Effect of Na2CO3 on the Microstructure and Macroscopic Properties and Mechanism Analysis of PVA/CMC Composite Film
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
 .
.2.2. Preparation of Samples
2.3. Fourier-Transform Infrared (FTIR) Spectroscopy
2.4. X-ray Diffraction (XRD)
2.5. Differential Scanning Calorimetry (DSC)
2.6. Atomic Force Microscopy (AFM) Studies
2.7. Dielectric Properties
2.8. Water Sorption
2.9. Solubility
3. Results and Discussion
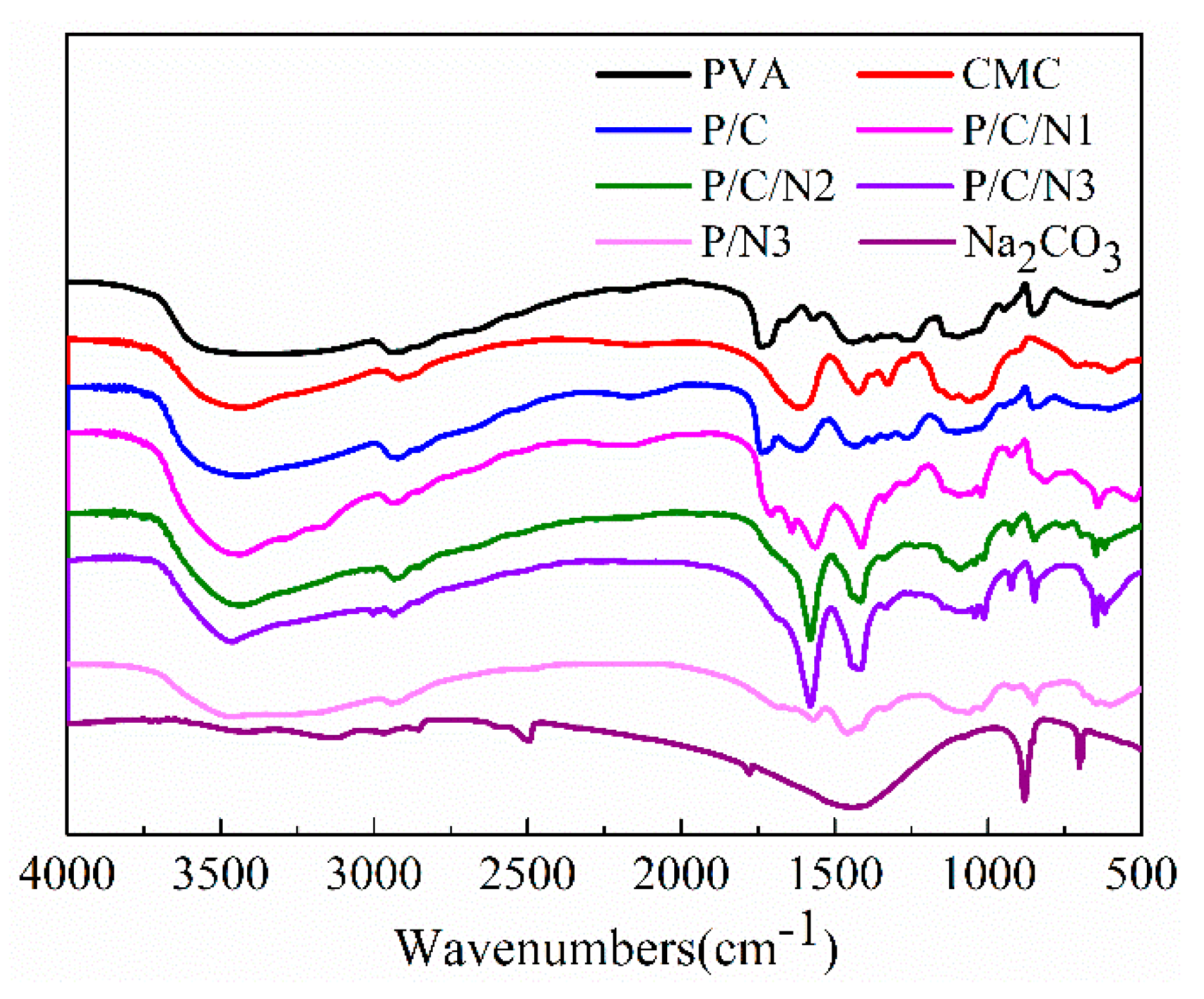
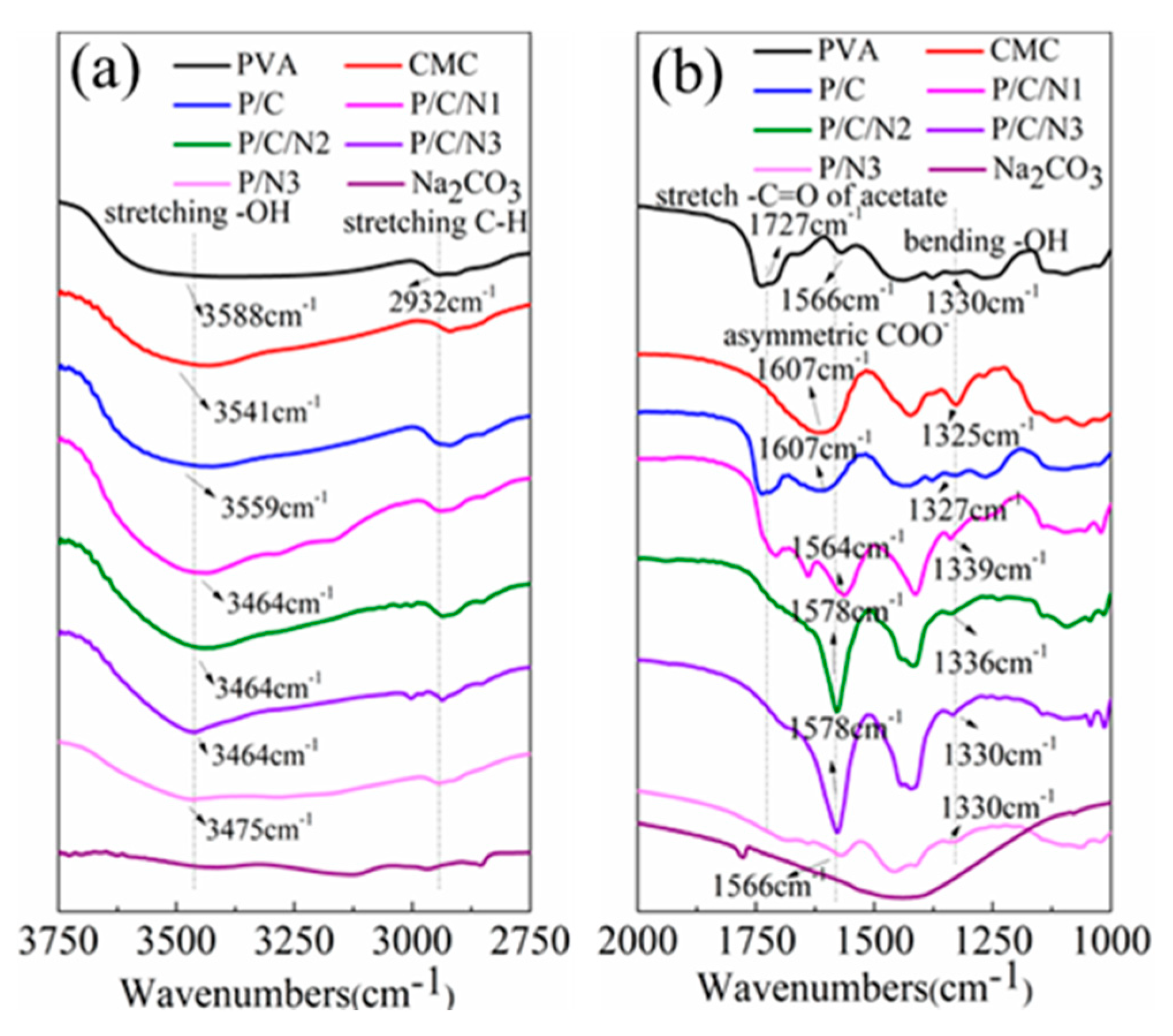
3.1. FTIR Spectroscopy
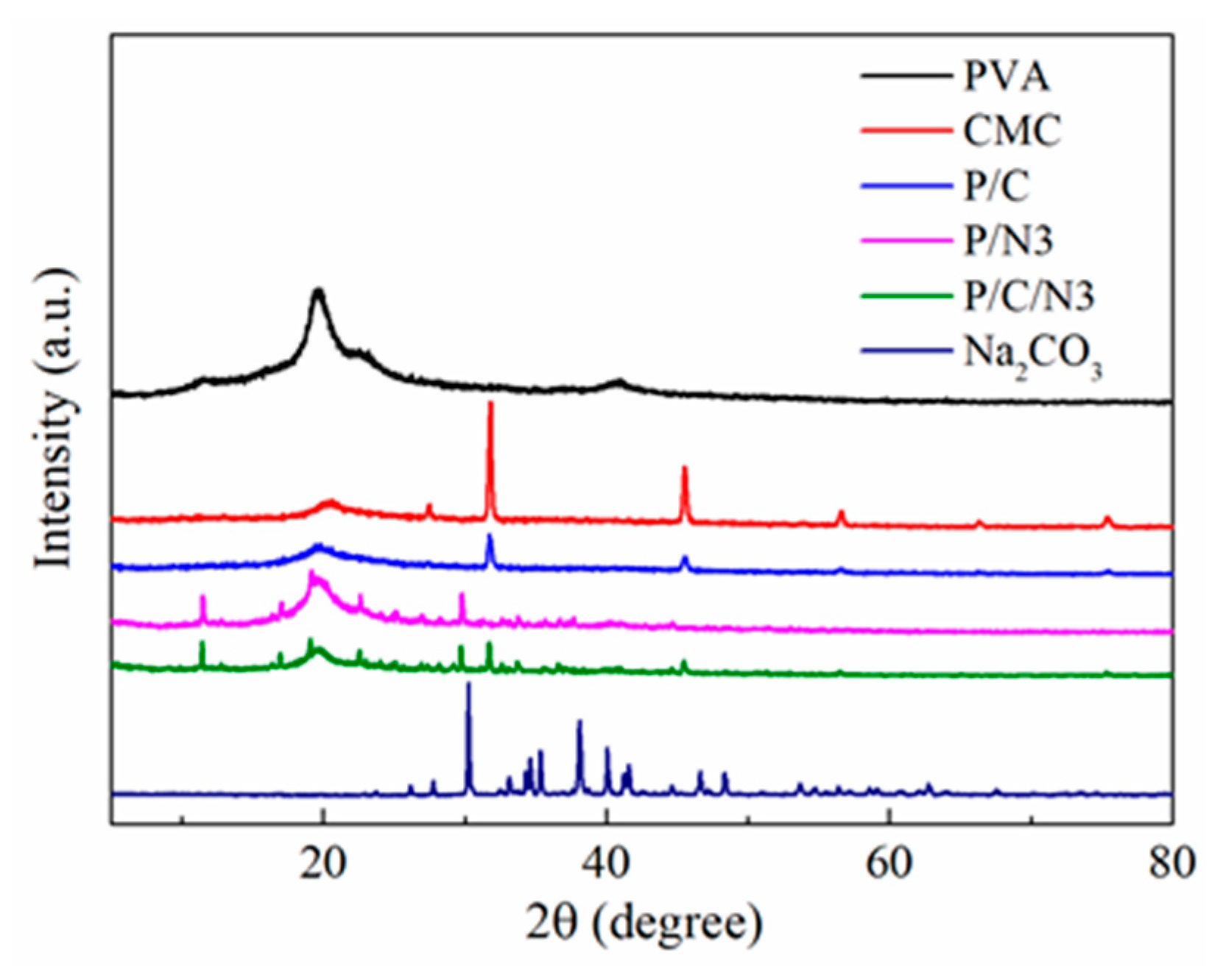
3.2. XRD Analysis
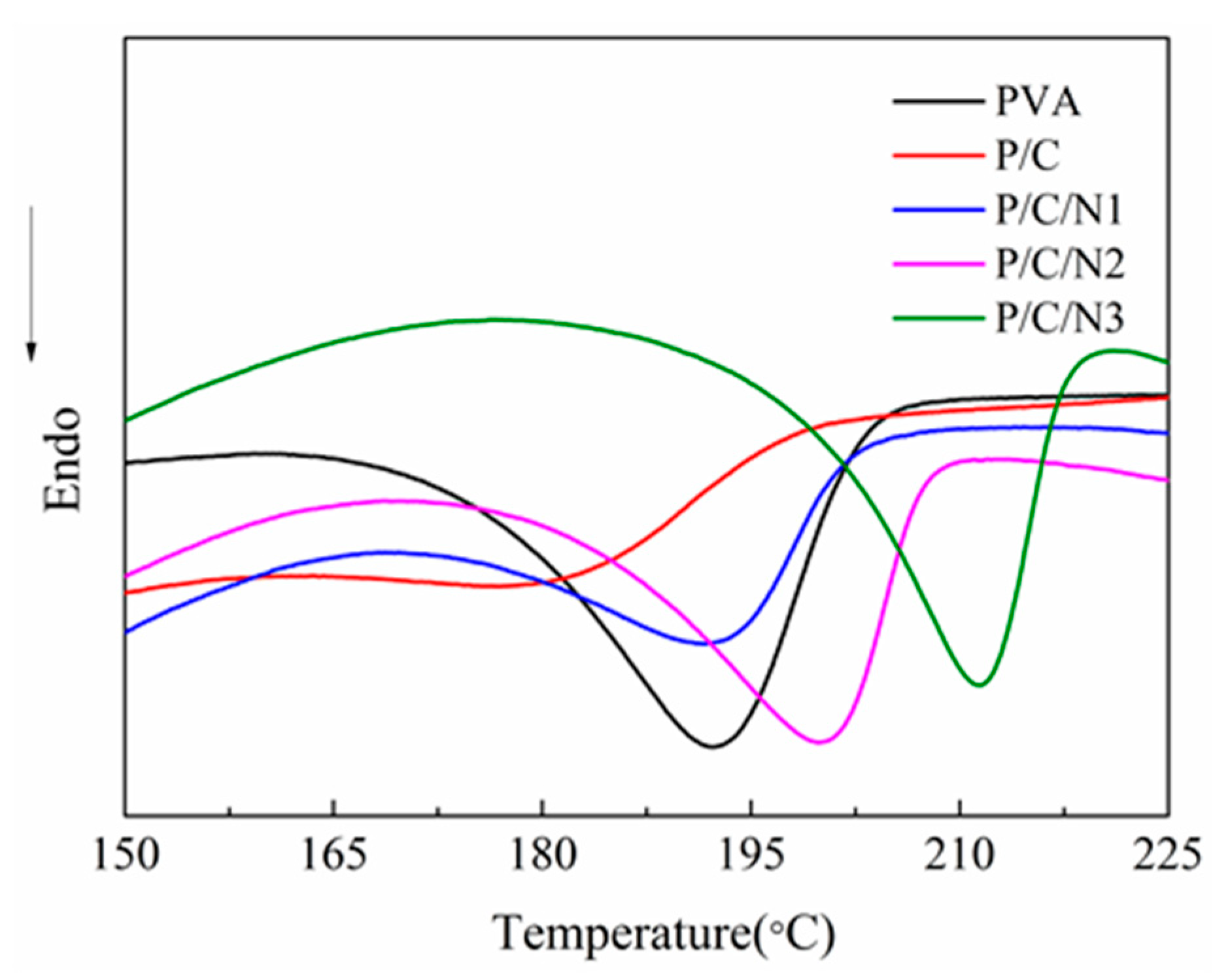
3.3. DSC Analysis
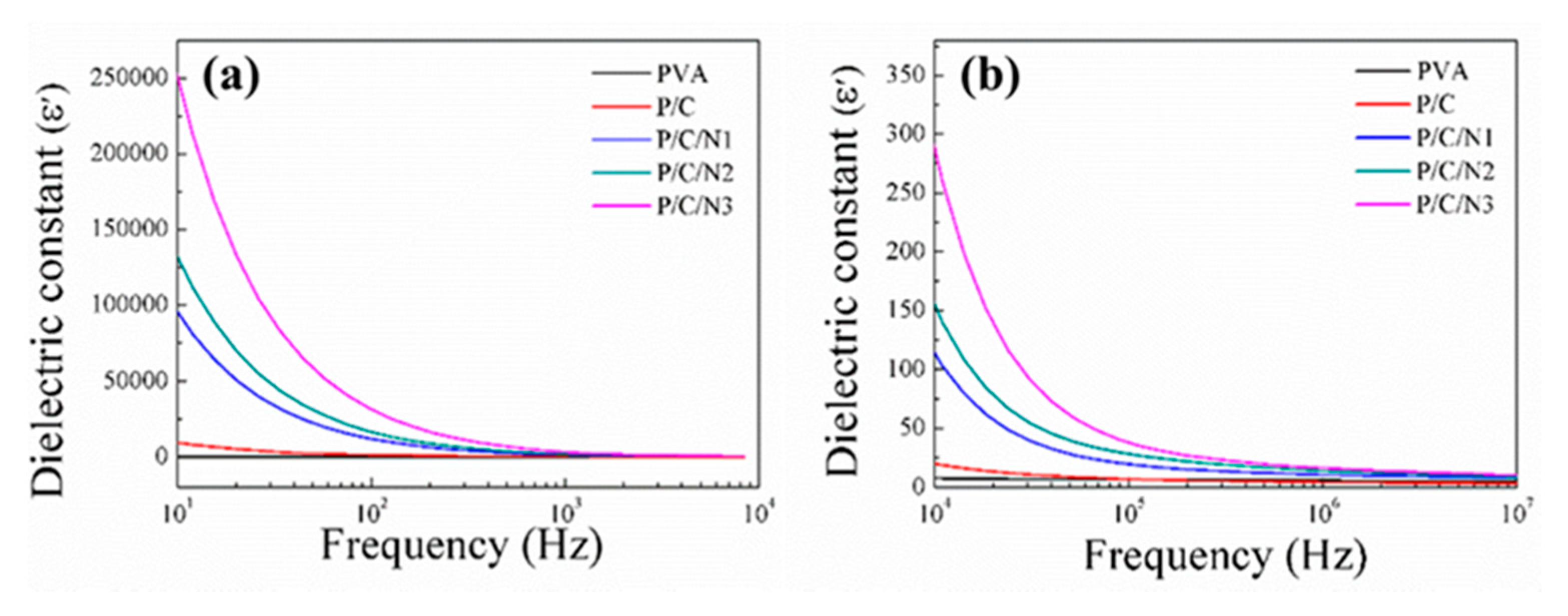
3.4. Dielectric Analysis
3.5. Water Sorption Analysis
3.6. Solubility Analysis
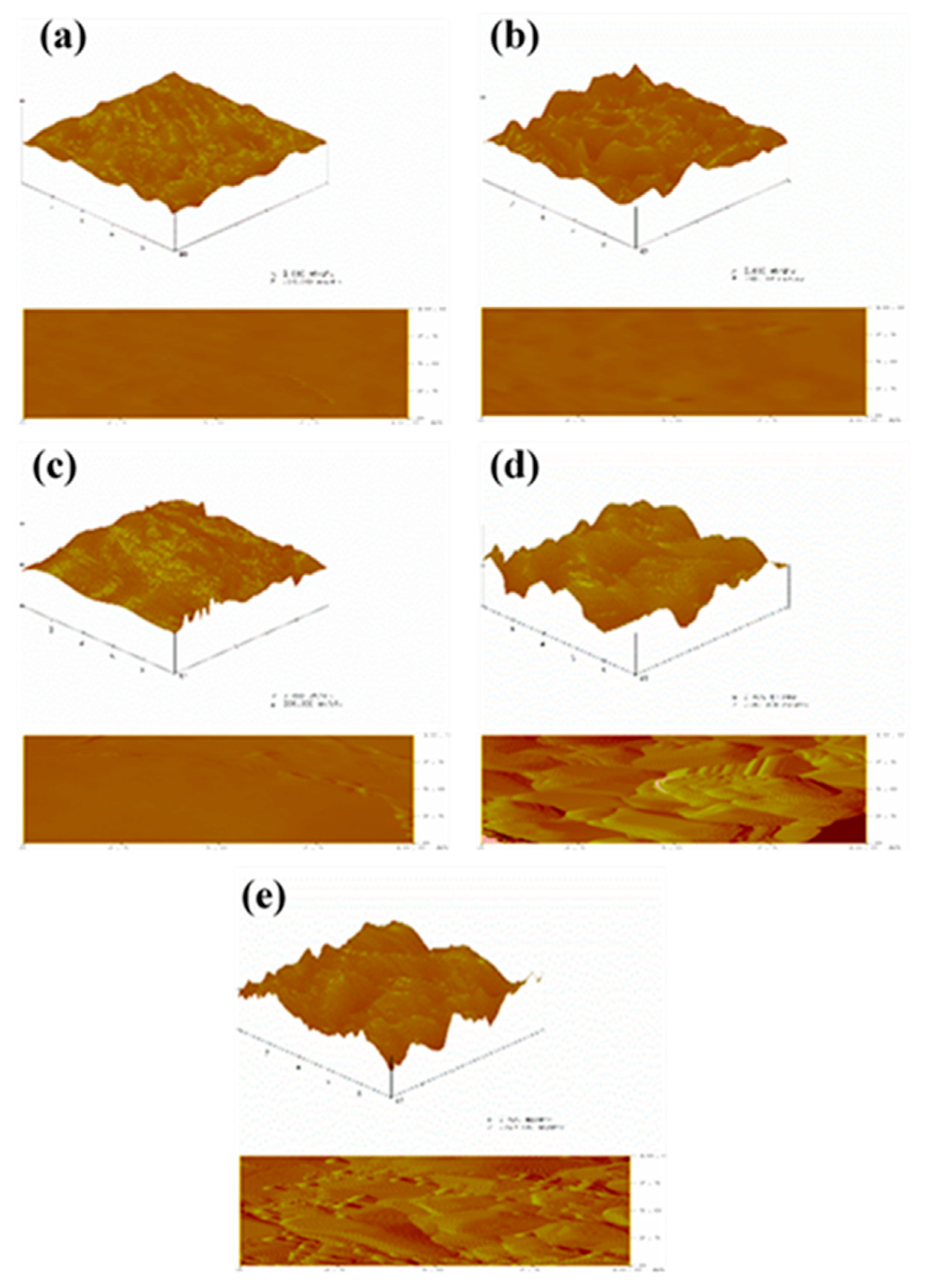
3.7. Surface Topography Analysis
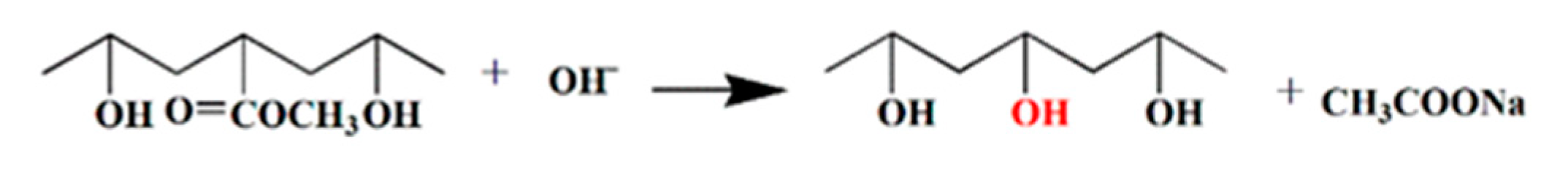
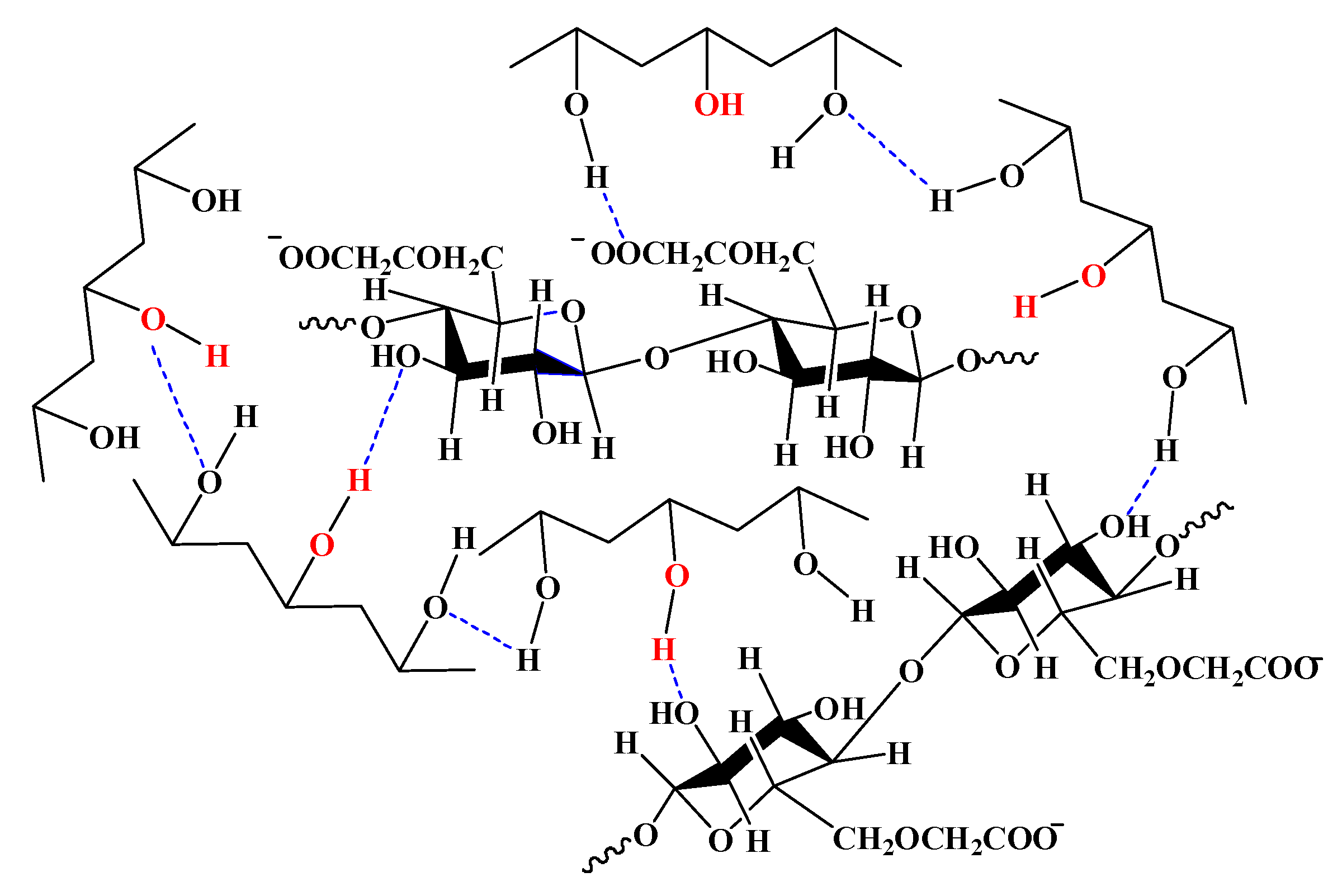
3.8. Mechanism Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Vlaia, L.; Coneac, G. Cellulose-derivatives-based hydrogels as vehicles for dermal and transdermal drug delivery. Emerg. Concepts Anal. Appl. Hydrogels 2016. [Google Scholar] [CrossRef]
- Kumar:, B.; Negi, Y.S. Water absorption and viscosity behaviour of thermally stable novel graft copolymer of carboxymethyl cellulose and poly(sodium 1-hydroxy acrylate). Carbohydr. Polym. 2018, 181, 862–870. [Google Scholar] [CrossRef] [PubMed]
