Takeru, B.; Grätzel, M. Combined Experimental and DFT-TDDFT Computational Study of Photoelectrochemical Cell Ruthenium Sensitizers. J. Am. Chem. Soc. 2005, 127, 16835–16847. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Lan, Z.; Hao, S.; Li, P.; Lin, J.; Huang, M.; Fang, L.; Huang, Y. Progress on the electrolytes for dye-sensitized solar cells. Pure Appl. Chem. 2008, 80, 2241. [Google Scholar] [CrossRef]
- Wang, P.; Zakeeruddin, S.M.; Moser, J.E.; Nazeeruddin, M.K.; Sekiguchi, T.; Grätzel, M. A stable quasi-solid-state dye-sensitized solar cell with an amphiphilic ruthenium sensitizer and polymer gel electrolyte. Nat. Mater. 2003, 2, 402. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Lan, Z.; Lin, J.; Huang, M.; Huang, Y.; Fan, L.; Luo, G. Electrolytes in Dye-Sensitized Solar Cells. Chem. Rev. 2015, 115, 2136–2173. [Google Scholar] [CrossRef] [PubMed]
- Khanmirzaei, M.H.; Ramesh, S.; Ramesh, K. Effect of different iodide salts on ionic conductivity and structural and thermal behavior of rice-starch-based polymer electrolytes for dye-sensitized solar cell application. Ionics 2015, 21, 2383–2391. [Google Scholar] [CrossRef]
- Khanmirzaei, M.H.; Ramesh, S. Nanocomposite polymer electrolyte based on rice starch/ionic liquid/TiO2 nanoparticles for solar cell application. Measurement 2014, 58, 68–72. [Google Scholar] [CrossRef]
- Lobregas, M.O.S.; Camacho, D.H. Gel polymer electrolyte system based on starch grafted with ionic liquid: Synthesis, characterization and its application in dye-sensitized solar cell. Electrochim. Acta 2019, 298, 219–228. [Google Scholar] [CrossRef]
- Nagaraj, P.; Sasidharan, A.; David, V.; Sambandam, A. Effect of Cross-Linking on the Performances of Starch-Based Biopolymer as Gel Electrolyte for Dye-Sensitized Solar Cell Applications. Polymers 2017, 9, 667. [Google Scholar] [CrossRef]
- Selvanathan, V.; Azzahari, A.D.; Halim, A.A.A.; Yahya, R. Ternary natural deep eutectic solvent (NADES) infused phthaloyl starch as cost efficient quasi-solid gel polymer electrolyte. Carbohydr. Polym. 2017, 167, 210–218. [Google Scholar] [CrossRef]
- Selvanathan, V.; Yahya, R.; Alharbi, H.F.; Alharthi, N.H.; Alharthi, Y.S.; Ruslan, M.H.; Amin, N.; Akhtaruzzaman, M. Organosoluble starch derivative as quasi-solid electrolytes in DSSC: Unravelling the synergy between electrolyte rheology and photovoltaic properties. Sol. Energy 2020, 197, 144–153. [Google Scholar] [CrossRef]
- Arof, A.K.; Aziz, M.F.; Noor, M.M.; Careem, M.A.; Bandara, L.R.A.K.; Thotawatthage, C.A.; Rupasinghe, W.N.S.; Dissanayake, M.A.K.L. Efficiency enhancement by mixed cation effect in dye-sensitized solar cells with a PVdF based gel polymer electrolyte. Int. J. Hydrog. Energy 2014, 39, 2929–2935. [Google Scholar] [CrossRef]
