High-Conductivity, Flexible and Transparent PEDOT:PSS Electrodes for High Performance Semi-Transparent Supercapacitors
Abstract
1. Introduction
2. Experimental Section
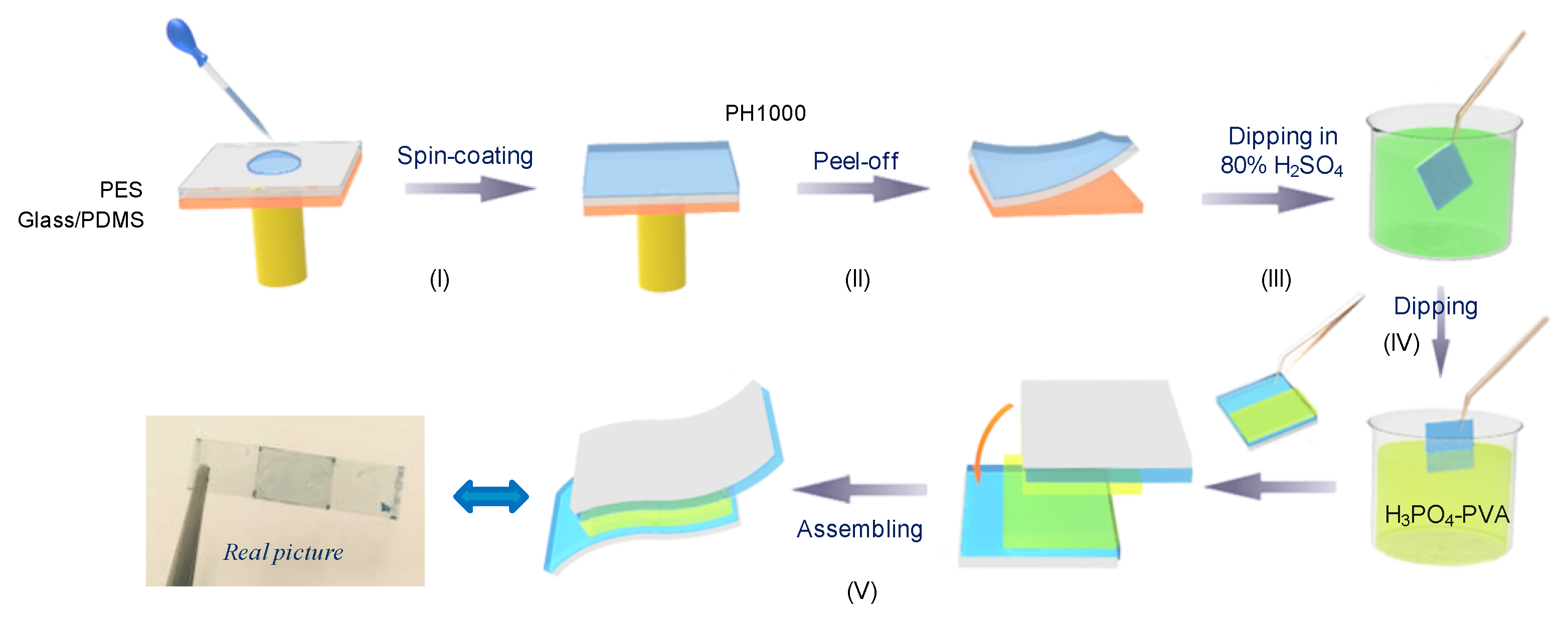
2.1. Preparation of the Flexible Highly-Conductive Transparent S-PH1000 Electrode
2.2. Fabrication and Characterization of Semi-Transparent Supercapacitors
3. Results and Discussion
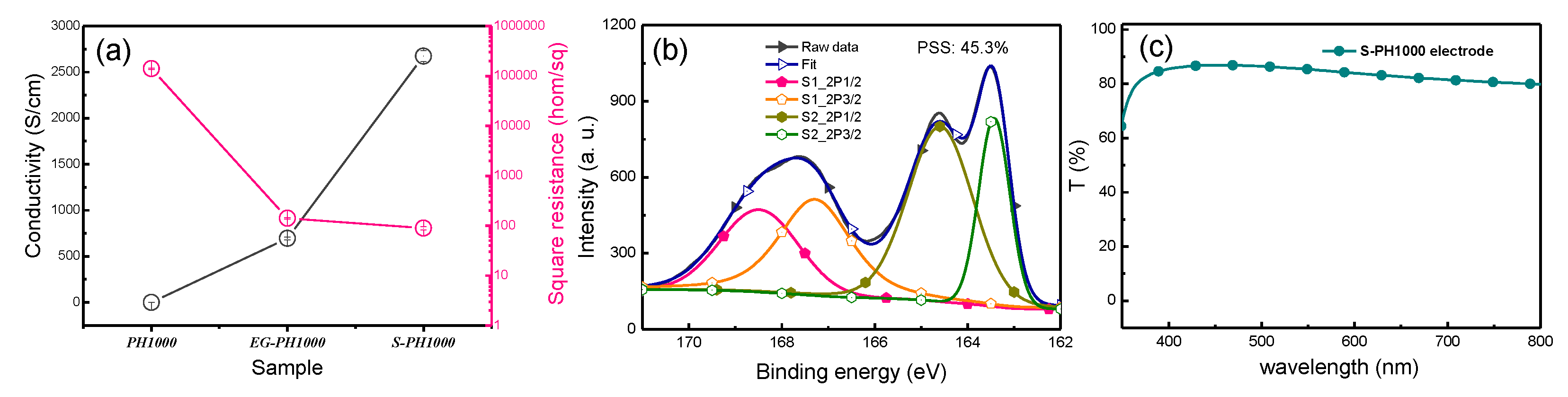
3.1. Preparation and Characterization of the High Conductivity S-PH1000 Films
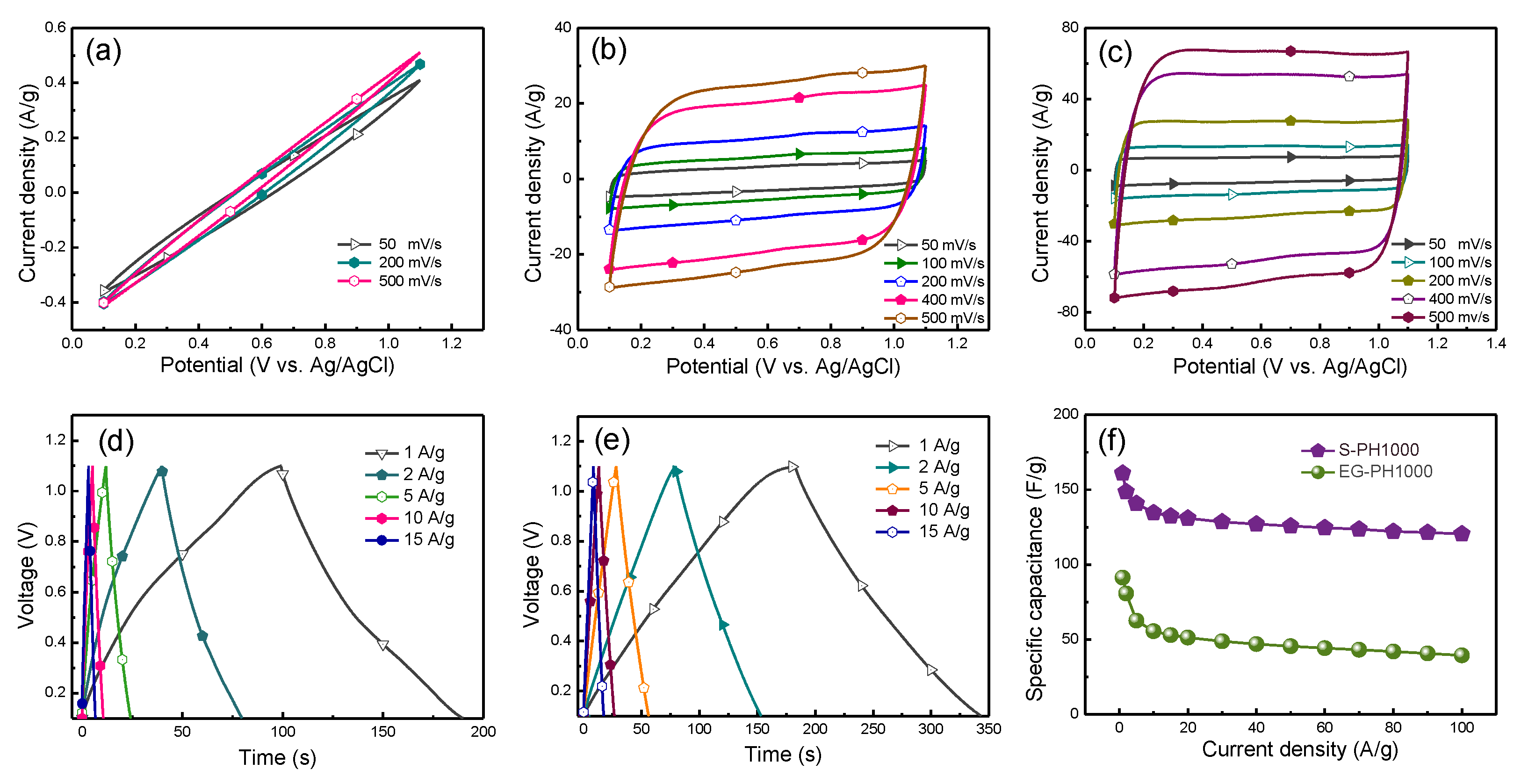
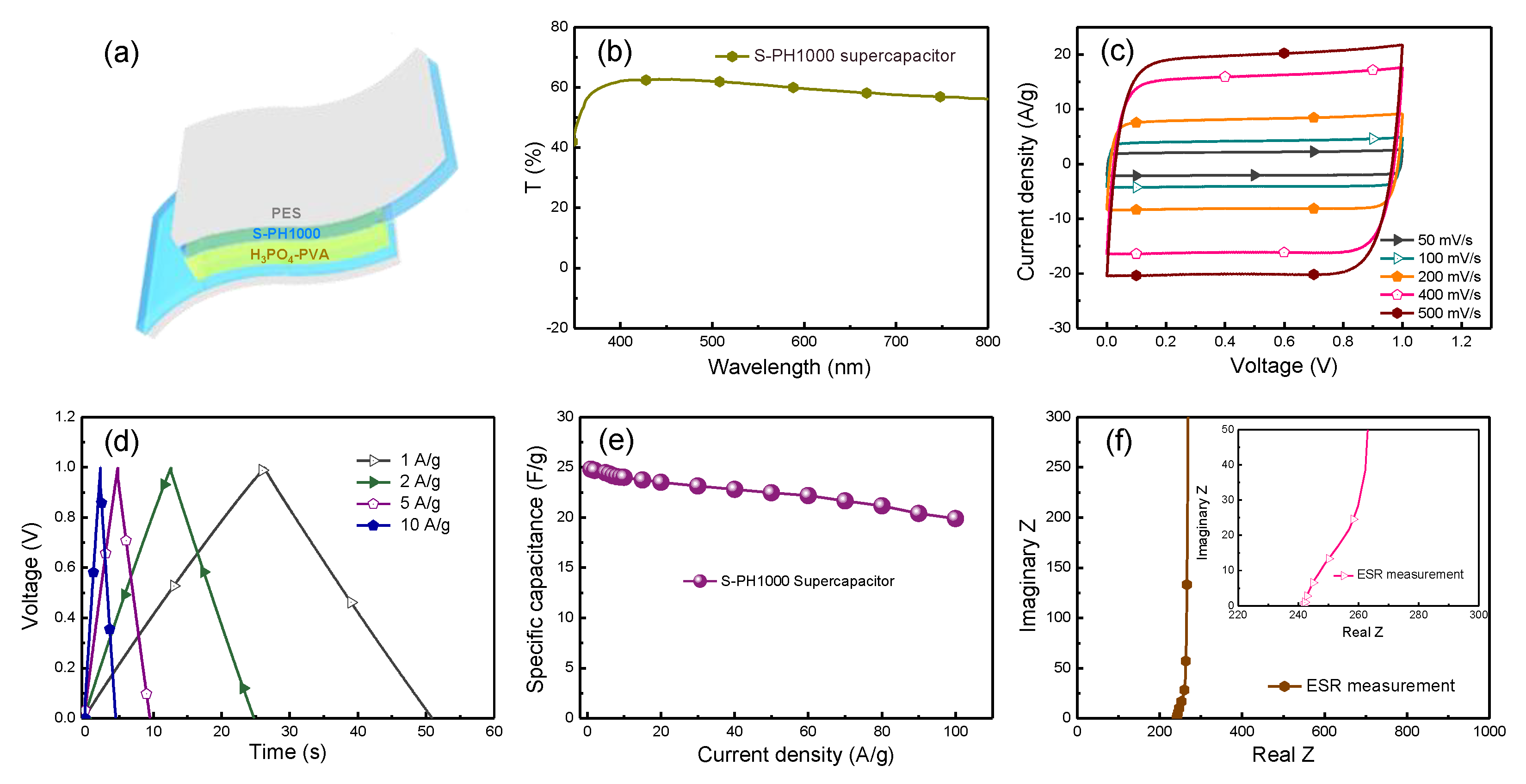
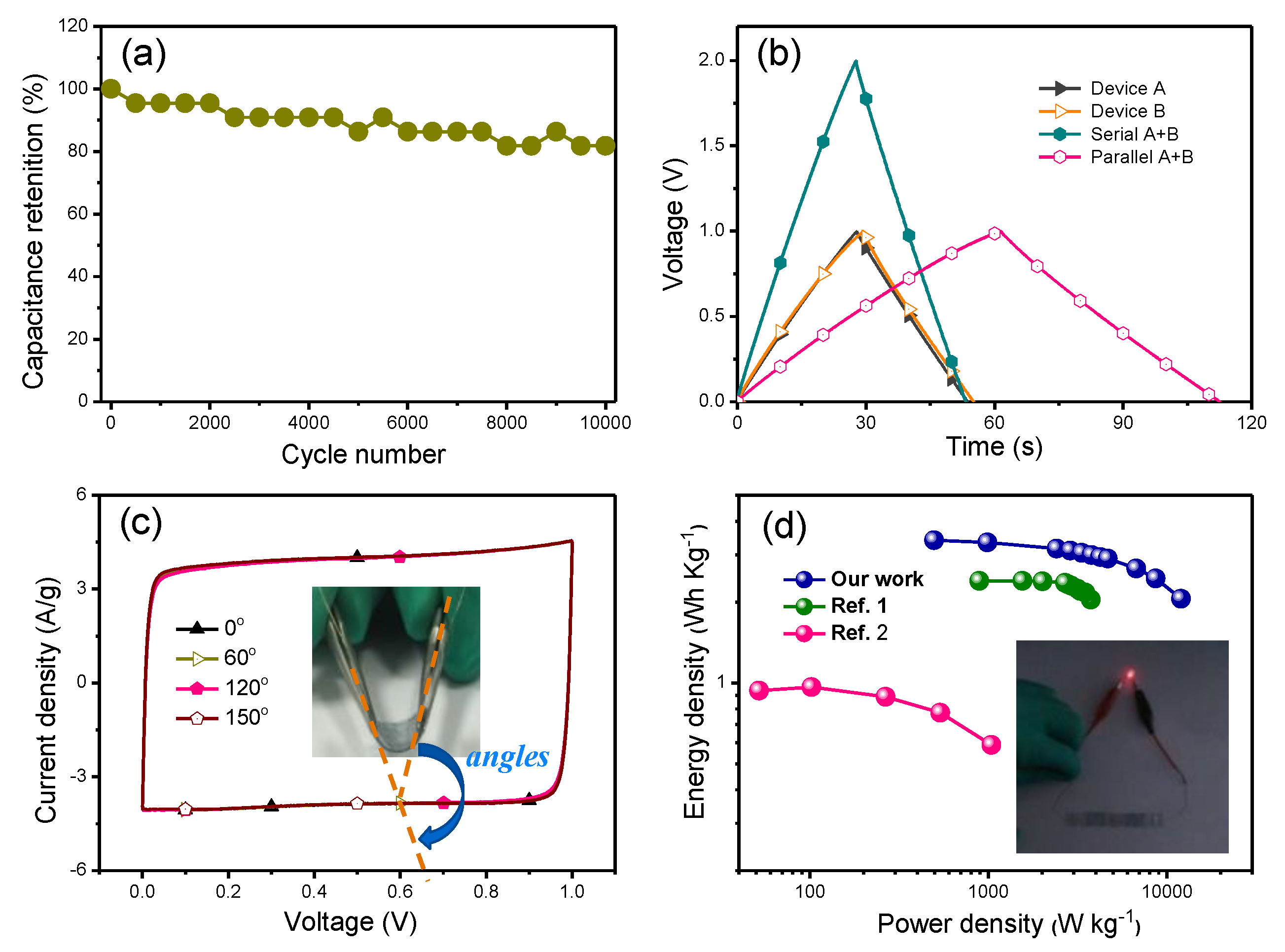
3.2. Application of S-PH1000 Electrodes for Semi-Transparent Flexible Supercapacitors
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Wu, H.; Kong, D.; Ruan, Z.; Hsu, P.-C.; Wang, S.; Yu, Z.; Carney, T.J.; Hu, L.; Fan, S.; Cui, Y. A transparent electrode based on a metal nanotrough network. Nat. Nanotechnol. 2013, 8, 421–425. [Google Scholar] [CrossRef] [PubMed]
- Donolato, M.; Tollan, C.; Porro, J.M.; Berger, A.; Vavassori, P. Flexible and stretchable polymers with embedded magnetic nanostructures. Adv. Mater. 2013, 25, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Gwon, H.; Hong, J.; Kim, H.; Seo, D.-H.; Jeon, S.; Kang, K. Recent progress on flexible lithium rechargeable batteries. Energy Environ. Sci. 2014, 7, 538–551. [Google Scholar] [CrossRef]
- Yu, W.J.; Lee, S.Y.; Chae, S.H.; Perello, D.; Han, G.H.; Yun, M.; Lee, Y.H. Small hysteresis nanocarbon-based integrated circuits on flexible and transparent plastic substrate. Nano Lett. 2011, 11, 1344–1350. [Google Scholar] [CrossRef]