- Entcheva, E.; Bien, H.; Yin, L.; Chung, C.Y.; Farrell, M.; Kostov, Y. Functional cardiac cell constructs on cellulose-based scaffolding. Biomaterials 2004, 25, 5753–5762. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Huang, S.; Li, X.; Zhang, L.; Tan, Y.; Wei, C.; Lv, J. Facile fabrication of novel polyhedral oligomeric silsesquioxane/carboxymethyl cellulose hybrid hydrogel based on supermolecular interactions. Mater. Lett. 2013, 90, 142–144. [Google Scholar] [CrossRef]
- Saha, N.; Shah, R.; Gupta, P.; Mandal, B.B.; Alexandrova, R.; Sikiric, M.D.; Saha, P. PVP-CMC hydrogel: An excellent bioinspired and biocompatible scaffold for osseointegration. Mater. Sci. Eng. 2019, 95, 440–449. [Google Scholar] [CrossRef]
- Agbovi, H.K.; Wilson, L.D. Design of amphoteric chitosan flocculants for phosphate and turbidity removal in wastewater. Carbohydr. Polym. 2018, 189, 360–370. [Google Scholar] [CrossRef]
- Holder, M.K.; Peters, N.V.; Whylings, J.; Fields, C.T.; Gewirtz, A.T.; Chassaing, B.; de Vries, G.J. Dietary emulsifiers consumption alters anxiety-like and social-related behaviors in mice in a sex-dependent manner. Sci. Rep. 2019, 9, 172. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Usha Rani, G.; Sen, G. Microwave initiated synthesis and application of polyacrylic acid grafted carboxymethyl cellulose. Carbohydr. Polym. 2012, 87, 2255–2262. [Google Scholar] [CrossRef]