- Ozawa, H.; Okuyama, Y.; Arakawa, H. Effects of cation composition in the electrolyte on the efficiency improvement of black dye-based dye-sensitized solar cells. RSC Adv. 2013, 3, 9175–9177. [Google Scholar] [CrossRef]
- Bandara, T.M.W.J.; Jayasundara, W.J.M.J.S.R.; Dissanayake, M.A.K.L.; Furlani, M.; Albinsson, I.; Mellander, B.E. Effect of cation size on the performance of dye sensitized nanocrystalline TiO2 solar cells based on quasi-solid state PAN electrolytes containing quaternary ammonium iodides. Electrochim. Acta 2013, 109, 609–616. [Google Scholar] [CrossRef]
- Dintcheva, N.T.; Furlani, M.; Jayasundara, W.J.M.J.S.R.; Bandara, T.M.W.J.; Mellander, B.-E.; La Mantia, F.P. Rheological behavior of PAN-based electrolytic gel containing tetrahexylammonium and magnesium iodide for photoelectrochemical applications. Rheol. Acta 2013, 52, 881–889. [Google Scholar] [CrossRef]
- Zhang, B.; Zhou, Y.; Li, X.; Wang, J.; Li, G.; Yun, Q.; Wang, X. Li+-molecule interactions of lithium tetrafluoroborate in propylene carbonate +N,N-dimethylformamide mixtures: An FTIR spectroscopic study. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 124, 40–45. [Google Scholar] [CrossRef]
- Jacob, M.M.E.; Arof, A.K. FTIR studies of DMF plasticized polyvinyledene fluoride based polymer electrolytes. Electrochim. Acta 2000, 45, 1701–1706. [Google Scholar] [CrossRef]
- Ståkhandske, C.M.V.; Mink, J.; Sandström, M.; Pápai, I.; Johansson, P. Vibrational spectroscopic and force field studies of N,N-dimethylthioformamide, N,N-dimethylformamide, their deuterated analogues and bis(N,N-dimethylthioformamide)mercury(II) perchlorate. Vib. Spectrosc. 1997, 14, 207–227. [Google Scholar] [CrossRef]
- Bar, N.; Basak, P. Quasi-Solid Semi-Interpenetrating Polymer Networks as Electrolytes: Part II. Assessing the Modes of Ion-Ion and Ion-Polymer Interactions Employing Mid-Fourier Transform Infrared Vibrational Spectroscopy. J. Phys. Chem. C 2014, 118, 10640–10650. [Google Scholar] [CrossRef]
- Echeverri, M.; Kim, N.; Kyu, T. Ionic Conductivity in Relation to Ternary Phase Diagram of Poly(ethylene oxide), Succinonitrile, and Lithium Bis(trifluoromethane)sulfonimide Blends. Macromolecules 2012, 45, 6068–6077. [Google Scholar] [CrossRef]
- Ratner, M.A.; Shriver, D.F. Ion transport in solvent-free polymers. Chem. Rev. 1988, 88, 109–124. [Google Scholar] [CrossRef]
- Petrowsky, M.; Frech, R. Salt concentration dependence of the compensated Arrhenius equation for alcohol-based electrolytes. Electrochim. Acta 2010, 55, 1285–1288. [Google Scholar] [CrossRef]
- Liu, Y.; Hagfeldt, A.; Xiao, X.-R.; Lindquist, S.-E. Investigation of influence of redox species on the interfacial energetics of a dye-sensitized nanoporous TiO2 solar cell. Sol. Energy Mater. Sol. Cells 1998, 55, 267–281. [Google Scholar] [CrossRef]