- Chen, T.; Hao, R.; Peng, H.; Dai, L. High-performance, stretchable, wire-shaped supercapacitors. Angew. Chem. 2015, 54, 618–622. [Google Scholar] [CrossRef]
- Guan, X.; Kong, D.; Huang, Q.; Cao, L.; Zhang, P.; Lin, H.; Lin, Z.; Yuan, H. In situ growth of a high-performance all-solid-state electrode for flexible supercapacitors based on a PANI/CNT/EVA composite. Polymers 2019, 11, 178. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, T.; Dai, L. Layer-by-layer growth of CH(3)NH(3)PbI(3-x)Clx for highly efficient planar heterojunction perovskite solar cells. Adv. Mater. 2015, 27, 1053–1059. [Google Scholar] [CrossRef]
- Xiao, X.; Peng, X.; Jin, H.; Li, T.; Zhang, C.; Gao, B.; Hu, B.; Huo, K.; Zhou, J. Freestanding mesoporous VN/CNT hybrid electrodes for flexible all-solid-state supercapacitors. Adv. Mater. 2013, 25, 5091–5097. [Google Scholar] [CrossRef]
- Ding, Z.; Hao, Z.; Meng, B.; Xie, Z.; Liu, J.; Dai, L. Few-layered graphene quantum dots as efficient hole-extraction layer for high-performance polymer solar cells. Nano Energy 2015, 15, 186–192. [Google Scholar] [CrossRef]
- Hu, Z.; Xiao, X.; Chen, C.; Li, T.; Huang, L.; Zhang, C.; Su, J.; Miao, L.; Jiang, J.; Zhang, Y.; et al. Al-doped α-MnO2 for high mass-loading pseudocapacitor with excellent cycling stability. Nano Energy 2015, 11, 226–234. [Google Scholar] [CrossRef]
- Cheng, Y.; Huang, L.; Xiao, X.; Yao, B.; Yuan, L.; Li, T.; Hu, Z.; Wang, B.; Wan, J.; Zhou, J. Flexible and cross-linked N-doped carbon nanofiber network for high performance freestanding supercapacitor electrode. Nano Energy 2015, 15, 66–74. [Google Scholar] [CrossRef]