- Toǧrul, H.; Arslan, N. Carboxymethyl cellulose from sugar beet pulp cellulose as a hydrophilic polymer in coating of mandarin. J. Food Eng. 2004, 62, 271–279. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, R.; Feng, W.; Zhou, X.; Chen, Z.; Wang, T. Carboxymethylcellulose/pectin inhibiting structural folding of rice proteins via trinary structural interplays. Int. J. Biol. Macromol. 2019, 133, 93–100. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, Y.; Luo, G.; Bai, S. Preparation of highly dispersed precipitated nanosilica in a membrane dispersion microreactor. Chem. Eng. J. 2014, 258, 327–333. [Google Scholar] [CrossRef]
- Harazono, K.; Nakamura, K. Decolorization of mixtures of different reactive textile dyes by the white-rot basidiomycete Phanerochaete sordida and inhibitory effect of polyvinyl alcohol. Chemosphere 2005, 59, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Yu, X.; Zhang, T.; Wang, X. Constructing zwitterionic coatings on thin-film nanofibrous composite membrane substrate for multifunctionality. Appl. Surf. Sci. 2019, 483, 979–990. [Google Scholar] [CrossRef]
- Follain, N.; Joly, C.; Dole, P.; Bliard, C. Properties of starch based blends. Part 2. Influence of poly vinyl alcohol addition and photocrosslinking on starch based materials mechanical properties. Carbohydr. Polym. 2005, 60, 185–192. [Google Scholar] [CrossRef]