- Wang, H.; Peter, L.M. Influence of Electrolyte Cations on Electron Transport and Electron Transfer in Dye-Sensitized Solar Cells. J. Phys. Chem. C 2012, 116, 10468–10475. [Google Scholar] [CrossRef]
- Yogananda, K.C.; Ramasamy, E.; Kumar, S.; Vasantha Kumar, S.; Navya Rani, M.; Rangappa, D. Novel Rice Starch based aqueous gel electrolyte for Dye Sensitized Solar Cell Application. Mater. Today Proc. 2017, 4, 12238–12244. [Google Scholar] [CrossRef]
- Khanmirzaei, M.H.; Ramesh, S.; Ramesh, K. Polymer electrolyte based dye-sensitized solar cell with rice starch and 1-methyl-3-propylimidazolium iodide ionic liquid. Mater. Des. 2015, 85, 833–837. [Google Scholar] [CrossRef]
- Yogananda, K.C.; Ramasamy, E.; Vasantha Kumar, S.; Rangappa, D. Synthesis, characterization, and dye-sensitized solar cell fabrication using potato starch- and potato starch nanocrystal-based gel electrolytes. Ionics 2019, 25, 6035–6042. [Google Scholar] [CrossRef]
- Kuwahara, S.; Taya, S.; Osada, N.; Shen, Q.; Toyoda, T.; Katayama, K. Effect of electrolyte constituents on the motion of ionic species and recombination kinetics in dye-sensitized solar cells. Phys. Chem. Chem. Phys. 2014, 16, 5242–5249. [Google Scholar] [CrossRef]





| Designation | Mass of Salt (g) | Wt % of Salt (%) | Mass of I2 (g) | |
|---|---|---|---|---|
| L Series | T Series | |||
| L0 | T0 | 0.00 | 0.0 | 0.000 |
| L1 | T1 | 0.04 | 2.5 | 0.008 |
| L2 | T2 | 0.08 | 5.0 | 0.016 |
| L3 | T3 | 0.13 | 7.5 | 0.025 |
| L4 | T4 | 0.18 | 10.0 | 0.034 |
| L5 | T5 | 0.23 | 12.5 | 0.043 |
| L6 | T6 | 0.28 | 15.0 | 0.054 |
| L7 | T7 | 0.34 | 17.5 | 0.064 |
| Sample | wt % of Salt | σ (×10−3 S·cm−1) | η (%) | JSC (mA cm−2) | VOC (V) | FF |
|---|---|---|---|---|---|---|
| LiI series | ||||||
| L1 | 2.5 | 3.38 | 3.07 | 7.69 | 0.59 | 0.68 |
| L2 | 5.0 | 3.85 | 2.89 | 8.13 | 0.56 | 0.67 |
| L3 | 7.5 | 4.83 | 3.29 | 8.85 | 0.54 | 0.69 |
| L4 | 10.0 | 5.50 | 3.67 | 10.68 | 0.51 | 0.65 |
| L5 | 12.5 | 5.35 | 2.74 | 7.77 | 0.51 | 0.68 |
| L6 | 15.0 | 4.40 | 1.87 | 5.78 | 0.48 | 0.67 |
| L7 | 17.5 | 4.29 | 1.57 | 5.03 | 0.48 | 0.65 |
| TPAI series | ||||||
| T1 | 2.5 | 1.69 | 1.98 | 5.15 | 0.61 | 0.63 |
| T2 | 5.0 | 3.41 | 3.02 | 9.02 | 0.57 | 0.60 |
| T3 | 7.5 | 4.35 | 3.29 | 9.16 | 0.57 | 0.60 |
| T4 | 10.0 | 4.54 | 3.40 | 9.46 | 0.56 | 0.64 |
| T5 | 12.5 | 4.97 | 3.94 | 10.11 | 0.56 | 0.69 |
| T6 | 15.0 | 4.35 | 3.21 | 8.92 | 0.52 | 0.68 |
| T7 | 17.5 | 3.57 | 2.16 | 5.82 | 0.52 | 0.71 |
| Electrolyte Composition (Polymer/Salt/Additive) | Dye | η (%) | JSC (mA cm−2) | VOC (V) | FF | Ref. |
|---|---|---|---|---|---|---|
| Rice starch/LiI/MPII//TiO2 | N3 | 0.17 | 0.49 | 0.45 | 0.75 | [7] |