- Hui, J.; Wei, D.; Chen, J.; Yang, Z. Polyaniline nanotubes/carbon cloth composite electrode by thermal acid doping for high-performance supercapacitors. Polymers 2019, 11, 2053. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, X.; Zi, Y.; Wang, S.; Li, Z.; Zheng, L.; Yi, F.; Li, S.; Wang, Z.L. A Flexible Fiber-Based Supercapacitor-Triboelectric-Nanogenerator Power System for Wearable Electronics. Adv. Mater. 2015, 27, 4830–4836. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Xu, H.; Lachman, N.; Ghaffari, M.; Wu, S.; Liu, Y.; Ugur, A.; Gleason, K.K.; Wardle, B.L.; Zhang, Q.M. Advanced asymmetric supercapacitor based on conducting polymer and aligned carbon nanotubes with controlled nanomorphology. Nano Energy 2014, 9, 176–185. [Google Scholar] [CrossRef]
- Yuan, L.; Xiao, X.; Ding, T.; Zhong, J.; Zhang, X.; Shen, Y.; Hu, B.; Huang, Y.; Zhou, J.; Wang, Z.L. Paper-based supercapacitors for self-powered nanosystems. Angew. Chem. 2012, 51, 4934–4938. [Google Scholar] [CrossRef]
- Niu, Z.; Zhou, W.; Chen, J.; Feng, G.; Li, H.; Hu, Y.; Ma, W.; Dong, H.; Li, J.; Xie, S. A repeated halving approach to fabricate ultrathin single-walled carbon nanotube films for transparent supercapacitors. Small 2013, 9, 518–524. [Google Scholar] [CrossRef]
- Jung, H.Y.; Karimi, M.B.; Hahm, M.G.; Ajayan, P.M.; Jung, Y.J. Transparent, flexible supercapacitors from nano-engineered carbon films. Sci. Rep. 2012, 2, 773. [Google Scholar] [CrossRef]