- El-Sayed, S.; Mahmoud, K.H.; Fatah, A.A.; Hassen, A. DSC, TGA and dielectric properties of carboxymethyl cellulose/polyvinyl alcohol blends. Phys. B Condens. Matter 2011, 406, 4068–4076. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, G.; Lu, J.; Liang, H. Preparation and characterization of carboxymethyl cellulose/polyvinyl alcohol blend film as a potential coating material. Polym.-Plast. Technol. Eng. 2013, 52, 163–167. [Google Scholar] [CrossRef]
- Ibrahim, M.M.; Koschella, A.; Kadry, G.; Heinze, T. Evaluation of cellulose and carboxymethyl cellulose/poly(vinyl alcohol) membranes. Carbohydr. Polym. 2013, 95, 414–420. [Google Scholar] [CrossRef]
- Bajpai, A.K.; Shrivastava, J. In vitro enzymatic degradation kinetics of polymeric blends of crosslinked starch and carboxymethyl cellulose. Polym. Int. 2005, 54, 1524–1536. [Google Scholar] [CrossRef]
- Paralikar, S.; Simonsen, J.; Lombardi, J. Poly(vinyl alcohol)/cellulose nanocrystal barrier membranes. J. Membr. Sci. 2008, 320, 248–258. [Google Scholar] [CrossRef]
- Sivakumar, M.; Malaisamy, R.; Sajitha, C.J.; Mohan, D. Preparation and performance of cellulose acetate–polyurethane blend membranes and their applications—II. J. Membr. Sci. 2000, 169, 215–228. [Google Scholar] [CrossRef]
- El-Sawy, N.M.; El-Arnaouty, M.B.; Ghaffar, A.M.A. γ-Irradiation effect on the non-cross-linked and cross-linked polyvinyl alcohol films. Polym.-Plast. Technol. Eng. 2010, 49, 169–177. [Google Scholar] [CrossRef]