| Rice starch/LiI/Distilled water | N719 | 0.35 | 0.83 | 0.92 | 0.46 | [25] |
| GMIC grafted starch/KI/DMSO | N719 | 0.63 | 0.49 | 0.55 | 0.61 | [8] |
| Rice starch/NaI | N719 | 0.78 | 2.40 | 0.49 | 0.67 | [6] |
| Crosslinked starch/LiI/Gly/DMF | N719 | 1.40 | 2.17 | 0.67 | 0.82 | [9] |
| Rice starch/NaI/MPII | N719 | 2.09 | 4.78 | 0.57 | 0.77 | [26] |
| PhSt/HEC/LiI/DMF | N719 | 3.02 | 9.02 | 0.57 | 0.60 | [11] |
| Potato starch nanocrystal/DMSO/NaI | N719 | 3.33 | 8.08 | 0.72 | 0.57 | [27] |
| PhSt/HEC/LiI/DMF | N719 | 3.94 | 10.11 | 0.56 | 0.69 | This work |
| Designation | RS (Ω) | RPT (Ω) | RCT (Ω) | RD (Ω) | (×10−7 cm2 s−1) | Σ (×10−3 cm−1) |
|---|---|---|---|---|---|---|
| LiI series | ||||||
| L1 | 13.92 | 8.17 | 18.58 | 8.29 | 6.71 | 3.38 |
| L2 | 15.91 | 8.12 | 17.62 | 5.48 | 6.46 | 3.85 |
| L3 | 14.78 | 5.88 | 17.58 | 3.26 | 7.73 | 4.83 |
| L4 | 12.68 | 6.36 | 11.97 | 1.89 | 8.76 | 5.50 |
| L5 | 16.11 | 12.07 | 11.23 | - | - | 5.35 |
| L6 | 13.21 | 32.90 | 12.95 | - | - | 4.40 |
| L7 | 12.81 | 39.80 | 10.42 | - | - | 4.29 |
| TPAI series | ||||||
| T1 | 23.79 | 24.15 | 41.82 | 25.12 | 6.58 | 1.69 |
| T2 | 13.43 | 9.16 | 22.59 | 15.72 | 7.23 | 3.41 |
| T3 | 17.06 | 6.00 | 22.56 | 11.99 | 7.33 | 4.35 |
| T4 | 12.37 | 5.62 | 21.58 | 9.00 | 8.38 | 4.54 |
| T5 | 11.10 | 5.11 | 23.05 | 4.49 | 11.30 | 4.97 |
| T6 | 15.03 | 23.50 | 26.47 | 6.09 | 4.15 | 4.35 |
| T7 | 27.00 | 39.05 | 20.66 | - | - | 3.57 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Selvanathan, V.; Yahya, R.; Ruslan, M.H.; Sopian, K.; Amin, N.; Nour, M.; Sindi, H.; Rawa, M.; Akhtaruzzaman, M. Organosoluble Starch-Cellulose Binary Polymer Blend as a Quasi-Solid Electrolyte in a Dye-Sensitized Solar Cell. Polymers 2020, 12, 516. https://doi.org/10.3390/polym12030516
Selvanathan V, Yahya R, Ruslan MH, Sopian K, Amin N, Nour M, Sindi H, Rawa M, Akhtaruzzaman M. Organosoluble Starch-Cellulose Binary Polymer Blend as a Quasi-Solid Electrolyte in a Dye-Sensitized Solar Cell. Polymers. 2020; 12(3):516. https://doi.org/10.3390/polym12030516
Chicago/Turabian StyleSelvanathan, Vidhya, Rosiyah Yahya, Mohd Hafidz Ruslan, Kamaruzzaman Sopian, Nowshad Amin, Majid Nour, Hatem Sindi, Muhyaddin Rawa, and Md. Akhtaruzzaman. 2020. "Organosoluble Starch-Cellulose Binary Polymer Blend as a Quasi-Solid Electrolyte in a Dye-Sensitized Solar Cell" Polymers 12, no. 3: 516. https://doi.org/10.3390/polym12030516
APA StyleSelvanathan, V., Yahya, R., Ruslan, M. H., Sopian, K., Amin, N., Nour, M., Sindi, H., Rawa, M., & Akhtaruzzaman, M. (2020). Organosoluble Starch-Cellulose Binary Polymer Blend as a Quasi-Solid Electrolyte in a Dye-Sensitized Solar Cell. Polymers, 12(3), 516. https://doi.org/10.3390/polym12030516