- Chen, T.; Peng, H.; Durstock, M.; Dai, L. High-performance transparent and stretchable all-solid supercapacitors based on highly aligned carbon nanotube sheets. Sci. Rep. 2014, 4, 3612. [Google Scholar] [CrossRef]
- Hiralal, P.; Wang, H.; Unalan, H.E.; Liu, Y.; Rouvala, M.; Wei, D.; Andrew, P.; Amaratunga, G.A.J. Enhanced supercapacitors from hierarchical carbon nanotube and nanohorn architectures. J. Mater. Chem. 2011, 21, 17810. [Google Scholar] [CrossRef]
- Li, H.; Zhao, Q.; Wang, W.; Dong, H.; Xu, D.; Zou, G.; Duan, H.; Yu, D. Novel planar-structure electrochemical devices for highly flexible semitransparent power generation/storage sources. Nano Lett. 2013, 13, 1271–1277. [Google Scholar] [CrossRef]
- Rodriguez-Moreno, J.; Navarrete-Astorga, E.; Dalchiele, E.A.; Schrebler, R.; Ramos-Barrado, J.R.; Martin, F. Vertically aligned ZnO@CuS@PEDOTcore@shellnanorod arrays decorated with MnO(2) nanoparticles for a high-performance and semi-transparent supercapacitor electrode. Chem. Commun. 2014, 50, 5652–5655. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Lee, J.S.; Cho, S. Comparative studies on two-electrode symmetric supercapacitors based on polypyrrole: Poly(4-styrenesulfonate) with different molecular weights of poly(4-styrenesulfonate). Polyme. 2019, 11, 232. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zhang, L.; Zhang, J. A review of electrode materials for electrochemical supercapacitors. Chem. Soc. Rev. 2012, 41, 797–828. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Luo, B.; Jia, Y.; Li, X.; Wang, B.; Song, Q.; Kang, F.; Zhi, L. Renewing functionalized graphene as electrodes for high-performance supercapacitors. Adv. Mater. 2012, 24, 6348–6355. [Google Scholar] [CrossRef] [PubMed]