- Dai, H.; Huang, Y.; Huang, H. Eco-friendly polyvinyl alcohol/carboxymethyl cellulose hydrogels reinforced with graphene oxide and bentonite for enhanced adsorption of methylene blue. Carbohydr. Polym. 2017, 185, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Houssein, A.; Claude, D. Chemical modification of poly(vinyl alcohol) in water. Appl. Sci. 2015, 5, 840–850. [Google Scholar]
- Kuanova, A.; Nurpeissova, Z.A.; Mangazbayeva, R.; Park, K. The obtaining of composite materials based on carboxymethylcellulose and polyvinyl alcohol. Int. J. Biol. Chem. 2017, 10, 62–68. [Google Scholar] [CrossRef]
- Mansur, H.S.; Sadahira, C.M.; Souza, A.N.; Mansur, A.A. FTIR spectroscopy characterization of poly (vinyl alcohol) hydrogel with different hydrolysis degree and chemically crosslinked with glutaraldehyde. Mater. Sci. Eng. C 2008, 28, 539–548. [Google Scholar] [CrossRef]
- Zhu, Y.; Xiao, S.; Li, M.; Chang, Z. Natural macromolecule based carboxymethyl cellulose as a gel polymer electrolyte with adjustable porosity for lithium ion batteries. Power Sources 2015, 288, 368–375. [Google Scholar] [CrossRef]
- Biswal, D.; Singh, R. Characterisation of carboxymethyl cellulose and polyacrylamide graft copolymer. Carbonhydrate Polym. 2004, 57, 379–387. [Google Scholar] [CrossRef]
- Mei, Y.; Wang, Z.; Fang, H. Na-containing mineral transformation behaviors during Na2CO3-catalyzed CO2 gasification of high-alumina coal. Energy Fuels 2017, 31, 1235–1242. [Google Scholar] [CrossRef]