- Gul, H.; Shah, A.-U.-H.A.; Bilal, S. Achieving ultrahigh cycling stability and extended potential window for supercapacitors through asymmetric combination of conductive polymer nanocomposite and activated carbon. Polymers 2019, 11, 1678. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Song, J.; Yin, X.; Su, Z.; Li, Z. Research progress on polymer solar cells based on PEDOT:PSS electrodes. Polymers 2020, 12, 145. [Google Scholar] [CrossRef]
- Kim, N.; Lee, B.H.; Choi, D.; Kim, G.; Kim, H.; Kim, J.R.; Lee, J.; Kahng, Y.H.; Lee, K. Role of interchain coupling in the metallic state of conducting polymers. Phys. Rev. Lett. 2012, 109, 106405. [Google Scholar] [CrossRef]
- Li, Z.; Sun, H.; Hsiao, C.-L.; Yao, Y.; Xiao, Y.; Shahi, M.; Jin, Y.; Cruce, A.; Liu, X.; Jiang, Y.; et al. A free-standing high-output power density thermoelectric device based on structure-ordered PEDOT: PSS. Adv. Electron. Mater. 2018, 4, 1700496. [Google Scholar] [CrossRef]
- Li, Z.; Qin, F.; Liu, T.; Ge, R.; Meng, W.; Tong, J.; Xiong, S.; Zhou, Y. Optical properties and conductivity of PEDOT: PSS films treated by polyethylenimine solution for organic solar cells. Org. Electron. 2015, 21, 144–148. [Google Scholar] [CrossRef]
- Fabretto, M.V.; Evans, D.R.; Mueller, M.; Zuber, K.; Hojati-Talemi, P.; Short, R.D.; Wallace, G.G.; Murphy, P.J. Polymeric Material with Metal-Like Conductivity for Next Generation Organic Electronic Devices. Chem. Mater. 2012, 24, 3998–4003. [Google Scholar] [CrossRef]