- Lagarón, J.M.; López-Rubio, A.; José Fabra, M. Bio-based packaging. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Zainuddin, N.K.; Samsudin, A.S. Investigation on the effect of NH4Br at transport properties in k–carrageenan based biopolymer electrolytes via structural and electrical analysis. Mater. Today Commun. 2018, 14, 199–209. [Google Scholar] [CrossRef]
- Alakanandana, A.; Subrahmanyam, A.R.; Siva Kumar, J. Structural and electrical conductivity studies of pure PVA and PVA doped with succinic acid polymer electrolyte system. Mater. Today Proc. 2016, 3, 3680–3688. [Google Scholar] [CrossRef]
- El-Gamal, S.; El Sayed, A.M.; Abdel-Hady, E.E. Effect of cobalt oxide nanoparticles on the nano-scale free volume and optical properties of biodegradable CMC/PVA films. J. Polym. Environ. 2017, 26, 2536–2545. [Google Scholar] [CrossRef]
- Uthayakumar, A.; Pandian, A.; Mathiyalagan, S. Interfacial effect of the oxygen-ion distribution on the conduction mechanism in strontium-added Ce0.8Sm0.2O2-δ/Na2CO3 nanocomposite. Phys. Chem. 2016, 120, 25068–25077. [Google Scholar] [CrossRef]
- Raza, R.; Wang, X.; Ma, Y.; Liu, X.; Zhu, B. Improved ceria-carbonate composite electrolytes. Hydrog. Energy 2010, 35, 2684–2688. [Google Scholar] [CrossRef]
- Blaine, R.L. Thermal applications note. TN 48. In Polymer Heats of Fusion; TA Instrument: New Castle, DE, USA, 2002. [Google Scholar]
- El-Zawawy, W.K. Blended graft copolymer of carboxymethyl cellulose and poly(vinyl alcohol) with banana fiber. J. Appl. Polym. Sci. 2006, 100, 1842–1848. [Google Scholar] [CrossRef]
- Peppas, N.A.; Wright, S.L. Solution diffusion in poly[(vinyl alcohol)/poly(acrylic acid)] interpenetrating networks. Macromolecules 1996, 29, 8798–8804. [Google Scholar] [CrossRef]
- Baig, M.I.; Ingole, P.G.; Jeon, J.-D.; Hong, S.U.; Choi, W.K.; Jang, B.; Lee, H.K. Water vapor selective thin film nanocomposite membranes prepared by functionalized silicon nanoparticles. Desalination 2019, 451, 59–71. [Google Scholar] [CrossRef]


| Sample | Sample Notation | PVA Solution | CMC Solution | Na2CO3 Solution | |||
|---|---|---|---|---|---|---|---|
| PVA (g) | Water (g) | CMC (g) | Water (g) | Na2CO3 (g) | Water (g) | ||
| PVA | PVA | 1 | 19 | ||||
| CMC | CMC | 0.4 | 19.6 | ||||
| PVA/CMC | P/C | 1 | 19 | 0.4 | 19.6 | ||
| PVA/CMC/Na2CO3 | P/C/N1 | 1 | 19 | 0.4 | 19.6 | 0.08 | 5 |
| P/C/N2 | 1 | 19 | 0.4 | 19.6 | 0.12 | 5 | |
| P/C/N3 | 1 | 19 | 0.4 | 19.6 | 0.16 | 5 | |
| PVA/Na2CO3 | P/N3 | 1 | 19 | 0.16 | 5 | ||
| Sample | Ac | Aa | Xc (%) |
|---|---|---|---|
| PVA | 2270 | 4223 | 34.96 |
| CMC | 373 | 1834 | 17.65 |
| P/C | 124 | 1536 | 7.47 |
| P/C/N3 | 214 | 953 | 18.34 |
| P/N3 | 501 | 1979 | 20.20 |
| Sample | PVA | P/C | P/C/N1 | P/C/N2 | P/C/N3 |
|---|---|---|---|---|---|