- Xia, Y.; Sun, K.; Ouyang, J. Solution-processed metallic conducting polymer films as transparent electrode of optoelectronic devices. Adv. Mater. 2012, 24, 2436–2440. [Google Scholar] [CrossRef]
- Kim, N.; Kee, S.; Lee, S.H.; Lee, B.H.; Kahng, Y.H.; Jo, Y.R.; Kim, B.J.; Lee, K. Highly conductive PEDOT:PSSnanofibrils induced by solution-processed crystallization. Adv. Mater. 2014, 26, 2268–2272. [Google Scholar] [CrossRef]
- Ouyang, J. “Secondary doping” methods to significantly enhance the conductivity of PEDOT: PSS for its application as transparent electrode of optoelectronic devices. Displays 2013, 34, 423–436. [Google Scholar] [CrossRef]
- Li, Z.; Meng, W.; Tong, J.; Zhao, C.; Qin, F.; Jiang, F.; Xiong, S.; Zeng, S.; Xu, L.; Hu, B.; et al. A nonionic surfactant simultaneously enhancing wetting property and electrical conductivity of PEDOT:PSS for vacuum-free organic solar cells. Sol. Energy Mater. Sol. Cells 2015, 137, 311–318. [Google Scholar] [CrossRef]
- Meng, W.; Ge, R.; Li, Z.; Tong, J.; Liu, T.; Zhao, Q.; Xiong, S.; Jiang, F.; Mao, L.; Zhou, Y. Conductivity Enhancement of PEDOT:PSS Films via Phosphoric Acid Treatment for Flexible All-Plastic Solar Cells. ACS Appl. Mater. Inter. 2015, 7, 14089–14094. [Google Scholar] [CrossRef]
- Rwei, S.-P.; Lee, Y.-H.; Shiu, J.-W.; Sasikumar, R.; Shyr, U.-T. Characterization of solvent-treated PEDOT:PSS thin films with enhanced conductivities. Polymers 2019, 11, 134. [Google Scholar] [CrossRef]
- Morris, J.D.; Wong, K.M.; Penaherrera, C.D.; Payne, C.K. Mechanism of the biomolecular synthesis of PEDOT:PSS: Importance of heme degradation by hydrogen peroxide. Biomater. Sci. 2016, 4, 331–337. [Google Scholar] [CrossRef]
- Li, Z.; Ma, G.; Ge, R.; Qin, F.; Dong, X.; Meng, W.; Liu, T.; Tong, J.; Jiang, F.; Zhou, Y.; et al. Free-Standing Conducting Polymer Films for High-Performance Energy Devices. Angew. Chem. 2016, 128, 991–994. [Google Scholar] [CrossRef]
- D’Arcy, J.M.; El-Kady, M.F.; Khine, P.P.; Zhang, L.; Lee, S.H.; Davis, N.R.; Liu, D.S.; Yeung, M.T.; Kim, S.Y.; Turner, C.L. Vapor-phase polymerization of nanofibrillar poly (3, 4-ethylenedioxythiophene) for supercapacitors. ACS Nano 2014, 8, 1500–1510. [Google Scholar] [CrossRef]
- Anothumakkool, B.; Soni, R.; Bhange, S.N.; Kurungot, S. Novel scalable synthesis of highly conducting and robust PEDOT paper for a high performance flexible solid supercapacitor. Energy Environ. Sci. 2015, 8, 1339–1347. [Google Scholar] [CrossRef]
- Zhang, C.; Higgins, T.M.; Park, S.-H.; O’Brien, S.E.; Long, D.; Coleman, J.N.; Nicolosi, V. Highly flexible and transparent solid-state supercapacitors based on RuO2/PEDOT:PSS conductive ultrathin films. Nano Energy 2016, 28, 495–505. [Google Scholar] [CrossRef]
- Cheng, T.; Zhang, Y.-Z.; Zhang, J.-D.; Lai, W.-Y.; Huang, W. High-performance free-standing PEDOT:PSS electrodes for flexible and transparent all-solid-state supercapacitors. J. Mater. Chem. A 2016, 4, 10493–10499. [Google Scholar] [CrossRef]
- Cai, G.; Darmawan, P.; Cui, M.; Wang, J.; Chen, J.; Magdassi, S.; Lee, P.S. Highly Stable Transparent Conductive Silver Grid/PEDOT:PSS Electrodes for Integrated Bifunctional Flexible Electrochromic Supercapacitors. Adv. Energy Mater. 2016, 6, 1501882. [Google Scholar] [CrossRef]