| Tm (°C) | 192 | 178 | 194 | 200 | 212 |
| Melting enthalpy (J/g) | 33.83 | 8.94 | 16.18 | 24.87 | 25.50 |
| Crystallinity (%) | 21.01 | 7.77 | 14.87 | 23.48 | 24.71 |
| PVA | P/C | P/C/N1 | P/C/N2 | P/C/N3 | |
|---|---|---|---|---|---|
| Rq | 10.205 | 19.963 | 16.160 | 295.52 | 264.09 |
| Ra | 7.756 | 15.115 | 11.991 | 233.14 | 210.62 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, J.; Li, Q.; Che, Y.; Liu, X.; Dong, C.; Chen, X.; Wang, C. Effect of Na2CO3 on the Microstructure and Macroscopic Properties and Mechanism Analysis of PVA/CMC Composite Film. Polymers 2020, 12, 453. https://doi.org/10.3390/polym12020453
Zhu J, Li Q, Che Y, Liu X, Dong C, Chen X, Wang C. Effect of Na2CO3 on the Microstructure and Macroscopic Properties and Mechanism Analysis of PVA/CMC Composite Film. Polymers. 2020; 12(2):453. https://doi.org/10.3390/polym12020453
Chicago/Turabian StyleZhu, Jufang, Qiuying Li, Yanchao Che, Xingchen Liu, Chengcheng Dong, Xinyu Chen, and Chao Wang. 2020. "Effect of Na2CO3 on the Microstructure and Macroscopic Properties and Mechanism Analysis of PVA/CMC Composite Film" Polymers 12, no. 2: 453. https://doi.org/10.3390/polym12020453
APA StyleZhu, J., Li, Q., Che, Y., Liu, X., Dong, C., Chen, X., & Wang, C. (2020). Effect of Na2CO3 on the Microstructure and Macroscopic Properties and Mechanism Analysis of PVA/CMC Composite Film. Polymers, 12(2), 453. https://doi.org/10.3390/polym12020453