- Kim, N.; Kang, H.; Lee, J.H.; Kee, S.; Lee, S.H.; Lee, K. Highly conductive all-plastic electrodes fabricated using a novel chemically controlled transfer-printing method. Adv. Mater. 2015, 27, 2317–2323. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, J.; Ma, G.; Qin, F.; Hu, L.; Luo, B.; Liu, T.; Yin, X.; Su, Z.; Zeng, Z.; Jiang, Y.; et al. High-Conductivity, Flexible and Transparent PEDOT:PSS Electrodes for High Performance Semi-Transparent Supercapacitors. Polymers 2020, 12, 450. https://doi.org/10.3390/polym12020450
Song J, Ma G, Qin F, Hu L, Luo B, Liu T, Yin X, Su Z, Zeng Z, Jiang Y, et al. High-Conductivity, Flexible and Transparent PEDOT:PSS Electrodes for High Performance Semi-Transparent Supercapacitors. Polymers. 2020; 12(2):450. https://doi.org/10.3390/polym12020450
Chicago/Turabian StyleSong, Jiaxing, Guoqiang Ma, Fei Qin, Lin Hu, Bangwu Luo, Tiefeng Liu, Xinxing Yin, Zhen Su, Zhaobing Zeng, Youyu Jiang, and et al. 2020. "High-Conductivity, Flexible and Transparent PEDOT:PSS Electrodes for High Performance Semi-Transparent Supercapacitors" Polymers 12, no. 2: 450. https://doi.org/10.3390/polym12020450
APA StyleSong, J., Ma, G., Qin, F., Hu, L., Luo, B., Liu, T., Yin, X., Su, Z., Zeng, Z., Jiang, Y., Wang, G., & Li, Z. (2020). High-Conductivity, Flexible and Transparent PEDOT:PSS Electrodes for High Performance Semi-Transparent Supercapacitors. Polymers, 12(2), 450. https://doi.org/10.3390/polym12020450




